Abstract
The effect of the hydrophilic ionic liquids (ILs) 1-butyl-2,3-dimethylimidazolium bromide [bdmim][Br] and 1-hexyl-2,3-dimethylimidazolium bromide [hdmim][Br] on the aggregation and surface active behaviour of the non-ionic surfactant Triton™ X-100 (TX-100) was studied in aqueous media. Several aggregation properties of TX-100 + IL/water systems, such as critical micelle concentration (CMC), surface active parameters, aggregation number (N agg) and aggregate size, were determined by surface tension, fluorescence and dynamic light scattering (DLS) techniques. It was found that the average micellar size and aggregation number decrease, whereas the CMC increases with increasing concentration of ILs. Interestingly, the CMC value of TX-100 is reduced slightly below 0.5 wt% of both the ILs in the medium. At higher wt% of IL in the system the CMC increases. It was demonstrated that ILs [bdmim][Br] and [hdmim][Br] can be judiciously used at different wt% for modifying the physico-chemical properties of TX-100.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactant self-assembly structures have been explored for various industrial and research purposes. Surfactant monomers tend to form micelles, vesicles or bilayers and many more nanostructures which provide a suitable media for numerous synthetic and commercial processes [1–3]. In employing surfactants for these applications, critical micelle concentration (CMC), thermodynamic parameters, surface properties and aggregation number etc. have prominent influence on their usage. These properties are frequently altered by using different types of additives, such as alcohols, co-surfactants, salts, organic solutes and recently ionic liquids (ILs) [4–9]. ILs are highly versatile class of compounds which have interesting and useful physico-chemical properties [10, 11]. ILs having long chain cations or anions possess aggregation properties and can act like a co-surfactant in surfactant media [12]. Many research groups including our own have explored the effect of ILs on surfactant properties. We have explored the effect of 1,2,3-trialkylimidazolium ILs on anionic and cationic surfactants [13–18]. Previous reports were confined to the effect of 1,3-dialkylsubstituted ILs on different surfactants [19–22].
The IL structure, constituent cation and anion, alkyl groups and functional groups dictate the extent of modulations in surfactant properties. In our earlier studies we showed that ILs 1-butyl-2,3-dimethylimidazolium chloride [bdmim][Cl] [13] and 1-butyl-2,3-dimethylimidazolium tetrafluoroborate [bdmim][BF4] [14], owing to the greater hydrophobic nature due to the presence of three alkyl chains in its structure, cause an increase in the CMC of sodium dodecylsulfate (SDS). The reverse trend in aggregation is observed when ILs [bdmim][Cl] [15] and 1,2-dimethyl-3-octylimidazolium chloride [odmim][Cl] [16] were employed to alter aggregation in dodecyltrimethylammonium bromide (DTAB). This behaviour is also shown by IL 1-butyl-2,3-dimethylimidazolium bromide when added to aqueous solution of tetradecyltrimethylammonium bromide (TTAB) [17] and cetyltrimethylammonium bromide (CTAB) [18]; however, in the case of TTAB and CTAB, TTAB causes a greater increase in surface activity on addition of IL than CTAB, as a result of lower solvophobicity.
In the present work we studied the effect of trisubstituted ILs 1-butyl-2,3-dimethylimidazolium bromide [bdmim][Br] and 1-hexyl-2,3-dimethylimidazolium bromide [hdmim][Br] on aggregation properties of a non-ionic surfactant. With ionic surfactants the major interactions are electrostatic and hydrophobic. For non-ionic surfactants, ion-induced dipole interactions are expected to have a significant effect on the results. We measured the CMC of TX-100 using tensiometry. The tensiometry results were used to obtain surface parameters. To the best of our knowledge, no study has reported the surface properties and surface parameters for TX-100 + IL/water systems. The CMC values of TX-100 in the presence of different wt% of ILs were also verified by fluorescence measurements using multiple probes. The fluorescence quenching of pyrene is used to obtain the aggregation number of TX-100 micelles on addition of various wt% of ILs in the surfactant solution. To obtain the average micellar size, dynamic light scattering has been used. Our results clearly indicate that ILs [bdmim][Br] and [hdmim][Br] have a significant effect on the properties of TX-100. The structure of ILs, [bdmim][Br] and [hdmim][Br], surfactant TX-100, fluorescent probes pyrene and pyrene-1-carboxaldehyde (PyCHO) and quencher cetylpyridinium chloride (CPC) are shown in Scheme 1.
Experimental Section
Materials
1,2-Dimethylimidazole (98 %), pyrene (99.9 %) and pyrene-1-carboxaldehyde (99 %) were purchased from Sigma-Aldrich. TX-100 (98 %, AR) was obtained from Merck-Schuchardt. 1-Bromobutane (>99 %) and 1-bromohexane (>99 %) were obtained from Acros Organics. Cetylpyridinium chloride (99 %) was purchased from Loba Chemie and methanol (99 %) from Rankem, IL; [bdmim][Br] was synthesized in our laboratory and is of the same origin and purity as described in our previous study [17].
Synthesis of 1-Hexyl-2,3-dimethylimidazolium Bromide [hdmim][Br]
The IL [hdmim][Br] was synthesized following the similar procedure as used for [bdmim][Br]; 1-bromohexane was used in place of 1-bromobutane. The crude product was washed with ether and dried under vacuum to get [hdmim][Br] in 84 % yield. Karl–Fisher examination of the IL indicated that the water content reduced to less than 280 ppm.
1H NMR (300 MHz, CDCl3, δ ppm): 0.85 (t, 3H), 1.27 (m, 2H), 1.71 (m, 2H), 2.51 (s, 3H), 3.59 (s, 3H), 4.03 (t, 2H), 7.23 (d, 1H), 7.27 (d, 1H).
Methods
All chemicals were weighed using an electronic balance (model GR-202, A&D Co. Ltd. Japan) with precision of ±0.1 mg. Doubly distilled de-ionized water obtained from a Milli-Q Academic water purification system (Millipore) was used to prepare all solutions. Each measurement was performed in triplicate and the average values reported.
Surface Tension Measurements
Surface tension measurements were made using a Du Noüy tensiometer (SD Hardsons Ltd) using the ring-detachment method with a precision of 0.01 mN m−1. The measurement temperature was maintained using a double jacketed vessel and a thermostatic bath with uncertainty of ±0.01 K. The platinum–iridium ring used in the study was properly cleaned and flame-dried before every experiment to get rid of any IL and surfactant residues on its surface. The maximal error of the surface tension measured related to water (71.99 mN m−1 at 25 °C [23]) is estimated to be less than ±0.2 mN m−1.
Fluorescence Measurements
A spectrofluorimeter (model RF-5301PC, Shimadzu) provided with blazed holographic grating excitation and emission monochromators and having a 150-W xenon lamp was used to obtain the fluorescence spectra. TX-100 and IL solutions at various concentrations were freshly prepared in aqueous media. The sample required for the measurements and stock solution of the probes was prepared as described in our previous study [17]. The data acquisition was also done as mentioned in earlier work [17].
Dynamic Light Scattering
The size of surfactant aggregates present in solution was obtained using dynamic light scattering. A Zetasizer Nano apparatus (Malvern, UK) was used to perform the experiments at 298 K. A 4-mW-power He–Ne laser light source was used. IL-surfactant solutions of particular concentration were prepared for the measurements.
Results and Discussion
Surface Tension Measurements
Critical Micelle Concentration and Surface Activity
The plots of surface tension, γ versus TX-100 concentration on addition of different wt% of ILs, [bdmim][Br] and [hdmim][Br], are shown in Figs. 1 and 2 respectively, (Fig. S1 shows the surface tension isotherms for 0.5 wt% IL). As the surfactant concentration increases surface tension gradually decreases and attains a minimum value after which surface tension remains nearly constant. The point at which γ becomes constant is considered the onset of micellization and taken as the CMC. At the CMC, the air–water interface is saturated with surfactant monomers and IL molecules, while the rest of the molecules of these two interacting species dissolve in the bulk to form the micellar aggregates. The CMC values thus obtained are reported in Table 1. It is observed that the CMC of TX-100 decreases on addition of ILs up to 0.5 wt%. From 2.0 wt% onwards the CMC value increases in the case of both [bdmim][Br] and [hdmim][Br]. This observed trend illustrates the dual nature of ILs.
In our previous studies we analysed the effect of trisubstituted ILs on anionic and cationic surfactants [13–18]. It was observed that the wt% at which IL switches from its electrolytic behaviour to co-solvent or co-surfactant behaviour highly depends on the surfactant under study. In a previous report on TX-100 + IL 1-butyl-3-methyimidazolium tetrafluoroborate [bmim][BF4] in aqueous media [9], Pandey et al. only observed an increase in surfactant CMC with increasing wt% of IL. They studied from 2.0 to 30.0 wt% of added IL. In our work, we covered the lower (0.1 and 0.5) as well as higher (2.0, 5.0 and 10.0) wt% region of added IL. We stopped after 10.0 wt% IL addition as only increasing CMC was observed.
The increase in CMC at higher (2.0, 5.0 and 10.0) wt% of IL can be understood on the basis of ion–dipole interactions between TX-100 polar head groups and IL cations/anions. The hydrogen bonding interactions will occur between hydroxyl groups of TX-100 and IL anions. The hydrophobic interactions will occur between the alkyl chains of IL and surfactant TX-100. In our previous study on cationic surfactants TTAB [17] and CTAB [18], the electrostatic interactions dominated over hydrophobic interactions until the IL concentration became very high; whereas here, the tendency of electrostatic interactions is negligible owing to the non-ionic nature of surfactant, and polar interactions like ion–dipole and hydrogen bonding will occur. In a study by Pandey et al. the IL [bmim][BF4] was disubstituted with an acidic hydrogen present on the carbon atom situated between the two nitrogen atoms of the imidazolium ring [9]. This hydrogen can interact well with the oxygen of ethoxy and hydroxyl groups of TX-100 molecules. The greater extent of these stabilizing interactions to surfactant monomers in that study as compared to our studied system TX-100 + [bdmim][Br]/[hdmim][Br] led to a much greater increase in CMC as compared to what is observed here.
The surface tension data are used to determine various surface parameters. The following equations were used to determine surface pressure, Π CMC, surface excess concentration, Γ max, and minimum area per surfactant molecule at the air–solution interface, A min [24]:
where the symbols have their usual meanings documented in the literature. The surface pressure decreases with increasing IL concentration, indicating that the aggregation efficiency decreases. The surface excess concentration also decreases with the addition of additive ILs; consequently the area per surfactant molecule at the interface increases.
The decrease in Γ max is greater in the case of short chain IL owing to greater ion–dipole interactions of [bdmim]+ ions with TX-100 molecules as compared to [hdmim]+ ions as a result of the small size of the former. This will render more TX-100 molecules in the bulk rather than at the air–solution interface. The low value of A min is also due to close packing of TX-100 monomers in pure water rather than in the presence of IL/water systems. The study by Ruiz et al. on TX-100 + ethylene glycol/water systems was considered for comparison where a decrease in Γ max and an increase in A min were also observed [25]. This behaviour was attributed to the structure-breaking property of ethylene glycol as well as solubilisation of the non-polar chain of surfactant. Our observation here also indicates the co-solvent nature of studied ILs at higher wt%.
The thermodynamic parameters of micellization and adsorption such as free energy change of micellization and free energy change of adsorption are obtained using the following equations:
where the symbols have the same meanings as mentioned in the literature. The \(\Delta G_{\text{m}}^{ \circ }\) values become more negative at 0.1 and 0.5 wt% and less negative at 2.0, 5.0 and 10.0 wt% IL than the value in pure water. This indicates more spontaneous micellization in the presence of 0.1 and 0.5 wt% of added ILs and the less spontaneous nature of micellization process observed in the presence of ILs from 2.0 wt% onwards. The \(\Delta G_{\text{ads}}^{ \circ }\) values are more negative than the corresponding \(\Delta G_{\text{m}}^{ \circ }\) values for both ILs. From this observation it can be inferred that work is required to transfer the surfactant monomers present in the monolayer at the surface to the micelle in aqueous solution. The \(\Delta G_{\text{ads}}^{ \circ }\) values for IL [bdmim][Br] are more negative than the TX-100 value in pure water while the converse trend is observed in the case of IL [hdmim][Br]. This indicates that more work is done in the presence of IL [hdmim][Br] than [bdmim][Br] for micellization of TX-100. It means that the adsorption of IL [hdmim][Br] on TX-100 surfactant is comparatively less spontaneous at the air–mixtures interface. For analysing mixing synergism between TX-100 and ILs, another thermodynamic quantity free energy of the given air–water interface—\(G_{{_{ \hbox{min} } }}^{{\left( {\text{s}} \right)}}\) was obtained as
where \(G_{ \hbox{min} }^{{^{{\left( {\text{s}} \right)}} }}\) is the free energy change accompanied by the transition from the bulk phase to the surface phase of the solution components. A lower value of this quantity indicates formation of a more thermodynamically stable surface. The \(G_{ \hbox{min} }^{{^{{\left( {\text{s}} \right)}} }}\) increases in the presence of IL [bdmim][Br] whereas it decreases in the presence of IL [hdmim][Br] compared to the TX-100 value in pure water. The CMC value and surface parameters of TX-100 in pure water obtained by us are consistent with the literature [25].
Fluorescence Measurements
Determination of CMC Using Pyrene Fluorescence
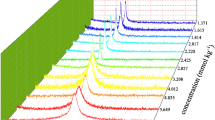
The fluorescent emission of pyrene was used to determine the CMC of aqueous TX-100 solutions in the presence of different wt% of ILs, [bdmim][Br] and [hdmim][Br]. It is well established in the literature that the fluorescent emission ratio of the 1st to 3rd vibronic peak of pyrene is sensitive to its surrounding environment. Pyrene shows significant changes in emission intensity of its peaks in pre- and post-micellar environments owing to the presence of micellar pseudo-phase post-micellization. A transition is observed in I I/I III ratio at micellization, resulting in a sigmoidal curve in all the TX-100 solutions in the presence of different wt% of additive ILs as shown in Figs. 3 and 4 for ILs [bdmim][Br] and [hdmim][Br], respectively (Fig.S2 shows data for 0.5 wt% addition of each IL). The midpoint of transition was considered the CMC as presented in Table 2. The CMC decreases initially at lower (0.1 and 0.5) wt% of both the ILs but starts increasing with higher (2.0, 5.0 and 10.0) wt% of these two ILs. The increase in CMC is comparatively larger for [hdmim][Br] than [bdmim][Br] as observed through tensiometric measurements, although the trend remains the same. This is due to the fact that use of different techniques leads to a slight variation in CMC. In tensiometry, the concentration assigned to the CMC value is the point where surface tension became constant. This occurs at the onset of micellization. In fluorimetry, at the start of micellization, I I/I III begins to decrease from a nearly constant value. This ratio decreases until micellization is completed. At the end, at the concentration when all the surfactant monomers underwent micellization, this ratio again becomes constant. Here the midpoint of the transition is taken as the CMC value. This point lies in the middle of the onset and completion of micellization. A different concentration point is assigned as CMC in different techniques, giving slightly different values.
We were surprised at the close agreement in CMC values due to addition of the two ILs. Here the interactions between TX-100 and IL molecules will be ion–dipole interactions between ions of ILs and TX-100 dipoles or hydrogen bonding interactions between the hydroxyl groups of TX-100 and [Br]− ions of ILs. As compared to the previous study done using pyrene fluorescence measurements on TX-100 + [bmim][BF4], the results show that in the presence of IL [bmim][BF4], the CMC value of aqueous TX-100 solution increased to about 0.75 mM at 2.0 wt%, 2.0 mM at 5.0 wt %, 3.75 mM at 10.0 wt% and to about 100 mM at 30.0 wt% of IL [9]. While in our study, the CMC of aqueous TX-100 solution doubled on addition of either of the two studied ILs as is clear from Table 2. As mentioned earlier, the hydrogen bonding interactions between hydroxyl and ethoxy groups of TX-100 and the acidic hydrogen present on the carbon situated between the two nitrogen atoms in the imidazolium ring lead to a major effect on the CMC.
These interactions are not possible in our study as the ILs are trisubstituted and no acidic hydrogen is available for interaction through hydrogen bonding. So IL cations [bdmim]+ and [hdmim]+ will mainly interact through ion–dipole interactions or through hydrophobic interactions via alkyl chains. As hexyl chains are longer than butyl chains, the CMC value increases slightly more in the case of TX-100 + [hdmim][Br] systems than TX-100 + [bdmim][Br] systems. This greater hydrophobic environment is also visible through the I I/I III ratio, which decreases considerably in the presence of [hdmim][Br]. Here the bromine anion of both ILs shows significant interactions and thereby influences the CMC value. Initially ILs behave like electrolytes, as salts like potassium chloride tend to decrease the CMC of TX-100 [26]. At higher concentrations, ILs behave more like co-solvents and increase the CMC by stabilizing surfactant monomers through various interactions as discussed.
Determination of CMC Using Pyrene-1-carboxaldehyde Fluorescence
In order to supplement our fluorescence data, we used another probe, PyCHO, to determine the CMC of aqueous TX-100 solutions. The fluorescence results thus obtained are presented in Figs. S3 and S4 corresponding to addition of ILs [bdmim][Br] and [hdmim][Br], respectively (Fig. S5 data shows for 0.5 wt% addition of each IL). These results were found in good agreement with the results obtained from pyrene probe and tensiometric measurements. The data points were fitted into a simplistic sigmoidal equation and the midpoint of the transition was taken as the CMC. The PyCHO fluorescence emission spectrum consists of a single peak whose intensity varies in the pre- and post-micellization regions. Normalized fluorescence intensity plots are used to determine the CMC as presented in Table 2.
Determination of Micellar Aggregation Number
The micellar aggregation number (N agg) of aqueous TX-100 solutions in the absence and presence of different wt% of ILs [bdmim][Br] and [hdmim][Br] was obtained by subsequent fluorescence quenching of probe pyrene by addition of quencher CPC in the medium. The following equation is used for this purpose [27]:
where the symbols used have their usual meanings. The aggregation number for different IL-containing TX-100 solutions were obtained by plotting ln(I O /I Q) versus [CPC]micelle. The linear fit of data is used to determine the N agg for 10 mmol kg−1 aqueous TX-100 solutions as depicted in Figs. S6 and S7, for addition of ILs, [bdmim][Br] and [hdmim][Br], respectively. An initial slight increase is observed in N agg up to 0.5 wt% addition of both the ILs, after this N agg starts decreasing and reaches to 66 and 62 for [bdmim][Br] and [hdmim][Br] respectively, from the initial value of 92. The N agg decreases with increase in CMC and vice versa is well documented in the literature. This is also observed in our study. The N agg followed the opposite trend as was observed in the case of CMC in this study. The initial increase is due to the electrolyte nature of ILs which reduces the repulsion among polyoxyethylene head groups due to ion–dipole interactions with IL ions as well as hydrogen bonding interactions with IL anion as discussed in earlier sections. At higher wt% of IL, the co-solvent nature of IL and greater number of IL moieties in solution make it difficult for TX-100 monomers to pack and form the micellar aggregate. Consequently N agg decreases with increasing wt% of IL in the medium. Pandey and co-workers also observed a decrease in N agg of TX-100 with higher wt% of IL [bmim][BF4] [9]. There the aggregation number decreased to about 16 ± 9 at 30.0 wt% of IL [bmim][BF4] from 94 ± 5 for 120 mM aqueous TX-100 solution.
Dynamic Light Scattering
The micellar size of TX-100 aggregates varies in the presence of ILs [bdmim][Br] and [hdmim][Br]. The variation in scattering intensity versus micellar diameter is shown in Figs. 5 and 6. The micellar size obtained for different TX-100 + IL solutions is included in Table 2. It is clearly evident that micellar size first increases in the presence of both ILs, but later on starts decreasing. This observation is highly consistent with the trend observed for aggregation number. The greater the number of surfactant monomers is in the micellar aggregate, the larger the aggregate size will be. At higher wt% of ILs [bdmim][Br] and [hdmim][Br] the aggregation number of TX-100 decreases as a result of assembly of lesser numbers of surfactant monomers so as per effect micellar size decreases. If compared to our previous studies on cationic surfactants TTAB/CTAB + [bdmim][Br] systems, there the micellar size increased irrespective of the increase or decrease in CMC on increasing wt% of IL. There colossal electrostatic interactions among IL anions and cationic head groups lead to water penetration in the aggregates which led to an overall increase in micellar size. The situation is different here; the head group of the non-ionic surfactant is polar but not charged, so the tendency for water penetration decreases even in the presence of ILs [bdmim][Br] and [hdmim][Br]. In the previous study done with TX-100 + [bmim][BF4] in aqueous media, the authors also observed a decrease in micellar size. The micellar size decreased to 5.3 ± 0.3 at 10.0 wt% of IL because of the significantly different behaviour of ILs compared to other additives [9].
Conclusions
The effect of trisubstituted ILs [bdmim][Br] and [hdmim][Br] on aggregation and surface properties of non-ionic surfactant TX-100 has been firmly established. By varying the IL chain length we studied the effect of long chain and short chain ILs. During this study we observed that both ILs produce similar effects but to different extents. This is due to interactions with the IL anion which is the same in both ILs; in contrast, interactions with the IL cations vary with the alkyl chain length. The magnitude of the interactions will vary from one IL to another depending upon the type of interaction involved. An initial lowering in the CMC is observed at lower (0.1 and 0.5) wt% of ILs. At higher IL concentrations (2.0, 5.0 and 10.0 wt%), the CMC increases. In general, surface pressure and surface excess concentration decrease with increasing wt% of IL, while minimum area available per molecule increases. The free energy change is less negative than the change observed for TX-100 in pure water, indicating a less spontaneous micellization process. Fluorescence probe studies coincided with the tensiometric observations. The I I/I III ratio of pyrene at high IL concentration decreases more for [hdmim][Br] than [bdmim][Br] as a result of greater hydrophobic interactions. The micellar aggregation number and micellar size further reinforced these results. The aggregate size increases with increase in aggregation number and vice versa. Our results clearly indicate the impact of the two ILs [bdmim][Br] and [hdmim][Br] on aqueous TX-100 solutions. As compared to the previous study [9], these ILs maintain the surface activity of TX-100 to a greater extent.
References
Morai Y (1992) Micelles: theoretical and applied aspects. Springer, New York
Fendler JH (1983) Membrane mimetic chemistry: characterizations and applications of micelles, microemulsions, monolayers, bilayers, vesicles and host-guest systems. Wiley, New York
Holmberg K, Johnsson B, Kronberg B, Lindman B (2003) Surfactants and polymers in aqueous solution. Wiley, Chichester
Christov NC, Denkov ND, Kralchevsky PA, Ananthapadmanabhan KP, Lips A (2004) Synergistic sphere-to-rod micelle transition in mixed solutions of sodium dodecylsulfate and cocoamidopropyl betaine. Langmuir 20:565–571
Wydro P (2007) The influence of the size of the hydrophilic group on the miscibility of zwitterionic and nonionic surfactants in mixed monolayers and micelles. J Colloid Interface Sci 316:107–113
Paul BC, Islam SS, Ismail K (1998) Effect of acetate and propionate co-ions on the micellization of sodium dodecylsulfate in water. J Phys Chem B 102:7807–7812
Neves ACS, Valente AJM, Burrows HD, Ribeiro ACF, Lobo VMM (2007) Effect of terbium(III) chloride on the micellization properties of sodium decyl- and dodecyl-sulfate solutions. J Colloid Interface Sci 306:166–174
Behera K, Dahiya P, Pandey S (2007) Effect of added ionic liquid on aqueous Triton X-100 micelles. J Colloid Interface Sci 307:235–245
Behera K, Pandey MD, Porel M, Pandey S (2007) Unique role of hydrophilic ionic liquid in modifying properties of aqueous Triton X-100. J Chem Phys 127:184501–184510
Earle MJ, Seddon KR (2000) Green solvents for the future. Pure Appl Chem 72:1391–1398
Huddleston JG, Visser AE, Reichert WM, Willauer HD, Broker GA, Rogers RD (2001) Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem 3:156–164
Dong B, Li N, Zheng L, Yu L, Inoue T (2007) Surface adsorption and micelle formation of surface active ionic liquids in aqueous solution. Langmuir 23:4178–4182
Pal A, Pillania A (2014) Effect of trisubstituted imidazolium based ionic liquid 1-butyl-2,3-dimethylimidazolium chloride on the aggregation behaviour of sodium dodecylsulphate in aqueous media. Colloids Surf A Physicochem Eng Asp 452:18–24
Pal A, Pillania A (2014) Modulating the aggregation behavior of aqueous sodium dodecylsulphate (SDS) with addition of trisubstituted imidazolium based ionic liquid 1-butyl-2,3-dimethylimidazolium tetrafluoroborate [bdmim][BF4]. Fluid Ph Equilib 375:23–29
Pal A, Pillania A (2015) Modulating effect of ionic liquid 1-butyl-2,3-dimethylimidazolium chloride on micellization behaviour of cationic surfactant dodecyltrimethylammonium bromide in aqueous media. Fluid Ph Equilib 389:67–73
Pal A, Pillania A (2015) Thermodynamic and aggregation properties of aqueous dodecyltrimethylammonium bromide in the presence of hydrophilic ionic liquid 1,2-dimethyl-3-octylimidazolium chloride. J Mol Liq 212:818–824
Pal A, Pillania A (2015) The effect of hydrophilic ionic liquid 1-butyl-2,3-dimethylimidazolium bromide on the aggregation behavior of tetradecyltrimethylammonium bromide in aqueous media. J Mol Liq 209:6–13
Pal A, Pillania A (2016) Thermodynamic and micellization properties of aqueous cetyltrimethylammonium bromide solution in presence of 1-butyl-2,3-dimethylimidazolium bromide. Fluid Ph Equilib 412:115–122
Beyaz A, Oh WS, Reddy VP (2004) Ionic liquids as modulators of the critical micelle concentration of sodium dodecyl sulphate. Colloids Surf B 35:119–124
Misonoa T, Sakaia H, Sakaia K, Abea M, Inoue T (2011) Surface adsorption and aggregate formation of nonionic surfactants in a room temperature ionic liquid, 1-butyl-3-methylimidazolium hexafluorophosphate (bmimPF6). J Colloid Interface Sci 358:527–533
Javadian S, Ruhi V, Heydari A, Shahir AA, Yousefi A, Akbari J (2013) Self-assembled CTAB nanostructures in aqueous/ionic liquid systems: effects of hydrogen bonding. Ind Eng Chem Res 52:4517–4526
Shi L, Jing X, Gao H, Gu Y, Zheng L (2013) Ionic liquid-induced changes in the properties of aqueous sodium dodecylsulfate solution: effect of acidic/basic functional groups. Colloid Polym Sci 291:1601–1612
Vargaftik NB, Volkov BN, Voljak LD (1983) International tables of the surface tension of water. J Phys Chem Ref Data 12:817–820
Jaycock MJ, Parfitt GD (1981) Chemistry of interfaces. Wiley, New York
Ruiz CC, Molina-Bolivar JA, Aguiar J, MacIssac G, Moroze S, Palepu R (2001) Thermodynamic and structural studies of Triton X-100 micelles in ethylene glycol-water mixed solvents. Langmuir 17:6831–6840
Molina-Bolivar JA, Aguiar J, Ruiz CC (2001) Light scattering and fluorescence probe studies on micellar properties of Triton X-100 in KCl solutions. Mol Phys 99:1729–1741
Turro NJ, Yekta A (1978) Luminescent probes for detergent solutions. A simple procedure for determination of the mean aggregation number of micelles. J Am Chem Soc 100:5951–5952
Acknowledgments
The University Grants Commission (UGC), New Delhi, India is been gratefully acknowledged by the authors for providing SRF fellowship to author Ankita Pillania through letter No. F.17-39/2008(SA-I). The authors also wish to acknowledge the Department of Chemistry, Panjab University, Chandigarh for providing the DLS research facility.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Pal, A., Pillania, A. Effect of Ionic Liquids on Surface and Aggregation Properties of Non-ionic Surfactant Triton™ X-100 in Aqueous Media. J Surfact Deterg 19, 1189–1198 (2016). https://doi.org/10.1007/s11743-016-1876-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1876-x