Abstract
The ultimate prospect of tissue engineering is to create autologous tissue grafts for future replacement therapies through utilization of cells and biomaterials simultaneously. Bio-printing is a novel technique, a growing field that is leading to the global revolution in medical sciences that has gained significant attention. Bio-printing has the potential to be used in producing human engineered tissues like bone and skin which then ultimately can be used in the clinics. In this paper, the 3D bio-printing applications of the engineered human tissues that are available (skin and bone) are reviewed. It is evident that various tissue engineering techniques have been applied in the fabrication of skin tissue; therefore, it leads to introduce tissue substitutes such as complementary, split-thickness skin graft, allografts, acellular dermal substitutes and cellularized graft-like commercial products, i.e., Dermagraft and Apligraf. Also, some bone scaffolds based on hydroxyapatite and biphasic calcium phosphate are available in the market. The technology of bio-printing has got validated for bone and skin tissue fabrication, and it is hoped that other tissues could be produced by this technique.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Regenerative medicine holds the promise to restitute the normal function of cells, tissues or organs lost due to disease or damage via replacing or regenerating [1]. In fact, there are three solutions for patients with organ impairment, based upon the condition and severity of the destruction, they are: graft implantation, substitution and restoration. Graft implantation has comprehensively extensive lists of anticipants all around the world, for example, organ transplant waiting list updated every 15 in the USA [2]. The ultimate prospect of tissue engineering is to create autologous tissue grafts for future replacement therapies through utilization of cells and biomaterials [3, 4]. Besides, tissue engineering has been seen as an efficient method to assist in rescuing lives and improving the quality of life. There are many methods to produce an appropriate scaffold that cells could seed on, like foam casting, electrospinning, phase separation, decellularizing, etc.
Recently human donor organs, such as kidney [5,6,7], lung [8,9,10], heart [11,12,13] and liver [14,15,16], have been experimented to obtain decellularized extracellular matrix scaffolds (dECM) in order to clarify their potential application in regenerative medicine. However, despite the important basic science achievements in the field, clinical applications of decellularized tissues are not available yet [17]. Also, the use of dECM scaffolds for bioengineering of human scale patient-specific organs using human pluripotent stem cells (hPSCs) has been considered as a major platform for therapeutic applications [18]. Interestingly, the concept of organ printing has lately taken the center stage due to recent 3D bio-printing (3DBP) advancements [19,20,21,22].
3DBP is considered to have significant impact in tissue engineering area. Bio-printing is a growing field that is leading to a global revolution in medical sciences and has gained significant attention worldwide because of tremendous transformation it will have in the treatment of diseases [23].
Bio-printing can be defined as the simultaneous printing of living cells and biomaterials like hydrogels, bioglasses, bio-ceramics and collagen by a prescribed layer-by-layer stacking organization, using a computer-aided transfer process for fabrication of bioengineered constructs. Initially the study of cells was performed applying 2D structures; nonetheless, with the initiation of 3D printing to the engineering of tissues, it became feasible for researchers to use 3D scaffolds. Even though vascularization, controlled cell dispersion, innervation and cell deposition with high resolution, in complicated 3D tissues are still current technical challenges faced in 3DBP, however, compared with other techniques it presents many advantages such as scalability, simplicity and cost efficiency. Due to these advantages, 3DBP development and application has been increasing constantly over the past few years [24]. Also, 3DBP techniques have extensive applications in tissue engineering [25,26,27,28], transplantation clinics [23,24,25,26,27,28,29], drug screening, high throughput assays [30] and cancer research [31].
Since the human organs are different in terms of the size, 3DBP is an appropriate and versatile method for fabrication that asserts micrometric cell patterning for extensive biomedical engineering applications [21]. Generating the printing paths, selecting appropriate bio-inks, printing control and performing quality control after printing are major steps that assure passable print [22].
Generating the printing pathways and verifying its feasibility by designers is the first step of the bio-printing process. By selecting appropriate cells and hydrogels, loading the bio-inks into the bio-printing system is needed. The next step is sending the designed paths to the controlling system and building structures by depositing bio-inks. The bio-printed constructs are examined through a microscope. As more researches are performed implementing the techniques of 3DBP, this will ensure an enhancement of the printing resolution and quality and also the actualization of the current challenges, furnishing the capacity to print more complex 3D constructs.
Due to the advances in the additive manufacturing, some of the most well-known for bone tissue engineering, including stereo-lithography, selective laser sintering, fused deposition modeling and three-dimensional printing are reviewed [32, 33]. Moreover, some basic physical principles along with primary applications of aforementioned techniques have been reviewed beside a list of bone tissue engineering-related biomaterials [33]. Various types of bio-inks used for bone bio-printing are reviewed as well as their major challenges and future strategies [34]. The recent advancements, limitations, challenges and future strategies in bone bio-printing are summarized in recently published reports [32, 34, 35].
There are several studies about 3DBP which cover diverse tissues [36,37,38,39,40,41,42,43]; meanwhile, to the best of our there isn’t any review study about bone and skin tissues bio-printing. In fact, this paper focuses on tissues that successfully passed the commercializing procedures not only by printing techniques, but also by the prior scaffold fabricating methods. The authors tried to include the skin and bone-related literature that published till preparing the paper with the “bio-printing, 3DBP, 3D printing” key words, whereas the unrelated papers in tissue engineering fields were excluded.
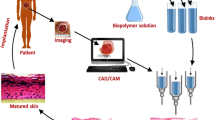
Our aim was to focus on studies with commonly employed cells, materials and printing methods in skin and bone bio-printing (Fig. 1).
Bio-inks and their performances
Among all the bio-inks used for 3DBP, there are some that are distinguished based on their merits. In some studies that refer to this issue, specific applications, new methods and spectacular properties have been reported [44,45,46,47,48,49,50]. In certain biomedical appliances, conductivity is of extreme importance while in scaffolds, cell support is vital [24]. In a recent study, a formidable bio-ink was designed for reconstructing cardiac tissue [51], it was produced to supply the correct conductivity and evade postponed electrical confluence in cardiac cells. This new gold nano-rod-integrated gelatin methacryloyl-based bio-ink was shown to be precisely printable, cytocompatible and boosted the performance of cardiac cells.
Nerve [52], kidney [53] and cartilage [54] rejuvenation and restoration including bionic ear [55] are other precise utilizations investigated for bio-ink improvement. Impressionable electronics for bioelectronic boundaries are also presently under comprehensive investigation [56, 57]. Also, 3DBP of commercialized tissue engineering products is a novel idea that can result in more effectiveness and lowering of costs.
Skin tissue 3D bio-printing
Many tissue fabrication techniques have been employed in the creation of skin tissues which culminate in the introduction of tissue substitutes, such as autologous split-thickness skin graft [58], allografts [59], acellular dermal substitutes and cellularized graft-like commercial products [59] such as Dermagraft and Apligraf [60]. New, bio-printing technology was utilized for skin tissue fabrication, Pourchet et al. [61] produced a two-layered (dermis and epidermis) 3D cell-printed full-thickness skin. The bio-ink was a mixture of gelatin and fibrinogen; moreover, they embedded human dermal fibroblasts in the mentioned mixture and printed to produce a dermis construct. Next stage was to generate the skin substitutes with 5 mm thickness which seeded the human epidermal keratinocytes over the dermis construct. Within 26 days of culture, natural human skin histological-like characteristics of the 3D cell-printed skin exhibited. Interestingly, immunofluorescence results using different differentiation markers exhibited high expression of Loricrin, showing the barrier function of the skin, which is related to the stratum corneum formation. Table 1 lists the studies on skin tissue 3DBP.
Cubo et al. [62] produced a full-thickness human skin similar to one-step manufacturing one, which used 3DBP technique. A combined structure was comprised of four different elements—human fibroblasts, human plasma supplemented with fibrinogen, calcium chloride (CaCl2) and human keratinocytes. Based on in vitro and in vivo evaluations, it was shown that the 3D cell-printed human skin substitute was very similar to natural human skin tissue, and extremely differentiated dermis and epidermis layers clearly were noticed. They constructed an intelligible and efficacious 3DBP technique and bio-inks that permitted the creation of a double-layer human skin, adopting human plasma and primary human fibroblasts (hFBs) and human keratinocytes (hKCs). The printed tissue was very similar to natural human skin and indistinguishable from dermo-epidermal analogs, precedent hand-crafted in their laboratory and employed successfully in the clinic. This method set up a novel technology that permitted the production of skin substitutes in reasonable amounts at shorter times. It showed decreased production cost and an improved production line by utilizing an automotive and standardized system of producing skin counterparts, thereby overcoming some of the challenges encountered by the present physical production process.
Zhao et al. [63] synthesized varying concentrations of gelatin methacryloyl (GelMA) hydrogels for a monolayer skin modernization process. GelMA hydrogels have good physical properties, and subsequently they systematically change the concentration of GelMA in order to control the adhesion, proliferation and differentiation of keratinocytes. A hydrogel scaffold was used to reach a keratinocyte suspension, which was used to develop the reconstruction of the classified and functional epitromes. The result of this study indicated that the physical and biological properties of the resultant hydrogels could be adequately controlled to meet the requirements for epidermis formation, by altering the concentration of GelMA pre-polymer solution. GelMA hydrogels supported the formation of a stratified epidermis with a certain barrier function, e.g., electrical resistance and prevention of water loss. Higher concentrations of hydrogels indicate the hardness of materials for cell adhesion and the formation of single-layer keratinocytes and cell adhesion, coupled with prolonged resistance to collagenase degradation.
Lee et al. [64] constructed a two-layer skin via a 3DBP procedure using skin dermal matrix formed by collagen. In fact, replacing the natural human skin by 3D-printed one is not impossible morphologically and biologically, while the 3D-printed one was reinforced via histological and immunofluorescence properties. In order to cover the full thickness of the transdermal and localized wound forms, this technique has a wide range of applications in skin design of toxicity investigations and wound healing. Their study illustrated that cell viability and function were affected little by proteins and printing cells in nano-droplets form. Both keratinocytes and fibroblasts enjoyed sufficiently high rate of viability in this study [66].
Xiong et al. [65] study revealed that the rate of full-thickness wound healing accelerated by utilizing from 3D-printed gelatin–silk fibroin base scaffolds. Also, incorporation of fibroblast growth factor 2 (FGF-2) could further enhance the treatment efficacy. Skin scaffolds shown to contain sulfonated moieties in order to raise scaffold hydrophilicity. The immobilized growth factor FGF-2 facilitated a sustained release kinetics, as well as being able to initiate cell proliferation and migration in vitro. Printed scaffolds exhibited favorable results in vivo. Proliferation rate in this study showed a significant raise from 40 to 75% by using FGF-2. Tissue morphology, collagen fibril assembly, blood vessel formation and the expression of various corresponding markers grew impressively. These data demonstrated that recombinant FGF-2 delivered by the scaffold could be a viable and innovative therapeutic strategy for severe skin wound.
Rutz et al. [66] introduced a versatile and cell-compatible bio-ink from a variety of amine-containing polymers and their mixtures, synthetically and naturally. It was shown that 35 formulations of bio-inks can be customized with regard to composition (additives and composites), the degree of crosslinking and polymer concentration in order to optimize structural and biological performance, while maintaining printability.
Also, Ng et al. [67] optimized a scaffold based on polyelectrolyte–gelatin–chitosan (PGC) hydrogel by 3DBP. In order to form polyelectrolytic compounds, the chitosan was modified with the different gelatin functional groups at pH of 6.5. In this work, to achieve an excellent biocompatibility with fibroblast skin cells, the PGC hydrogels were modified at room temperature for the 3DBP procedure. In fact, PGC hydrogels have a high viscosity that is suitable for the printing procedure at room temperature. The PGC hydrogels were optimized for the bio-printing of skin designs. Their scaffolds in 400 μm for three layers were representative of the outer epidermal layer and part of the dermal area. Their findings suggested the potential use of polyelectrolyte–gelatin–chitosan hydrogels for skin bio-printing applications.
Min et al. [68] developed a 3DBP procedure that there was capability of producing a thick skin with pigmentations. Multiple layers of collagen hydrogel precursors with fibroblasts were printed using sodium bicarbonate as the cross-linker. For the skin pigmentation induction on the subsequent air–liquid interface, melanocytes and keratinocytes were sequentially printed on top of the dermal layer. To clarify the formation of distinct skin layers also to recognize the pigmentation presence, histological analysis was done. The printed skin product illustrated that final differentiation of the keratinocytes caused the formation of the stratum corneum in the dermal and epidermal layers. Moreover, the epidermal layer included melanocytes that showed the dermal–epidermal junction with freckle-like pigmentations, without the use of chemical stimuli or external ultraviolet light. For therapeutic and research purposes, explanation of 3DBP technique as a productive on-demand option is available and presented the ability of engineering ephelides production in the biomimetic skin. Additionally, Li et al. [69] designed and manufactured the gelatin/alginate scaffolds via 3D bio-printer and investigated its biocompatibility and the histocompatibility over the skin wound healing duration using bone marrow-derived mesenchymal stem cells (BMSCs).
Huang et al. [70] produced a 3D ECM mimic construct for the sweat glands regeneration. Sweat glands perform a vital thermoregulatory function in mammals; like other skin attachments, they are made up of epidermal progenitors. Also, adult epidermal progenitors could be specified to differentiate to a sweat gland cell lineage but this remains largely unexplored and whether they have low regenerative potential in response to injury is still questionable.
Additionally, in order to create a functional in vitro cell-laden 3D extracellular matrix mimics (3D-ECM), 3D printing technology was used with composite hydrogels based on gelatin and sodium alginate, due to their chemical and structural similarities to ECM components. Facilitating cell spreading and matrix formation because of the biological 3D structure could maintain cell viability. In this study, Ng et al. [71] demonstrated a bio-printing technique that can be utilized for the production of films for skin wound healing. Application of these bio-printed films could be in skin tissue engineering area.
For the first time, Rimann et al. [72] provided an all-in-one solution for the production of a skin-like soft tissue model with human primary fibroblasts and keratinocytes. The defined printing method and the advanced bio-ink were cell compatible and allowed long-term culture models to be generated. Further optimization of their model can possibly promote the full standardization of the production of 3D tissue model. Also, future advances in this technology will depend on standardizing bio-printing equipment and tailored bio-inks to support the correct functioning of cells. Reliable in vitro skin models are urgently required, especially in the cosmetic business, for testing cosmetic ingredients as required by legislation in Europe.
In a novel study, Skardal et al. [73] applied bio-printing technique for the full-thickness skin wounds treatment in nu/nu mice. They used fibrin-collagen gel filled with amniotic fluid-based stem cell (AFSs) and mesenchymal stem cells (MSCs) to print them on the wound site.
Although Kim et al. [74] invented a novel 3D cell-printing single-step process approach for human skin engineering with a functional transwell system. They established a hybrid 3D cell-printing system that facilitated the employment of extrusion and inkjet modules simultaneously. The collagen-based construct facilitated this procedure with polycaprolactone (PCL) mesh that interrupted collagen contraction during tissue maturation. Moreover, the inkjet-based disbursing unit was used to evenly distribute keratinocytes. This skin model disclosed promising biological properties that comprised of a steady fibroblast-stretched dermis and stratified epidermis layers 14 days thereon. This method also possessed 50 times lower cost and 10 times less consumption of medium than in a stereotyped culture. All in all, due to one-step procedure possibilities, authors advised their cell-printing approach for different human skin replicas engineering.
Michael et al. [75] produced a completely cellularized auxiliary skin by employing a laser-assisted bio-printing (LaBP) method. The unique aspect of LaBP is the opportunity to situate diverse types of cell in a precise three-dimensional pattern in space. They fixed fibroblasts and keratinocytes atop a sustaining matrix Matriderm in order to construct skin surrogates. These skin surrogates were later verified in vivo, using the dorsal skinfold chamber in nude mice. Full-thickness skin wounds were then implanted with the grafts, and these were completely bound to the neighboring tissue when explanted after 11 days. The printed keratinocytes established a multiple-folded epidermis with a commencement of differentiation and stratum corneum. The proliferation of the keratinocytes was principally identified in the percussive fundamental layers. Test tube controls, which were cultured at the air–liquid interface, also revealed proliferative cells, but these were somewhat situated in the entire epidermis. The presence of E-cadherin as an indication for adherens junctions and consequently the formation of tissue could be seen in the epidermis both in vivo and in vitro. In both conditions, the printed fibroblasts partially remained above the substratal Matriderm where they formed collagen, while a part of them wandered into the Matriderm. The blood vessels were seen to develop from the base of the wound and its edges toward the printed cells. In summary, Michael et al. [75] successfully exhibited the 3D printing of a cell composite through LaBP and the successive formation of tissue in vivo. These discoveries denote the precondition for the generation of a composite tissue-like skin, comprising of various types of cells in a sophisticated 3D pattern.
In vivo skin 3DBP procedure is shown in Fig. 2.
In a different study, Binder [76] aimed at developing a prototype skin bio-printer that could act as a test bed for the core components of an in situ printing system. A movement system was constructed capable of 1.57 μm precision. The printer enjoyed a cartridge-based delivery system capable of delivering up to eight different types of cells. This study promoted the idea of utilizing bio-printing in the clinics.
In fact, skin bio-printing is a novel technique which must be brought to the clinic (Fig. 3).

Reproduced with permission from Universidad Carlos III de Madrid (UC3M) and Lawrence [139]. Based on skin wound area, the wound repair strategy is selectable. Whether printing on wound or printing on a feeder layer and assembling it on wound area is available
3DBP skin procedure.
After a bio-ink pre-cellularization using a novel passive blending unit method, Thayer et al. [77] produced skin analogs. This method was designed to simplify the blending steps of a cell suspension into an extremely viscous bio-ink. In this study, a bio-ink based on nano-cellulose/alginate was used. The analogs of skin tissue could be grown for up to 4 weeks. Histological results showed both tissue-specific extracellular matrix (ECM) markers deposition and cell viability.
Albanna et al. [78] described a novel model of a mobile skin bio-printing process that quickly manages comprehensive injuries on site. The biomaterials used consisted of fibrinogen from bovine plasma and thrombin from bovine plasma lyophilized powder. Immunohistochemistry for human cells showed that 3–6 weeks after printing, as well as endogenous cells, human fibroblasts and keratinocytes were present in the dermis and epidermis of the wound, respectively. This research discussed the proof-of-concept validation of mobile in situ skin bio-printing process with embedded imaging technology to quickly manage full-thickness wounds on site. It was observed that the treatment with autologous fibroblasts and keratinocytes, supplied immediately to targeted wound places depending on wound size and topology, consisting of improved wound healing and standard in situ skin formation.
Admane et al. [79] demonstrated that the special undulated pattern of the dermal–epidermal junction in the 3D human skin is physiologically relevant to human skin and the bio-printed skin structure was dimensionally stable compared to the serious contraction associated with the collagen-based skin structure. At the other side, extensive keratinocyte migration is observed by day 21 with the observed self-assembly of keratinocytes by full coverage of the 3D bio-printed construct’s pore. Proteomics data analysis demonstrating striking resemblance of the developed 3D human skin model with a number of skin-specific pathways and required expression of proliferation and cornification markers depicting full stratification of the advanced 3D human skin model and extensive transcriptomics.
All the mentioned artificial materials are usually non-biodegradable, and subsequent removal of such temporary wound dressings from the wound site is mandatory. Progression of biodegradable films is necessary in the skin tissue engineering field [80]. Albeit solvent casting is a simple construction technique to produce such films, requirement qualities such as mechanical strength and water transmission rate cannot easily guarded through a solvent casting technique. Hence, the bio-printing technique could be used to manipulate the ultimate tensile strength, moisture permeability and the water uptake ability of the film.
3D bio-printing of bone tissue
Bone tissue engineering has been widely studied using 3DBP. Leukers et al. [81] studied scaffolds based on hydroxyapatite by using 3D printing for engineering of bone tissue. They brought forth a special test-part in which mouse calvaria 3T3-E1 (MC3T3-E1) cells were cultured on the scaffolds and maintained under static and dynamic setups, followed by a histological examination which was performed to determine the growth of these cells. In brief, the dynamic culture process resulted in a potent population versus the static culture process. The cells were developed into the structure forming a nearly indirect communication with the hydroxyapatite (HA) granules, by creating a scaffold layout with inclined layers of 45°. This design facilitated the seeding procedure and increased cell attachment because the cells made the structure more integrated and prevented them from sliding down the structure. In a static culture, cells are deposited on the interface of the HA granules in layers, whereas in a dynamic seeding process, the cells grow within the cavities of the HA granules.
The dynamic cultured cells are significantly different from the static cultured cells. The powder-based 3D printing process developed micro-porosity of the scaffold and as a result, it enhanced the scaffold surface available for the medium flow and dissolution. Cox et al. [82] also presented the property of bone tissue scaffolds manufactured by 3D printing from a composite of HA and polyvinyl alcohol (PVOH). However, mechanical stability, microstructure and porosity of scaffolds produced by 3D printing were affected by the presence of HA: PVOH ratio precursor materials. By testing their comprehensive strengths, these constructs showed anisotropic behavior and partly failed at the interface of their interlayer bonds. This study used 3D printing, in other words, a print-based additive layer manufacturing (ALM) technique for fabricating an applicable porous scaffold adequate for the applications of the engineering of bone tissue. In brief, the characterization of precursor flow ability, using two common funnel tests, qualitatively comparable with observed printability can be assumed as an exclusive vital prerequisite since it required recoating of the build bed which finally distinguished several critical physical criteria such as mechanical strength, microstructure and porosity.
A glance at Table 2 provided reveals that there are several studies in bone tissue engineering field. Brunello et al. [83] experimented with 3D printing of powder for the purpose of the bone tissue engineering. Powder-based 3D printing is propounded as a special encouraging bone remodeling technique, as the exterior frame, interior structure, permeability and 3D-printed physical properties of bone replacements can be modified and hence used for particular purposes. 3DBP of stem cells and polymer/bioactive glass compound scaffolds for the engineering of bone tissue was accomplished by Murphy et al. [84]. They used 3DBP techniques for manufacturing of PCL/bioactive borate glass composite, as well as human adipose stem cells (ASCs) in their work, by applying a two-syringe system for fabrication of a scaffold with a bio-polymer/bio-glass composite. As a scaffold, material of this composite dissolved in an organic solvent, whereas concurrently printed cells remained suspended in the Matrigel®. They noted that the borate glass content could have an impact on the printability of composite paste, the scaffold temporal bioactivity, degradability and cell survival in the scaffold. The extrusion bio-printing technique has important features, which can produce a scaffolding structure that supports cells and provides shape and mechanical integrity. Extrusion bio-printers normally have more than one syringe, one of them dedicated to print scaffolding structures. Molten deposition of polymer and fused deposition modeling (FDM) with a polymer wire feed were the options applied for this matter, also the pore size factor is considerable because it has a potential to affect the bone growth after implantation.
Byambaa et al. [85] produced bone-like microstructure tissue constructs which contained perfusable vascular lumen by 3DBP. The bio-printed constructs were used as biomimetic in vitro matrices to co-culture human umbilical vein endothelial cells (hUVECs) together with human mesenchymal stem cells (hMSCs) in a naturally derived hydrogel. A central cylinder with %5 GelMA hydrogel and low methacryloyl substitution was printed. For the purpose of osteogenesis induction and synthesizing hydrogel formulations with a chemically conjugated vascular endothelial growth factor (VEGF) to promote vascular spreading, cell-laden GelMA cylindrical parts loaded with silicate nano-platelets were applied, the engineered construct could support cell survival and proliferation during in vitro maturation.
Kim and Kim [86] examined a combination of 3D printing, electrospinning and physical punching process techniques to provide a composite of PCL/alginate construct along nano-fibrous content also modified mechanical strength. This was achieved by sandwiching layers of micro-sized PCL structures between electrospun layers of PCL/alginate and punching the final scaffold to produce micro-sized pores moving through the stack of 3D-printed and electrospun materials. Interestingly, PCL/alginate composite scaffolds against pure PCL scaffolds showed a considerable 7 days increased cell viability, calcium deposition and alkaline phosphatase (ALP) activity at 14 days and a high increase in water absorption capacity due to the improved hydrophilicity contributed by the content of alginate scaffold.
PLA-based scaffold employing an integrated precipitation modeling 3D printer has been developed by Holmes et al. [87]. To support reconstruction of the ossified bone, like vascular cell growth, scaffolds were designed with highly interconnected 3D microvascular-mimicking channels. The constructed scaffolds were also chemically conjugated with nano-hydroxyapatite (nHA) to induce osteodifferentiation of seeded hMSCs. SEM imaging illustrated printing of vertical micro-channels having both a 500 and 250 mm radius, surrounded by a porous bone matrix.
Gao et al. [88] applied inkjet 3DBP to co-print an acrylated polyethylene glycol (PEG) hydrogel with acetylated peptides. Composite hydrogel filled with hMSCs applied to initiate simultaneous photo-polymerization of the hydrogel during printing following exposure to ultraviolet light. The constructed scaffold demonstrated high biocompatibility with a cell viability of 87.9 ± 5.3% 24 h after printing. Mentioned constructs containing hMSCs were cultured for 21 days in both osteogenic and chondrogenic media. Osteogenic and chondrogenic gene expressions were noticed to be highly enhanced from day 7–21, as well as a major collagen and extracellular matrix deposition was observed.
Kang et al. [89] developed an interwoven scaffold which was containing cell-laden hydrogels, PCL polymer and a sacrificial pluronic F127 hydrogel used as a multi-head bio-printer. The Pluronic F127 has been included in several other composite constructs to facilitate the development of provisional structural support or vascular channels.
Wang et al. [90] constructed a 3D-printed bio-ceramic scaffold with phage nano-fibers to dominate obstacle of bone tissue formation. The 3D-printed scaffold contained biphasic calcium phosphate (BCP) with a composition of HA and b-tri-calcium phosphate (b-TCP) at a mass ratio of 60/40. Uniform structure along interconnected macroscale and microscale pores on the columns of the scaffold are features of the mentioned construct. To achieve modification of scaffold osteogenesis and also its vascularization, nano-fiber phages loaded with Arg-Gly-Asp (RGD) were combined with chitosan and adhered to the construct pores through electrostatic interactions. After implantation of this construct in an animal model, it was observed that the host cells interfered with the scaffold and established a vasculature, with MSCs undergoing osteogenesis. Even though the host cells had their survival within the cell-laden construct impaired; however, they slowly formed the vasculature. Costantini et al. [91] constructed a 3D-printed biomimetic hydrogel scaffold containing different combinations of GelMA, chondroitin sulfate amino ethyl methacrylate (CS-AEMA) and hyaluronic acid methacrylate (HAMA). By applying of two coaxial-needle bio-printing system, they reached cell high density, increased cell viability, high printing resolution and post-printing.
Kim et al. [92] manufactured a SF/HA composite of hydrogel, made by hyaluronic acid-dopamine and also with surface modification of HA nanoparticles, managed to facilitate distribution of the HA content. The hydrogel composite demonstrated excellent cell proliferation, an in vivo analysis required to entirely investigate its bone tissue engineering potential.
Bendtsen et al. [93] created a modern formulation of a scaffold made of alginate/PVA/HA hydrogel. This scaffold had the appropriate rheological property for 3D printing of MC3T3 cells. Nyberg et al. [94] printed a 3D porous PCL scaffold applying a FDM process. To functionalize them, mineral additives were mixed in that had been widely utilized commercially and clinically: TCP, HA, Bio-oss (BO) and decellularized bone matrix (DCB). In order to investigate properties of osteoconductivity, each scaffold composites were seeded with an adipose-derived stromal/stem cells in vitro and their differentiation into osteoblasts was evaluated. The content of calcium—normalized to DNA—was especially elevated in PCL-TCP, PCL-BO and PCL-DCB groups relative to PCL only.
Buyuksungur et al. [95] printed 3D PCL scaffolds adapted with HA and poly-propylene fumarate (PPF). In order to produce a mechanically strong implant with uniform pore size and porosity, governable surface hydrophilicity and osteoconductivity, cylindrical disks of PCL were printed by FDM and modified with nHA and PPF. The cytotoxicity, irritation and inflammation analyses showed that the scaffolds were biocompatible. Also, PCL/nHA and PCL/nHA/PPF scaffolds were implanted in the rabbit’s femurs for in vivo testing, with and without seeding of rabbit BMSCs and evaluated after 4 and 8 weeks by histological test, micro-CT and mechanical test. As determined by bone mineral density and micro-CT, scaffolds that were seeded by BMSC demonstrated progress in bone tissue regeneration. After eight weeks of implantation, the values obtained from mechanical analysis were remarkably better than those of the healthy rabbit femur and demonstrated a high capacity for patient-specific bone defect.
In addition to these researches, at present time many researchers are attracted to the study of bone 3DBP [96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113]. The 3DBP bone transplantation procedure is shown in Fig. 4. Based on this figure, a computer-aided design of bone graft is an initial phase for fabricating of bone grafts procedure.
3DBP bone transplantation procedure. Patients suffering from bone disorder, supposed to data gathering by computer-aided instruments such as MRI. Provided bone graft design will be transmitted to a bone scaffold by selecting appropriate cells and growth factors in order to produce a well printable bio-ink. After completion of printing procedure, transplantation surgery is the final step
Instances of successful 3D printing for bone application are that of the 3D printing of bone scaffolds with hybrid biomaterials. Oladapo et al. [114] designed a new hybrid material bone implant, by combination of polylactic acid (PLA) matrix-reinforced with carbohydrate particles (cHA) using the additive manufacturing (AM) technology. A software application was used for digital surfacing in the mass proportions of 100/0, 95/5, 90/10 and 80/20 for application in bone tissue engineering, seeking higher proposition strength of PLA. As it is evident, biomimetic application can produce high-strength biomechanical implants with appropriate mechanical features. The combination of polymers leads to a rise in diversity of components and applications of biomaterials increased.
In another additive manufacturing technique for scaffold fabricating, Zhao et al. [115] constructed bionic porous titanium scaffolds by the selective laser melting (SLM) procedure. In those studies, different bionic bone scaffolds were manufactured by computer-aided design (CAD). This structure with novel porosity was plotted by using parameterization modeling. At all stages of construction from the design phase of the evaluation of the scaffold porous structures, the parametric modeling of porous titanium bone scaffold with competent mechanical and biological virtues was obtained. Meanwhile, in another study, Lai et al. [116] created a novel porous poly(lactide-co-glycolide) (PLGA)/TCP/Mg (PTM) scaffolds using low temperature, rapid prototyping (LT-RP) technology with the formulation of Mg powder, PLGA and β-TCP. The release of Mg ions was studied, and physical characterization of PTM scaffold in vitro assay was performed. The PTM scaffolds were implanted in a rabbit model for evaluation of the osteogenic and angiogenic properties of the implant. Their results proved that the PTM scaffold had designed in the bio-mimic the structure and modified mechanical properties. Ultimately it was confirmed that the PTM scaffolds are desirable composite biomaterials for solving challenging bone defect that would have great excellent property for its clinical usage.
Roopavath et al. [117] prepared a 3D-printed hydrogels by using an extrusion-based 3D printing technology. The material of this hydrogel is based on HA that had been approved clinically. SEM and Micro-CT results provided revealed that the scaffold enjoys from a better rate of porosity. Mechanical tests were employed to evaluate the porosity effects on the compressive properties of 3D-printed structures. Eventually printed HA hydrogel made of a patient-specific bone graft was tested in a series of studies in patients. The results confirmed the promising potential of this 3D-printed material in manufacturing a bio-mimic porous structure-based anatomical bone models and preoperative 3D planning.
3D bio-printing challenges
The 3DBP has created a huge impact in the tissue analysis field and is turning into a practical strong tool to produce tissues of human body.
The main challenge of 3DBP is the need for in vivo vascularization in order to provide the cells with adequate nutrition, growth factors, oxygen and remove carbon dioxide and wastes. Of course, the future developments of bio-printing can also potentially overcome these vascularization challenges [118]. In vivo, capillaries are mostly located at a 100 mm distance from a majority of cells in order to enable enough diffusion for the cells to survive [119]. For greater distances, like in thick tissues in printed organs, supplementary modes for diffusion may be required. To surpass this huddle, Hutmacher et al. [120] suggested an artificial vascular system.
Also, the bio-printing process is not currently automated and plenty of manual operations separated in various steps may result in slow processing speed, thereby increasing the prospect for mistakes and errors [21]. In order to form a highly mimetic tissue or organ on a macroscale, bio-printed cells should proliferate. When selecting cells, two main factors are considered: how the bio-printed cells can imitate the physiological state of cells in vivo and how much the bio-printed cells can perform their in vivo functions under optimized microenvironments [22]. Artificial tissues are seeded by either printing functional primary cells with supporting cells [75, 121,122,123,124,125,126] or printing progenitors or stem cells for further differentiation [127,128,129,130,131,132]. Direct printing of primary cells can rapidly increase the complexness of bio-printing.
Prospective market
3DBP is presently increasing greatly toward a large industry as a result of its variation and potential implementations. 3DBP market size is hoped to obtain a $10.8 billion worth in 2021 from a $2.2 billion stance in 2012 [133]. Presently, many companies engage in 3DBP production, for the purpose of tissue engineering applications [134].
Furthermore, in 2014, a bio-printed human liver tissue, named exVive3D™ Liver, which was manufactured for drug toxicity evaluation, was introduced [135]. However, the product provided for in vitro drug screening, the successful development of a commercially available liver tissue, is still pending [24]. Microfluidic systems [136] and layer-by-layer assembly [137] will have an effect on the bio-fabrication of microstructures in the future. It is inevitable that advances in bio-fabrication will also profit related fields such as imaging and diagnostic applications too [24]. It is predicted that the progressive trend of 3DBP methods’ prevalence will lead to in situ bio-printing developing, which could be considered as an upward procedure from benchside to the bedside [78, 138].
Conclusion
In this paper, the different procedures of 3DBP of skin and bone tissues, results, advantages and disadvantages have been reviewed with details. This review emphasizes on the 3D bio-printed skin as a novel technology that provides the scaffold using biomaterials such as gelatin, fibrinogen, GelMA, chitosan and collagen. In this technology, applying cells, such as fibroblast, keratinocyte, melanocyte and HUVEC by growth factors like FGF-2, thrombin and hydrocortisone into a 3D environment, provides a similar setting close to the natural skin tissue. Skin constructs can be beneficial for patients, whom interfere with extensive burns and full-thickness skin wounds. Skin bio-printer can decrease healing time and less pain, and this technology has the potential of creating the fully functional skin constructs.
For bone tissue 3D printing, materials such as HA, PVOH, PCL, GelMA, PLA and PLGA were used by utilization of various cell types such as human adipose stem cells (ASCs), HUVEC and hMSCs. This fabricating process could involve powder-based 3D printing and extrusion bio-printing.
The bio-printing technology is a growing field that is leading to a global revolution in the medical sciences and has gained significant attention worldwide. However, bio-printing technology has been adopted for skin and bone tissue fabrication and it is hoped that other tissues could be produced by this technique.
References
Mason C, Dunnill P (2008) A brief definition of regenerative medicine. Regen Med 3(1):1–5. https://doi.org/10.2217/17460751.3.1.1
Abouna GM (2008) Organ shortage crisis: problems and possible solutions. Transpl Proc 40(1):34–38. https://doi.org/10.1016/j.transproceed.2007.11.067
Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R (2014) 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu Rev Biomed Eng 16:247–276. https://doi.org/10.1146/annurev-bioeng-071813-105155
Mao AS, Mooney DJ (2015) Regenerative medicine: current therapies and future directions. Proc Natl Acad Sci USA 112(47):14452–14459. https://doi.org/10.1073/pnas.1508520112
Orlando G, Booth C, Wang Z, Totonelli G, Ross CL, Moran E, Salvatori M, Maghsoudlou P, Turmaine M, Delario G, Al-Shraideh Y, Farooq U, Farney AC, Rogers J, Iskandar SS, Burns A, Marini FC, De Coppi P, Stratta RJ, Soker S (2013) Discarded human kidneys as a source of ECM scaffold for kidney regeneration technologies. Biomaterials 34(24):5915–5925. https://doi.org/10.1016/j.biomaterials.2013.04.033
Peloso A, Petrosyan A, Da Sacco S, Booth C, Zambon JP, O’Brien T, Aardema C, Robertson J, De Filippo RE, Soker S, Stratta RJ, Perin L, Orlando G (2015) Renal extracellular matrix scaffolds from discarded kidneys maintain glomerular morphometry and vascular resilience and retains critical growth factors. Transplantation 99(9):1807–1816. https://doi.org/10.1097/tp.0000000000000811
Yu YL, Shao YK, Ding YQ, Lin KZ, Chen B, Zhang HZ, Zhao LN, Wang ZB, Zhang JS, Tang ML, Mei J (2014) Decellularized kidney scaffold-mediated renal regeneration. Biomaterials 35(25):6822–6828. https://doi.org/10.1016/j.biomaterials.2014.04.074
Balestrini JL, Gard AL, Liu A, Leiby KL, Schwan J, Kunkemoeller B, Calle EA, Sivarapatna A, Lin T, Dimitrievska S, Cambpell SG, Niklason LE (2015) Production of decellularized porcine lung scaffolds for use in tissue engineering. Integr Biol 7(12):1598–1610. https://doi.org/10.1039/C5IB00063G
Gilpin SE, Guyette JP, Gonzalez G, Ren X, Asara JM, Mathisen DJ, Vacanti JP, Ott HC (2014) Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J Heart Lung Transpl 33(3):298–308. https://doi.org/10.1016/j.healun.2013.10.030
Stabler CT, Lecht S, Mondrinos MJ, Goulart E, Lazarovici P, Lelkes PI (2015) Revascularization of decellularized lung scaffolds: principles and progress. Am J Physiol Lung Cell Mol Physiol 309(11):L1273–L1285. https://doi.org/10.1152/ajplung.00237.2015
Kim TH, Jung Y, Kim SH (2018) Nanofibrous electrospun heart decellularized extracellular matrix-based hybrid scaffold as wound dressing for reducing scarring in wound healing. Tissue Eng Part A 24(9–10):830–848. https://doi.org/10.1089/ten.TEA.2017.0318
Sánchez PL, Fernández-Santos ME, Costanza S, Climent AM, Moscoso I, Gonzalez-Nicolas MA, Sanz-Ruiz R, Rodríguez H, Kren SM, Garrido G, Escalante JL, Bermejo J, Elizaga J, Menarguez J, Yotti R, Pérez del Villar C, Espinosa MA, Guillem MS, Willerson JT, Bernad A, Matesanz R, Taylor DA, Fernández-Avilés F (2015) Acellular human heart matrix: a critical step toward whole heart grafts. Biomaterials 61:279–289. https://doi.org/10.1016/j.biomaterials.2015.04.056
Seo Y, Jung Y, Kim SH (2018) Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis. Acta Biomater 67:270–281. https://doi.org/10.1016/j.actbio.2017.11.046
Lee H, Han W, Kim H, Ha DH, Jang J, Kim BS, Cho DW (2017) Development of liver decellularized extracellular matrix bioink for three-dimensional cell printing-based liver tissue engineering. Biomacromolecules 18(4):1229–1237. https://doi.org/10.1021/acs.biomac.6b01908
Mattei G, Magliaro C, Pirone A, Ahluwalia A (2017) Decellularized human liver is too heterogeneous for designing a generic extracellular matrix mimic hepatic scaffold. Artif Organs 41(12):E347–E355. https://doi.org/10.1111/aor.12925
Mazza G, Rombouts K, Rennie Hall A, Urbani L, Vinh Luong T, Al-Akkad W, Longato L, Brown D, Maghsoudlou P, Dhillon AP, Fuller B, Davidson B, Moore K, Dhar D, De Coppi P, Malago M, Pinzani M (2015) Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci Rep 5:13079. https://doi.org/10.1038/srep13079
Garreta E, Oria R, Tarantino C, Pla-Roca M, Prado P, Fernández-Avilés F, Campistol JM, Samitier J, Montserrat N (2017) Tissue engineering by decellularization and 3D bioprinting. Mater Today 20(4):166–178. https://doi.org/10.1016/j.mattod.2016.12.005
Moser PT, Ott HC (2014) Recellularization of organs: what is the future for solid organ transplantation? Curr Opin Organ Transpl 19(6):603–609. https://doi.org/10.1097/mot.0000000000000131
Cheung DYC, Duan B, Butcher JT (2015) Chapter 21—Bioprinting of cardiac tissues. In: Atala A, Yoo JJ (eds) Essentials of 3D biofabrication and translation. Academic Press, Boston, pp 351–370. https://doi.org/10.1016/B978-0-12-800972-7.00021-9
Groll J, Boland T, Blunk T, Burdick JA, Cho D-W, Dalton PD, Derby B, Forgacs G, Li Q, Mironov VA, Moroni L, Nakamura M, Shu W, Takeuchi S, Vozzi G, Woodfield TBF, Xu T, Yoo JJ, Malda J (2016) Biofabrication: reappraising the definition of an evolving field. Biofabrication 8(1):013001. https://doi.org/10.1088/1758-5090/8/1/013001
Mandrycky C, Wang Z, Kim K, Kim D-H (2016) 3D bioprinting for engineering complex tissues. Biotechnol Adv 34(4):422–434. https://doi.org/10.1016/j.biotechadv.2015.12.011
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32(8):773–785. https://doi.org/10.1038/nbt.2958
Ozbolat IT (2015) Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol 33(7):395–400. https://doi.org/10.1016/j.tibtech.2015.04.005
Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M (2018) 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact Mater 3(2):144–156. https://doi.org/10.1016/j.bioactmat.2017.11.008
Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G (2010) Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2(2):022001. https://doi.org/10.1088/1758-5082/2/2/022001
Moroni L, de Wijn JR, van Blitterswijk CA (2006) 3D fiber-deposited scaffolds for tissue engineering: influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials 27(7):974–985. https://doi.org/10.1016/j.biomaterials.2005.07.023
Zhu W, Ock J, Ma X, Li W, Chen S (2015) “Chapter 2—3D printing and nanomanufacturing. In: Zhang LG, Fisher JP, Leong KW (eds) 3D bioprinting and nanotechnology in tissue engineering and regenerative medicine. Academic Press, New York, pp 25–55. https://doi.org/10.1016/B978-0-12-800547-7.00002-3
Shanjani Y, Pan CC, Elomaa L, Yang Y (2015) A novel bioprinting method and system for forming hybrid tissue engineering constructs. Biofabrication 7(4):045008. https://doi.org/10.1088/1758-5090/7/4/045008
Lim G, Choi D, Richardson EB (2014) 3-D printing in organ transplantation. Hanyang Med Rev 34(4):158–164. https://doi.org/10.7599/hmr.2014.34.4.158
Snyder JE, Hamid Q, Wang C, Chang R, Emami K, Wu H, Sun W (2011) Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication 3(3):034112. https://doi.org/10.1088/1758-5082/3/3/034112
Perkins JD (2007) Are we reporting the same thing? Liver Transpl: Off Publ Am Assoc Study Liver Dis Int Liver Transpl Soc 13(3):465–466
Midha S, Dalela M, Sybil D, Patra P, Mohanty S (2019) Advances in three-dimensional bioprinting of bone: progress and challenges. J Tissue Eng Regen Med 13(6):925–945. https://doi.org/10.1002/term.2847
Moreno Madrid AP, Vrech SM, Sanchez MA, Rodriguez AP (2019) Advances in additive manufacturing for bone tissue engineering scaffolds. Mater Sci Eng, C 100:631–644. https://doi.org/10.1016/j.msec.2019.03.037
Ashammakhi N, Hasan A, Kaarela O, Byambaa B, Sheikhi A, Gaharwar AK, Khademhosseini A (2019) Advancing frontiers in bone bioprinting. Adv Healthc Mater 8(7):1801048. https://doi.org/10.1002/adhm.201801048
Atala A, Forgacs G (2019) Three-dimensional bioprinting in regenerative medicine: reality, hype, and future. Stem Cells Transl Med 8(8):744–745. https://doi.org/10.1002/sctm.19-0089
Dasgupta Q, Black LD (2019) A fresh slate for 3D bioprinting. Science 365(6452):446. https://doi.org/10.1126/science.aay0478
Kuss M, Duan B (2019) Chapter 2—Extrusion-based bioprinting. In: Cho D-W (ed) Biofabrication and 3D tissue modeling. The Royal Society of Chemistry, London, pp 22–48. https://doi.org/10.1039/9781788012683-00022
Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT (2019) Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 226:119536. https://doi.org/10.1016/j.biomaterials.2019.119536
Miri AK, Khalilpour A, Cecen B, Maharjan S, Shin SR, Khademhosseini A (2019) Multiscale bioprinting of vascularized models. Biomaterials 198:204–216. https://doi.org/10.1016/j.biomaterials.2018.08.006
Moldovan F (2019) Recent trends in bioprinting. Procedia Manuf 32:95–101. https://doi.org/10.1016/j.promfg.2019.02.188
Shafiee A, Ghadiri E, Ramesh H, Kengla C, Kassis J, Calvert P, Williams D, Khademhosseini A, Narayan R, Forgacs G, Atala A (2019) Physics of bioprinting. Appl Phys Rev 6(2):021315. https://doi.org/10.1063/1.5087206
Zhang B, Gao L, Ma L, Luo Y, Yang H, Cui Z (2019) 3D bioprinting: a novel avenue for manufacturing tissues and organs. Engineering 5(4):777–794. https://doi.org/10.1016/j.eng.2019.03.009
Zhou D, Chen J, Liu B, Zhang X, Li X, Xu T (2019) Bioinks for jet-based bioprinting. Bioprinting 16:e00060. https://doi.org/10.1016/j.bprint.2019.e00060
Unagolla JM, Jayasuriya AC (2019) Hydrogel-based 3D bioprinting: a comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl Mater Today. https://doi.org/10.1016/j.apmt.2019.100479
Liu P, Shen H, Zhi Y, Si J, Shi J, Guo L, Shen SG (2019) 3D bioprinting and in vitro study of bilayered membranous construct with human cells-laden alginate/gelatin composite hydrogels. Colloids Surf, B 181:1026–1034. https://doi.org/10.1016/j.colsurfb.2019.06.069
Cheng L, Yao B, Hu T, Cui X, Shu X, Tang S, Wang R, Wang Y, Liu Y, Song W, Fu X, Li H, Huang S (2019) Properties of an alginate-gelatin-based bioink and its potential impact on cell migration, proliferation, and differentiation. Int J Biol Macromol 135:1107–1113. https://doi.org/10.1016/j.ijbiomac.2019.06.017
Gonzalez-Fernandez T, Rathan S, Hobbs C, Pitacco P, Freeman FE, Cunniffe GM, Dunne NJ, McCarthy HO, Nicolosi V, O’Brien FJ, Kelly DJ (2019) Pore-forming bioinks to enable spatio-temporally defined gene delivery in bioprinted tissues. J Control Release 301:13–27. https://doi.org/10.1016/j.jconrel.2019.03.006
Kim W, Kim G (2019) A functional bioink and its application in myoblast alignment and differentiation. Chem Eng J 366:150–162. https://doi.org/10.1016/j.cej.2019.02.071
Oliveira EP, Malysz-Cymborska I, Golubczyk D, Kalkowski L, Kwiatkowska J, Reis RL, Oliveira JM, Walczak P (2019) Advances in bioinks and in vivo imaging of biomaterials for CNS applications. Acta Biomater 95:60–72. https://doi.org/10.1016/j.actbio.2019.05.006
Kajave NS, Schmitt T, Nguyen T-U, Kishore V (2019) Dual crosslinking strategy to generate mechanically viable cell-laden printable constructs using methacrylated collagen bioinks. Materials Science and Engineering: C 107:110290. https://doi.org/10.1016/j.msec.2019.110290
Zhu K, Shin SR, van Kempen T, Li Y-C, Ponraj V, Nasajpour A, Mandla S, Hu N, Liu X, Leijten J, Lin Y-D, Hussain MA, Zhang YS, Tamayol A, Khademhosseini A (2017) Gold nanocomposite bioink for printing 3D cardiac constructs. Adv Func Mater 27(12):1605352. https://doi.org/10.1002/adfm.201605352
Johnson BN, Lancaster KZ, Zhen G, He J, Gupta MK, Kong YL, Engel EA, Krick KD, Ju A, Meng F, Enquist LW, Jia X, McAlpine MC (2015) 3D printed anatomical nerve regeneration pathways. Adv Funct Mater 25(39):6205–6217. https://doi.org/10.1002/adfm.201501760
Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, Lewis JA (2016) Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep 6:34845. https://doi.org/10.1038/srep34845. https://www.nature.com/articles/srep34845#supplementary-information
Kesti M, Eberhardt C, Pagliccia G, Kenkel D, Grande D, Boss A, Zenobi-Wong M (2015) Bioprinting complex cartilaginous structures with clinically compliant biomaterials. Adv Funct Mater 25(48):7406–7417. https://doi.org/10.1002/adfm.201503423
Mannoor MS, Jiang Z, James T, Kong YL, Malatesta KA, Soboyejo WO, Verma N, Gracias DH, McAlpine MC (2013) 3D printed bionic ears. Nano Lett 13(6):2634–2639. https://doi.org/10.1021/nl4007744
Khan Y, Pavinatto FJ, Lin MC, Liao A, Swisher SL, Mann K, Subramanian V, Maharbiz MM, Arias AC (2016) Inkjet-printed flexible gold electrode arrays for bioelectronic interfaces. Adv Funct Mater 26(7):1004–1013. https://doi.org/10.1002/adfm.201503316
Shin SR, Farzad R, Tamayol A, Manoharan V, Mostafalu P, Zhang YS, Akbari M, Jung SM, Kim D, Comotto M, Annabi N, Al-Hazmi FE, Dokmeci MR, Khademhosseini A (2016) A bioactive carbon nanotube-based ink for printing 2D and 3D flexible electronics. Adv Mater 28(17):3280–3289. https://doi.org/10.1002/adma.201506420
Coruh A, Yontar Y (2012) Application of split-thickness dermal grafts in deep partial- and full-thickness burns: a new source of auto-skin grafting. J Burn Care Res: Off Publ Am Burn Assoc 33(3):e94–e100. https://doi.org/10.1097/BCR.0b013e31823499e9
Leon-Villapalos J, Eldardiri M, Dziewulski P (2010) The use of human deceased donor skin allograft in burn care. Cell Tissue Bank 11(1):99–104. https://doi.org/10.1007/s10561-009-9152-1
Metcalfe AD, Ferguson MWJ (2007) Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 4(14):413–437. https://doi.org/10.1098/rsif.2006.0179
Pourchet LJ, Thepot A, Albouy M, Courtial EJ, Boher A, Blum LJ, Marquette CA (2017) Human skin 3D bioprinting using scaffold-free approach. Adv Healthc Mater. https://doi.org/10.1002/adhm.201601101
Cubo N, Garcia M, del Cañizo JF, Velasco D, Jorcano JL (2016) 3D bioprinting of functional human skin: production andin vivoanalysis. Biofabrication 9(1):015006. https://doi.org/10.1088/1758-5090/9/1/015006
Zhao X, Lang Q, Yildirimer L, Lin ZY, Cui W, Annabi N, Ng KW, Dokmeci MR, Ghaemmaghami AM, Khademhosseini A (2016) photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv Healthc Mater 5(1):108–118. https://doi.org/10.1002/adhm.201500005
Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, Yoo S-S, Dai G, Karande P (2014) Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C, Methods 20(6):473–484. https://doi.org/10.1089/ten.TEC.2013.0335
Xiong S, Zhang X, Lu P, Wu Y, Wang Q, Sun H, Heng BC, Bunpetch V, Zhang S, Ouyang H (2017) A gelatin-sulfonated silk composite scaffold based on 3D printing technology enhances skin regeneration by stimulating epidermal growth and dermal neovascularization. Sci Rep 7(1):4288. https://doi.org/10.1038/s41598-017-04149-y
Rutz AL, Hyland KE, Jakus AE, Burghardt WR, Shah RN (2015) A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv Mater 27(9):1607–1614. https://doi.org/10.1002/adma.201405076
Ng WL, Yeong WY, Naing MW (2016) Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int J Biopr 2(1):10. https://doi.org/10.18063/ijb.2016.01.009
Min D, Lee W, Bae IH, Lee TR, Croce P, Yoo SS (2018) Bioprinting of biomimetic skin containing melanocytes. Exp Dermatol 27(5):453–459. https://doi.org/10.1111/exd.13376
Li J, Chi J, Liu J, Gao C, Wang K, Shan T, Li Y, Shang W, Gu F (2017) 3D printed gelatin-alginate bioactive scaffolds combined with mice bone marrow mesenchymal stem cells: a biocompatibility study. Int J Clin Exp Pathol 10(6):6299–6307
Huang S, Yao B, Xie J, Fu X (2016) 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomater 32:170–177. https://doi.org/10.1016/j.actbio.2015.12.039
Ng WL, Yeong WY, Win Naing M (2014) Potential of bioprinted films for skin tissue engineering. In: Paper presented at the 1st international conference on progress in additive manufacturing
Rimann M, Bono E, Annaheim H, Bleisch M, Graf-Hausner U (2016) Standardized 3D bioprinting of soft tissue models with human primary cells. J Lab Autom 21(4):496–509. https://doi.org/10.1177/2211068214567146
Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, Soker S (2012) Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem cells Transl Med 1(11):792–802. https://doi.org/10.5966/sctm.2012-0088
Kim BS, Lee J-S, Gao G, Cho D-W (2017) Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 9(2):025034. https://doi.org/10.1088/1758-5090/aa71c8
Michael S, Sorg H, Peck C-T, Koch L, Deiwick A, Chichkov B, Vogt PM, Reimers K (2013) Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS ONE 8(3):e57741
Binder KW (2011) In situ bioprinting of the skin. Wake Forest University, Winston-Salem
Thayer PS, Orrhult LS, Martínez H (2018) Bioprinting of cartilage and skin tissue analogs utilizing a novel passive mixing unit technique for bioink precellularization. J Vis Exp JoVE 3(131):56372. https://doi.org/10.3791/56372
Albanna M, Binder KW, Murphy SV, Kim J, Qasem SA, Zhao W, Tan J, El-Amin IB, Dice DD, Marco J, Green J, Xu T, Skardal A, Holmes JH, Jackson JD, Atala A, Yoo JJ (2019) In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci Rep 9(1):1856. https://doi.org/10.1038/s41598-018-38366-w
Admane P, Gupta AC, Jois P, Roy S, Chandrasekharan Lakshmanan C, Kalsi G, Bandyopadhyay B, Ghosh S (2019) Direct 3D bioprinted full-thickness skin constructs recapitulate regulatory signaling pathways and physiology of human skin. Bioprinting 15:e00051. https://doi.org/10.1016/j.bprint.2019.e00051
Mao JS, Zhao LG, Yin YJ, Yao KD (2003) Structure and properties of bilayer chitosan-gelatin scaffolds. Biomaterials 24(6):1067–1074
Leukers B, Gülkan H, Irsen SH, Milz S, Tille C, Schieker M, Seitz H (2005) Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med 16(12):1121–1124. https://doi.org/10.1007/s10856-005-4716-5
Cox SC, Thornby JA, Gibbons GJ, Williams MA, Mallick KK (2015) 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater Sci Eng, C 47:237–247. https://doi.org/10.1016/j.msec.2014.11.024
Brunello G, Sivolella S, Meneghello R, Ferroni L, Gardin C, Piattelli A, Zavan B, Bressan E (2016) Powder-based 3D printing for bone tissue engineering. Biotechnol Adv 34(5):740–753. https://doi.org/10.1016/j.biotechadv.2016.03.009
Murphy C, Kolan K, Li W, Semon J, Day D, Leu M (2017) 3D bioprinting of stem cells and polymer/bioactive glass composite scaffolds for bone tissue engineering. Int J Biopr 3(1):11. https://doi.org/10.18063/ijb.2017.01.005
Byambaa B, Annabi N, Yue K, Trujillo-de Santiago G, Alvarez MM, Jia W, Kazemzadeh-Narbat M, Shin SR, Tamayol A, Khademhosseini A (2017) Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv Healthc Mater. https://doi.org/10.1002/adhm.201700015
Kim MS, Kim G (2014) Three-dimensional electrospun polycaprolactone (PCL)/alginate hybrid composite scaffolds. Carbohydr Polym 114:213–221. https://doi.org/10.1016/j.carbpol.2014.08.008
Holmes B, Bulusu K, Plesniak M, Zhang LG (2016) A synergistic approach to the design, fabrication and evaluation of 3D printed micro and nano featured scaffolds for vascularized bone tissue repair. Nanotechnology 27(6):064001. https://doi.org/10.1088/0957-4484/27/6/064001
Gao G, Schilling AF, Yonezawa T, Wang J, Dai G, Cui X (2014) Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol J 9(10):1304–1311. https://doi.org/10.1002/biot.201400305
Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A (2016) A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 34(3):312–319. https://doi.org/10.1038/nbt.3413
Wang J, Yang M, Zhu Y, Wang L, Tomsia AP, Mao C (2014) Phage nanofibers induce vascularized osteogenesis in 3D printed bone scaffolds. Adv Mater 26(29):4961–4966. https://doi.org/10.1002/adma.201400154
Costantini M, Idaszek J, Szoke K, Jaroszewicz J, Dentini M, Barbetta A, Brinchmann JE, Swieszkowski W (2016) 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication 8(3):035002. https://doi.org/10.1088/1758-5090/8/3/035002
Kim HH, Park JB, Kang MJ, Park YH (2014) Surface-modified silk hydrogel containing hydroxyapatite nanoparticle with hyaluronic acid-dopamine conjugate. Int J Biol Macromol 70:516–522. https://doi.org/10.1016/j.ijbiomac.2014.06.052
Bendtsen ST, Quinnell SP, Wei M (2017) Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J Biomed Mater Res A 105(5):1457–1468. https://doi.org/10.1002/jbm.a.36036
Nyberg E, Rindone A, Dorafshar A, Grayson WL (2017) Comparison of 3D-printed poly-varepsilon-caprolactone scaffolds functionalized with tricalcium phosphate, hydroxyapatite, bio-oss, or decellularized bone matrix. Tissue Eng Part A 23(11–12):503–514. https://doi.org/10.1089/ten.TEA.2016.0418
Buyuksungur S, Endogan Tanir T, Buyuksungur A, Bektas EI, Torun Kose G, Yucel D, Beyzadeoglu T, Cetinkaya E, Yenigun C, Tonuk E, Hasirci V, Hasirci N (2017) 3D printed poly(epsilon-caprolactone) scaffolds modified with hydroxyapatite and poly(propylene fumarate) and their effects on the healing of rabbit femur defects. Biomater Sci 5(10):2144–2158. https://doi.org/10.1039/c7bm00514h
Bose S, Vahabzadeh S, Bandyopadhyay A (2013) Bone tissue engineering using 3D printing. Mater Today 16(12):496–504. https://doi.org/10.1016/j.mattod.2013.11.017
Daly AC, Pitacco P, Nulty J, Cunniffe GM, Kelly DJ (2018) 3D printed microchannel networks to direct vascularisation during endochondral bone repair. Biomaterials 162:34–46. https://doi.org/10.1016/j.biomaterials.2018.01.057
Fedorovich NE, Schuurman W, Wijnberg HM, Prins HJ, van Weeren PR, Malda J, Alblas J, Dhert WJ (2012) Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng Part C, Methods 18(1):33–44. https://doi.org/10.1089/ten.TEC.2011.0060
Huang B, Bártolo PJ (2018) Rheological characterization of polymer/ceramic blends for 3D printing of bone scaffolds. Polym Test 68:365–378. https://doi.org/10.1016/j.polymertesting.2018.04.033
Hung BP, Naved BA, Nyberg EL, Dias M, Holmes CA, Elisseeff JH, Dorafshar AH, Grayson WL (2016) Three-dimensional printing of bone extracellular matrix for craniofacial regeneration. ACS Biomater Sci Eng 2(10):1806–1816. https://doi.org/10.1021/acsbiomaterials.6b00101
Kebede MA, Asiku KS, Imae T, Kawakami M, Furukawa H, Wu CM (2018) Stereolithographic and molding fabrications of hydroxyapatite-polymer gels applicable to bone regeneration materials. J Taiwan Inst Chem Eng 92:91–96. https://doi.org/10.1016/j.jtice.2018.01.034
Khanarian NT, Jiang J, Wan LQ, Mow VC, Lu HH (2012) A hydrogel-mineral composite scaffold for osteochondral interface tissue engineering. Tissue Eng Part A 18(5–6):533–545. https://doi.org/10.1089/ten.TEA.2011.0279
Kim YC, Min KH, Choi JW, Koh KS, Oh TS, Jeong WS (2018) Patient-specific puzzle implant preformed with 3D-printed rapid prototype model for combined orbital floor and medial wall fracture. J Plast Reconstr Aesthet Surg: JPRAS 71(4):496–503. https://doi.org/10.1016/j.bjps.2017.11.016
Lee H, Yang GH, Kim M, Lee J, Huh J, Kim G (2018) Fabrication of micro/nanoporous collagen/dECM/silk-fibroin biocomposite scaffolds using a low temperature 3D printing process for bone tissue regeneration. Mater Sci Eng, C 84:140–147. https://doi.org/10.1016/j.msec.2017.11.013
Luo Y, Lode A, Wu C, Chang J, Gelinsky M (2015) Alginate/nanohydroxyapatite scaffolds with designed core/shell structures fabricated by 3D plotting and in situ mineralization for bone tissue engineering. ACS Appl Mater Interfaces 7(12):6541–6549. https://doi.org/10.1021/am508469h
Müller M, Becher J, Schnabelrauch M, Zenobi-Wong M (2013) Printing thermoresponsive reverse molds for the creation of patterned two-component hydrogels for 3D cell culture. JoVE 77:e50632. https://doi.org/10.3791/50632
Ni J, Li D, Mao M, Dang X, Wang K, He J, Shi Z (2018) A method of accurate bone tunnel placement for anterior cruciate ligament reconstruction based on 3-dimensional printing technology: a cadaveric study. Arthroscopy 34(2):546–556. https://doi.org/10.1016/j.arthro.2017.08.288
Park J, Lee SJ, Lee H, Park SA, Lee JY (2018) Three dimensional cell printing with sulfated alginate for improved bone morphogenetic protein-2 delivery and osteogenesis in bone tissue engineering. Carbohydr Polym 196:217–224. https://doi.org/10.1016/j.carbpol.2018.05.048
Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH (2008) In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J Biomed Mater Res, Part A 86(1):1–12. https://doi.org/10.1002/jbm.a.32073
Tayebi L, Rasoulianboroujeni M, Moharamzadeh K, Almela TKD, Cui Z, Ye H (2018) 3D-printed membrane for guided tissue regeneration. Mater Sci Eng, C 84:148–158. https://doi.org/10.1016/j.msec.2017.11.027
Trauner KB (2018) The emerging role of 3D printing in arthroplasty and orthopedics. J Arthroplasty 33(8):2352–2354. https://doi.org/10.1016/j.arth.2018.02.033
Zhang B, Pei X, Zhou C, Fan Y, Jiang Q, Ronca A, D’Amora U, Chen Y, Li H, Sun Y, Zhang X (2018) The biomimetic design and 3D printing of customized mechanical properties porous Ti6Al4 V scaffold for load-bearing bone reconstruction. Mater Des 152:30–39. https://doi.org/10.1016/j.matdes.2018.04.065
Hernández-González AC, Téllez-Jurado L, Rodríguez-Lorenzo LM (2019) Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: a review. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2019.115514
Oladapo BI, Zahedi SA, Adeoye AOM (2019) 3D printing of bone scaffolds with hybrid biomaterials. Compos B Eng 158:428–436. https://doi.org/10.1016/j.compositesb.2018.09.065
Zhao L, Pei X, Jiang L, Hu C, Sun J, Xing F, Zhou C, Fan Y, Zhang X (2019) Bionic design and 3D printing of porous titanium alloy scaffolds for bone tissue repair. Compos B Eng 162:154–161. https://doi.org/10.1016/j.compositesb.2018.10.094
Lai Y, Li Y, Cao H, Long J, Wang X, Li L, Li C, Jia Q, Teng B, Tang T, Peng J, Eglin D, Alini M, Grijpma DW, Richards G, Qin L (2019) Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials 197:207–219. https://doi.org/10.1016/j.biomaterials.2019.01.013
Roopavath UK, Malferrari S, Van Haver A, Verstreken F, Rath SN, Kalaskar DM (2019) Optimization of extrusion based ceramic 3D printing process for complex bony designs. Mater Des 162:263–270. https://doi.org/10.1016/j.matdes.2018.11.054
Rupnick MA, Panigrahy D, Zhang C-Y, Dallabrida SM, Lowell BB, Langer R, Folkman MJ (2002) Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA 99(16):10730–10735. https://doi.org/10.1073/pnas.162349799
Kaully T, Kaufman-Francis K, Lesman A, Levenberg S (2009) Vascularization–the conduit to viable engineered tissues. Tissue Eng Part B, Rev 15(2):159–169. https://doi.org/10.1089/ten.teb.2008.0193
Hutmacher DW, Schantz T, Zein I, Ng KW, Teoh SH, Tan KC (2001) Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J Biomed Mater Res 55(2):203–216
Cui X, Breitenkamp K, Finn MG, Lotz M, D’Lima DD (2012) Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A 18(11–12):1304–1312. https://doi.org/10.1089/ten.TEA.2011.0543
Dolati F, Yu Y, Zhang Y, De Jesus AM, Sander EA, Ozbolat IT (2014) In vitro evaluation of carbon-nanotube-reinforced bioprintable vascular conduits. Nanotechnology 25(14):145101. https://doi.org/10.1088/0957-4484/25/14/145101
Duan B, Hockaday LA, Kang KH, Butcher JT (2013) 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res, Part A 101(5):1255–1264. https://doi.org/10.1002/jbm.a.34420
Keriquel V, Guillemot F, Arnault I, Guillotin B, Miraux S, Amedee J, Fricain JC, Catros S (2010) In vivo bioprinting for computer- and robotic-assisted medical intervention: preliminary study in mice. Biofabrication 2(1):014101. https://doi.org/10.1088/1758-5082/2/1/014101
Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ, Atala A (2013) Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 5(1):015001. https://doi.org/10.1088/1758-5082/5/1/015001
Zhang T, Yan KC, Ouyang L, Sun W (2013) Mechanical characterization of bioprinted in vitro soft tissue models. Biofabrication 5(4):045010. https://doi.org/10.1088/1758-5082/5/4/045010
Duarte Campos DF, Blaeser A, Weber M, Jakel J, Neuss S, Jahnen-Dechent W, Fischer H (2013) Three-dimensional printing of stem cell-laden hydrogels submerged in a hydrophobic high-density fluid. Biofabrication 5(1):015003. https://doi.org/10.1088/1758-5082/5/1/015003
Gruene M, Pflaum M, Deiwick A, Koch L, Schlie S, Unger C, Wilhelmi M, Haverich A, Chichkov BN (2011) Adipogenic differentiation of laser-printed 3D tissue grafts consisting of human adipose-derived stem cells. Biofabrication 3(1):015005. https://doi.org/10.1088/1758-5082/3/1/015005
Hong S, Song SJ, Lee JY, Jang H, Choi J, Sun K, Park Y (2013) Cellular behavior in micropatterned hydrogels by bioprinting system depended on the cell types and cellular interaction. J Biosci Bioeng 116(2):224–230. https://doi.org/10.1016/j.jbiosc.2013.02.011
Owens CM, Marga F, Forgacs G, Heesch CM (2013) Biofabrication and testing of a fully cellular nerve graft. Biofabrication 5(4):045007. https://doi.org/10.1088/1758-5082/5/4/045007
Visser J, Peters B, Burger TJ, Boomstra J, Dhert WJ, Melchels FP, Malda J (2013) Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 5(3):035007. https://doi.org/10.1088/1758-5082/5/3/035007
Xu F, Sridharan B, Wang S, Gurkan UA, Syverud B, Demirci U (2011) Embryonic stem cell bioprinting for uniform and controlled size embryoid body formation. Biomicrofluidics 5(2):22207. https://doi.org/10.1063/1.3580752
Kannan S (2014) The 3D bio printing revolution. Harv Sci Rev. https://harvardsciencereview.com/2014/05/01/the-3d-bioprinting-revolution/
Arslan-Yildiz A, El Assal R, Chen P, Guven S, Inci F, Demirci U (2016) Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication 8(1):014103. https://doi.org/10.1088/1758-5090/8/1/014103
Vaidya M (2015) Startups tout commercially 3D-printed tissue for drug screening. Nat Med 21(1):2. https://doi.org/10.1038/nm0115-2
Wang CC, Yang KC, Lin KH, Liu HC, Lin FH (2011) A highly organized three-dimensional alginate scaffold for cartilage tissue engineering prepared by microfluidic technology. Biomaterials 32(29):7118–7126. https://doi.org/10.1016/j.biomaterials.2011.06.018
Chang R, Emami K, Wu H, Sun W (2010) Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication 2(4):045004. https://doi.org/10.1088/1758-5082/2/4/045004
Singh S, Choudhury D, Yu F, Mironov V, Naing MW (2019) In situ bioprinting–bioprinting from benchside to bedside? Acta Biomater. https://doi.org/10.1016/j.actbio.2019.08.045
Murr LE (2015) Bioprinting and biofabrication of organs. In: Handbook of materials structures, properties, processing and performance. Springer, Cham, pp 629–638. https://doi.org/10.1007/978-3-319-01815-7_36
Acknowledgements
A word of appreciation must be given to Aron Munggela Foma for his invaluable assistance and comments on the first phase of paper editing. Also, we are extremely grateful to Mariam Sharifi Sistani for her precision and insightful comments on the second draft edition.
Funding
This study was not funded by any institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beheshtizadeh, N., Lotfibakhshaiesh, N., Pazhouhnia, Z. et al. A review of 3D bio-printing for bone and skin tissue engineering: a commercial approach. J Mater Sci 55, 3729–3749 (2020). https://doi.org/10.1007/s10853-019-04259-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04259-0