Abstract
The challenges and potential of using tissue engineering and regenerative medicine to create functional skin tissue constructs. While traditional skin grafts have limitations, advancements in biofabrication technology and natural polymers such as collagen offer promising avenues for creating skin tissue that mimics the cellular architecture of native tissue and organs. Laser-assisted bioprinting is a high-resolution printing method that can print delicate substrates with high precision, but it is not well-suited for printing large-scale tissue constructs and has a slower printing speed compared to other bioprinting methods. Creating ideal biomaterials and including skin appendages in the fabrication process are still challenges that need to be addressed, but the potential applications for tissue-engineered constructs in congenital defect surgery, surgical reconstruction, and epidermolysis bullosa treatment could improve patient outcomes and reduce overall costs. Overall, the development of functional skin tissue constructs with a prevascularized network and an effective barrier function has significant potential to impact patient care and advance the field of regenerative medicine.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
Skin envelops the entire body and is the identification of individual human beings. It also reflects the body’s internal condition. Its perfect conformity to the body’s contour maintains dynamic equilibrium while continuously replenishing the outer surface by underneath stem cells. As the skin is the outer part of the body, damage by chemicals, burns, mechanical injury is common and different skin diseases due to polluted environment occur in daily life. Although the regeneration capacity of skin is relatively higher than other organs, it is limited by the degree and frequency of injury and the appendages can hardly regenerate under natural conditions. In the case of large-scale skin defects caused by accident or burn, if the effective treatment is not taken timely, it can threaten patient’s lives due to loss of excess tissue fluid (Vaezi and Yang 2015). The current effective treatment for skin tissue is mainly autograft as it has no immune response, but it is limited by donor site morbidity and number of donors. Allograft and xenograft can be the ultimate strategies to treat large-scale skin damage, though immune rejection is the primary cause of its failure (Wang et al. 2018).
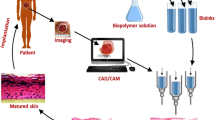
3D bioprinting, also called bio-additive manufacturing, is a layer-by-layer manufacturing process that fabricates tissue analogous to predefined architectural design. The fundamental idea of 3D bioprinting is the deposition of cell-laden bioink with a highly bionic structure like native tissue with computer-aided design (CAD) modeling. The advancement of bioprinting has the characteristics of user-defined controllability, short production time, and patient-specific customization that open a tremendous possibility for manufacturing complex tissue structures.
Human organs are not just a collection of cells and extracellular matrix; cells reside in a specific niche and manner embedded in the ECM matrix. The spatial context of cellular arrangement enables cells to respond to specific chemical cues, leading to the activation of a specific pathway and the inhibition of others. In 3D cell culture, the biomaterials support a provisional extracellular matrix to create a 3D microenvironment for imparting cellular and tissue level activity for a specific function. The cell-encapsulated biomaterial formulation (referred to as “bioink”) supports the seeded cells and can be used for bioprinting to fabricate tissue analogs. The advancement of materials science in tissue engineering unlocks the possibility of using different biomaterials that mimic the 3D microenvironment at the cellular level and the native ECM and support the seeded cells, inducing proliferation, differentiation, migration, and ultimate tissue remodeling.
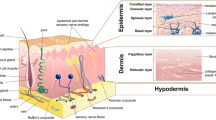
Bioprinting allows the deposition of bioink on receiving substrate in a predefined position in the x-, y-, and z-axes with the potential to mimic the cellular architecture like native tissue and organs. The flexibility of design and manufacturing enables the production of various forms of tissue construction, including tubes, patches, sheets, and organoid structures with high shape and size fidelity. With respect to skin tissue engineering, the dermis layer contains a more elevated amount of extracellular matrix (mainly type 1 collagen) (Montagna et al. 1992). In the epidermis, the keratinocyte cell layer is present without much extracellular matrix with several types of appendages (Fig. 5.1). The basement membrane presents between the dermis and epidermis, bonded tightly with each side, and prevents vascularization of the epidermis. Skin tissue has very high strength and elastic properties due to the presence and orientation of collagen fiber in the dermis that response to external stress and strain. The innermost layer, called hypodermis, comprises adipocytes, lipocytes, and macrophages. The function of the hypodermis is to provide insulation, vascularize the dermis, and padding that acts as a shock absorber.
In this chapter, we briefly review different bioprinting strategies for skin tissue fabrication, outlining the importance of structural requirements, highlighting the limitation of available engineered skin tissue, and pointing to the need for bioprinting of human skin tissue. Finally, the future challenges and future scope are also discussed.
5.2 Ultrastructure and ECM Composition
Skin is the largest and heaviest organ of our body. Though the thickness of skin up to a few millimeters only, its weighs from 3.5 to 10 kg depending upon height and body mass index. The surface area of 1.5–2 m2 makes it about one-seventh of our body weight. The skin mirrors the internal body’s condition and provides primary information about a person’s age and health conditions. Changes in skin color and structure can signify medical conditions and display initial signals for chronic diseases.
5.2.1 Epidermis
Epidermis, the outer layer of the body, is maintained in a dynamic equilibrium state with continuously renewing by underneath cells. Depending on the site, the epidermis varies in thickness, and it is only 0.3 mm thick on elbows and in soles of our feet and palms of our hands up to 4 mm. The predominant cells in the epidermis are keratinocytes with different layers (Table 5.1) containing viable inner cells called stratum basale or stratum Malpighi and outer anucleated horny cells layer are called stratum corneum (Fig. 5.2). The keratinocytes in basale are continuously renewing and pushing up the upper layer, where they harden and eventually die off. The hardened keratinocyte (corneum) is closely packed and sealed from the outside environment. This continuously renewing keratinocyte replaces the cells, which shed off as tiny flakes. The cells in the epidermis grow faster, become thicker, hardened, and develop a callus to withstand pressure or rubbing to protect itself (Montagna et al. 1992).
The epidermis also contains other cell types with special functions:
-
Melanocytes: Melanocytes secrete melanin, the color pigment of skin in response to sunlight. This protects the skin from harmful UV rays.
-
Lymphocytes and Langerhans Cells: The predominant immune cells present in epidermis.
-
Merkel Cells: Merkel cells are special nerve cells in the skin that get activated in response to pressure and send signal to the central nervous system.
5.2.2 Basement Membrane
A periodic acid–Schiff (PAS) reactive membrane separates epidermis from underneath dermis by physically and functionally at the dermal–epidermal junction. In the epidermal side, basal keratinocyte cells produce hemidesmosomes, a junction between basal keratinocytes and lamina densa that attached basal keratinocytes to the basement membrane matrix. The keratin filament forms a cytoskeleton within the keratinocyte that attaches to the hemi desmosome, and anchoring fibrils bind to the lamina densa to the dermis collagen matrix. In the lamina densa, type IV collagen is predominant to anchor with the dermis and epidermis side, and the anchoring plaque in the dermis matrix is composed of collagen type VII.
5.2.3 Dermis
The dermis, a connective tissue matrix predominantly type 1 collagen, makes up 20% of the total body weight. It comprises fibrous proteins embedded in amorphous ground substances like collagen, elastin, and reticulin. The matrix is penetrated by the blood vessels, nerves, and lymphatics from the subcutis layer that reach the epidermal appendages like the eccrine and apocrine sweat glands.
Grossly, the dermis is rigid and flexible tissue with viscoelastic properties, mainly due to collagen. Based on the orientation of collagen fiber and fibroblast cells, the dermis can be arbitrarily divided into two layers: the papillary layer and the reticular layer (Fig. 5.3). Papillary dermis is the uppermost layer of dermis that lies immediately beneath the basement membrane, and it protruded to epidermis called rete ridges that serve to anchor tightly with increase in the surface area for interaction between epidermis and dermis. The papillary dermis is demarcated as it contains more elongated shaped fibroblast cells and the presence of a vascular plexus in rete subpapillae. Papillary dermal fibroblasts have higher growth rate and less contractile properties and synthesize more decorin than the reticular dermis. In the reticular dermis, the fibroblast cells have more finger-like projection, less growth kinetics, and higher contractile properties. Reticular dermal fibroblast produced less decorin and higher versican both in vivo and in vitro. Despite their dissimilarity, in respect to synthesis of matrix protein collagen types I and III, there are no differences between the two fibroblast subpopulations (Sriram et al. 2015). The composition and orientation of ECM also vary in papillary and reticular dermis based upon their secretion of ECM and functionality. In the papillary layer, the collagen fibers are thin with random orientation, whereas the reticular layer composes thick collagen fibers with orientation along the dermis–epidermis junction (DEJ). As the papillary layer contains more delicate collagen fiber, the inter-fibrillar space has a greater ground substance composed of proteoglycan.
5.2.4 Hypodermis
The hypodermis, innermost layer of skin containing fatty layer, differs in thickness with sex, race, hormonal, and individual nutritional status. The primary functions of the hypodermis layer are thermoregulation, contouring the body shape, cushioning from the outside mechanical force, filling space, and serving as an instantly available source of energy (Fig. 5.3). The hypodermis consists of three fatty layers separated from each other by connective tissue sheaths. The upper first layer is thicker and has a smaller number of connective tissue septa in women than in men.
5.3 3D Bioprinting Methods Applied in Skin Bioprinting
Bioprinting is a promising technology for the commercial fabrication of tissue constructed by layer-by-layer deposition of cells and biomaterials with computer-controlled precision. The bioink is mainly composed of live cells and biomaterials. Additional biological substances like growth factors and cytokines are often mixed in the bioink for better functionality of seeded cells. This technique enables the creation of a construct with patient-specific architecture using CT-scan data. The bioprinted construct aims to support and promote the seeded cells for maturation, proliferation, and differentiation, ensuring tissue remodeling. With the advances in additive manufacturing, researchers have developed different 3D bioprinting methods to fabricate a complex tissue construct. Three methods commonly used for the bioprinting of skin tissue are extrusion-based bioprinting, inkjet bioprinting, and laser-assisted bioprinting (Fig. 5.4).
5.3.1 Extrusion-Based Bioprinting
Extrusion-based bioprinting is the most popular and well-established fabrication process for the fabrication of tissue construct. The bioink is extruded out from the nozzle tip by using pneumatic pressure pushed by a plunger or mechanical screw. Though hydrogel-based biomaterials are generally used as a bioink for extrusion-based bioprinting, thermoplastic materials also can be used for printing scaffolds using a temperature-controlled extruder. As the melting temperatures of thermoplastic materials are much higher than the physiological temperature, the cells can only be seeded on the scaffolds after printing and used for various tissue engineering applications. Extrusion-based 3D bioprinters can print different hydrogels with shear thinning behavior and natural polymers like collagen, decellularized extracellular matrix, and synthetic biopolymers like poly (lactic-co-glycolic acid).
The resolution of extrusion-based bioprinting is fundamentally governed by the diameter of nozzle diameter, mechanical and physical properties of hydrogels, and the printing parameters (Fig. 5.4). The extrusion pressure, either pneumatic or screw-based, applied on hydrogel, generates shear stress that in turn causes reduction in viscosity of bioink intended to dispense from the nozzle. The volumetric flow rate of bioink increases with increasing the applied pressure, which needs to be optimized. The printed line width correlates with the inner diameter of the nozzle. Further, it directly correlates with the applied pressure and inversely correlates with the printing speed. Although the final resolution (in X-, Y-, and Z-directions) depends on the gelation kinetics of the bioink and the viscosity (how quickly the material spread before it got gelled), the possible way to increase the printing resolution for hydrogel is to choose a minimum diameter of the nozzle and low applied pressure with high printing speed. The applied shear stress on bioink and cells is directly related to the applied pressure, printing speed, and nozzle diameter, which limits the further printing resolution as higher shear stress directly damages the cells.
Bioinks often used in extrusion-based bioprinting are not robust enough to support themselves and hold the weight of the additional layer printed on the top of it. Recently, researchers developed a support bath to counter this by providing support to the printed structure. This support bath, made up of protein or polysaccharide with very small size of particles, behaves as a solid substrate under zero or low shear. As the nozzle passes through the media, it generates shear strain to decrease storage modulus (G’) and increase loss modulus (G”), resulting in it behaving like a liquid while allowing dispensing of bioink. The technology has expanded the printability of low viscous materials for fabricating complex tissue.
5.3.2 Inkjet-Based Bioprinting
Inkjet-based bioprinting techniques have been developed simultaneously along with extrusion bioprinting. The bioink forms into picometer-level droplets and is dispensed by the nozzle. The pressure pulse, generated by microvalve, thermal, piezoelectric, or acoustic actuators, is the driving force to generate bioink droplets (Fig. 5.4). The bioinks needed for inkjet-based bioprinting typically have low viscosity with a high gelation rate as they become gel before deposition on the receiving substrate. Low viscosity and a high surface area-to-volume ratio are needed for extrusion, and fast gelation is required to prevent shape deformation, but these limit the number of materials used in droplet-based bioprinting. Ideally, the bioink droplets should form a gel after they are ejected from the nozzle to prevent nozzle clogging. Methods have also been developed to print bioink droplets into other liquids that induce gelation containing cross-linking agents to preserve the shape and volume. This printing strategy is beneficial for controlling the porosity of printed scaffold by allowing micelle formation of sacrificial materials with precise size and shape. This high controllable porosity is being applied for bioprinting vascular tissue fabrication.
The bioinks used in inkjet printing typically have low viscosity compared to extrusion-based bioprinting. They also need less pressure to extrude from the nozzle, resulting in high cell viability compared to extrusion-based bioprinting. Inkjet printing also benefits from the small size of bioink droplet formation that offers excellent printing resolution.
5.3.3 Laser-Assisted Bioprinting
Laser-assisted bioprinting (LAB) is an emerging technique to engineer tissue mimics by direct writing based on laser-induced forward transfer. The three important major parts of a laser-assisted bio printer include a ribbon, a pulse laser, and a receiving substrate (Fig. 5.4). The multilayered ribbon comprises a transparent glass, a fine layer of gold or titanium (laser-absorbing), and a bioink layer. A pulsed laser beam focuses on the ribbon to heat the thin metal layer, which in turn creates a high-pressure bubble to push the bioink on the receiving substrate. Culture media present on the receiving substrate supports the newly formed droplets that are transferred from the ribbon. The resolution of LAB varies from picometer to micrometer depending upon several factors like thickness of bioink coating on the ribbon, viscosity, surface tension of bioink, laser intensity, and the gap between the ribbon and substrate. LAB has high precision and resolution and can print viscous bioink-containing cells without imparting mechanical stress to the cells.
5.4 Bioprinted Skin Tissue
Standard 3D skin models are significantly advanced from the traditional 2D skin cell culture. Still, their regulation does not reflect like in vivo situation. Production of automated functional engineered skin tissue is possible by the advancements in current 3D tissue development technology. Commercially available skin-like tissue constructs for in vivo applications are Apligraf® (Eaglstein and Falanga 1998), TheraSkin®, Dermagraft (Hart et al. 2012), and OrCelR and in Vitro applications such as EpiSkin (L'Oréal) (Roguet et al. 1994), EpiDerm™ (MatTek corp.) (Cannon et al. 1994), Leiden epidermal skin model and total thickness human skin model (biomimetic Aeon Astron Europe), EpiCSR RHE (CellAystemsR GmbH and Atera SAS), and LabCyte EPI-MODEL (Japan Tissue Engineering Co., Ltd). The developed model underlines the progress of tissue models toward more physiologically relevant tissue models through collaboration between industry and academia (Fig. 5.5).
Histological and immunohistochemical assessment of 3D printed skin equivalent. (a) H&E-stained cross-sectional image of cultured multilayered skin. Scale bar (i) 1 mm, (ii) 200 μm, and (iii) 50 μm. (b–e) Expression of skin-specific markers, keratinocyte differentiation: anti-involucrin (b), cell proliferation: anti-Ki-67 (c), anti-vimentin for viable epidermal/dermal layer (d), and anti-collagen 1 for type 1 collagen production (e) (scale bar: 20 μm). Reproduced from reference Lee et al. (2021) with permission from ELSEVIER
5.4.1 Toward Well-Defined Physiological Matrix
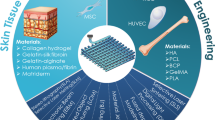
The microenvironment of living tissue is a mechano-physiological space provided to tissue, maintaining its structural and functional integrity. Owing to the versatility and heterogeneity of multicellular organisms, it is difficult to define the native microenvironment of specific tissue and cellular activity is lost when we modify their microenvironment during in vitro cellular study. Mimicking the physiological microenvironments is the primary target for fabricating functional tissue analogs in vitro. Natural polymers like collagen, hyaluronic acid, and alginate; natural polymer blends like gelatin/chitosan, collagen/alginate, gelatin/silk fibroin; and composites like PCL/collagen are commonly used biomaterials for 3D bioprinting skin constructs.
Collagen is the structural and functional unit of skin ECM; in particular, type 1 and type III collagens are the significant elements of skin ECM. Content and ratio of these two types of collagens vary with respect to age and injury. In fetal skin, type III collagen accounts for 34–65%, while in adults, type I collagen is 80–85% (Sriram et al. 2015). The changes in ECM content with time infer that it evolves to support the residing cells and architecture that is highly dynamic and heterogeneous. Collagen is most extensively explored in tissue engineering and regenerative medicine due to its abundance in target organs and in nature and because of its cells’ supporting behavior. Although pure collagen has low mechanical strength and slow gelation properties, 5% collagen solution is printable with extrusion-based bioprinting, but 10% collagen was used rarely because in higher collagen concentration, the dense fibrous architecture limits cell migration and viability (Hospodiuk et al. 2017; Yoon et al. 2016; Cross et al. 2010). The possible approach to increase the mechanical strength is to either increase NaCl concentration, temperature, and collagen concentration or mix with other higher modulus biomaterials (Duan et al. 2013; Lai et al. 2008; Rhee et al. 2016; Ng et al. 2016; Kim et al. 2011; Xiong et al. 2017).
Designing ideal biomaterials for tissue engineering application is extremely challenging as the tissue-specific microenvironment undergoes an evolutionary development from embryo to maturity. Additionally, the composition and ultrastructure of ECM are still yet to be fully characterized using the most sophisticated approach; the synthetic approach cannot generate tissue construct in vitro. Decellularized extracellular matrices could be better materials to mimic the tissue-specific microenvironment with respect to ECM composition and ultrastructure. Additionally, the ECM acts as a reservoir of various growth factors and protects them from degradation while controlling their release profile (Shpichka et al. 2019). Multiple decellularization processes remove cells from the target tissue while maintaining the ECM composition and ultrastructure. Detergent-based decellularization can limit the ECM composition and ultrastructure, further restricting cellular activity (Bera et al. 2022).
5.4.2 Toward Well-Defined Cellular Architecture
Cellular architectural design is the key regulatory factor for functional in vitro tissue construction. Skin is a multicellular and multilayered complex organ, and it is challenging to build a skin tissue model with high reproducibility. The current technology focuses on emulating skin anatomy by co-culturing major cell types such as fibroblasts, keratinocytes, and melanocytes. In general, keratinocyte cells are top seeded on the engineered dermis scaffold by manually or by bioprinting techniques that mature upon exposure to air. Additionally, growth factors or calcium ions can be used to promote keratinocyte cells to differentiation and stratification to mature stratum corneum (Pruniéras et al. 1983; Bikle 2014; Bikle et al. 2012).
To fabricate a more stable and reliable skin model, the high-resolution positioning and arrangement of keratinocyte cells are crucial that mimic the epidermal–dermal junction and further helps in the formation of mature corneocyte. Although the extracellular matrix is not present in the epidermis, the bioink-containing cell suspension can be printed with inkjet or extrusion-based printing for precise patterning. A controlled volume of cell-laden bioink can be used. The human epidermal keratinocytes (HEKs) suspended in a culture medium have been extruded uniformly as mono-layered basal keratinocytes on top of the fibroblast cells encapsulated decellularized dermal matrix. Notably, the keratinocyte-suspended culture medium needs to extrude with a small diameter nozzle as it has very low viscosity and requires very low pressure for dispensing. Typically, the epidermis is very thin and bioprinting of dense epithelium on the bulky dermis layer limits reproducibility and is the limitation of extrusion-based technology. The piezoelectric inkjet printing technology may be appropriate for depositing very thin layers of keratinocyte cells (Lee et al. 2021). Collagen has been used for laser-assisted bioprinting as a bioink for printing NIH 3 T3 fibroblast cells on top of the supportive MatriDerm scaffold. The keratinocyte cells (HaCaT) were printed on top of the NIH 3 T3 layer to generate a bi-layer dermis and epidermis. After 10 days of culture, the epidermis becomes intact and the presence of Connexin 43 confirms the formation of gap junction (Koch et al. 2012).
In connective tissue, the predominant cells are fibroblasts that secrete extracellular matrix (ECM) and maintain their architectural design. The epidermis layer is underpinned by its connection to the dermis, which contains the dermal fibroblast, blood vessels, immune cells, nerve fiber, hair follicles, and secretory gland. The spindle-shaped fibroblasts cells have capability to attach to tissue culture plastics and express vimentin and collagen 1. Traditionally, fibroblasts maintain a static population that maintains and supports the skin by secretion and degradation of ECM. Still, it plays crucial roles in almost every process throughout life: embryogenesis and pathology like psoriasis, aging, fibrosis healing, and skin cancer. The fibroblast in the skin resides as morphologically and functionally heterogeneous subpopulations in different dermis compartments. Despite their typical phenotype, dermal fibroblasts in the superficial layer (papillary dermis) exhibit different gene expression patterns than deeper layer (reticular dermis) fibroblasts (Chang et al. 2002; Fries et al. 1994; Sorrell et al. 2004; Rinn et al. 2006; Sorrell and Caplan 2004). In the papillary dermis, the collagen fibers are thin and poorly organized, while in the reticular dermis it is well-organized and thick (Sorrell and Caplan 2004).
Additionally, the papillary dermis has a higher collagen III to collagen I ratio and a higher level of decorin than the reticular dermis and undergoes better remodeling during wound healing (Sorrell et al. 2004; Sorrell and Caplan 2004; Jahoda 2003; Taylor et al. 2000). The differences in composition and organization of the ECM in different sublayers play an important role in functionalizing the fibroblast cells in response to wound healing and scar formation. Despite the heterogeneous fibroblast subpopulation in papillary and reticular subtypes, the fibroblasts in association with hair follicles are also subdivided into follicular dermal papilla (FDP) and dermal sheath (DS) fibroblast. Fibroblasts associated with hair follicles are the regulatory factor to maintain homeostasis and generation of epithelial including epidermis and hair (Jahoda 2003; Taylor et al. 2000).
Various cells have been used for 3D bioprinting for skin tissue engineering. The primary choice to fabricate dermis is human neonatal dermal fibroblasts (hNDFs) due to their high expansion rate, relatively low immunogenicity, secretion of bioactive molecules like basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and keratinocyte growth factor (Shpichka et al. 2019). Some studies also use other types of cells for bioprinting dermal substitutes listed in Table 5.2.
5.4.3 Toward Well-Defined Skin Appendages
The advancement of tissue engineering and regenerative medicine has made a breakthrough in the fabrication of engineered skin but still it needs to incorporate many functional units like sweat glands, blood vessels, sensory neurons, hair follicles, and pigmentation. The ideal tissue-engineered skin must have all kinds of skin appendages and could develop pigmentation followed by transplantation. Creating a microenvironment with matrix and stem cells could regenerate appendages like sweat glands and hair follicles in a biofabricated skin tissue graft. The embryonic ectoderm develops all the skin appendages in developmental stages, and the epidermis acts as a reservoir of stem cell niches. However, compared to relatively well-known skin appendages like hair follicles, cell inductive response about sweat glands is less known. Recreating the microenvironment with adult epidermal progenitor cells through bioprinting technology is suitable for regenerating and restoring the sweat gland function (Todd 2015). In another study, a bioprinted matrix with different pore sizes has been shown to induce epidermal progenitor cells to differentiate into sweat glands (Liu et al. 2016).
Like other skin appendages, skin pigmentation is essential in protecting from damage by harmful UV rays. The melanocyte in the epidermis secretes melanin, and the pigment determines the skin color. The biofabrication of pigmented skin construct remains a bottleneck in skin tissue engineering. However, incorporating melanocytes into the 3D bioprinted skin construct has been used for developing pigmented skin models for cosmetics and toxicological studies. The sequential printing of keratinocyte and melanocyte on top of the collagen layer induces pigmentation when it is exposed to air–liquid interface culture (Min et al. 2018).
Hair follicles are another important skin appendage that plays an important role in thermoregulation, barrier function, secretion, and wound healing (Schneider et al. 2009). Compared to other skin appendages, regeneration of hair follicles from specialized dermal papilla cells (DPCs) is relatively slow. Although there have been some proof of concepts established for inducing hair follicles in mice when the DPCs are transplanted intracutaneously, developing therapeutic strategies of integrating hair follicles within artificial skin graft is challenging (Toyoshima et al. 2012). 3D bioprinting has been used for recreating specialized 3D microenvironments to develop hair follicles from dermal papilla cells (DPCs). Seeding DPCs in the microwell formed aggregation and developed hair follicles, creating micro-fabricated plastics containing hair follicles shaped extensions in the collagen I matrix (Abaci et al. 2018).
5.4.4 Toward Well-Defined Vascular Bed
Vascularization is an important indicator of engineered tissue to reach application level in the field of clinical and in tissue engineering and regenerative medicine. The bioengineered tissue constructs need a well-connected capillary network to develop as a drug testing model and to use as a graft. Additionally, when the bioengineered construct is used as a graft, it needs a prevascularized capillary network for maturation and functionalization. The diffusion limit of oxygen and nutrients is approximately 100–200 μm, also called Krogh length. When the fabricated tissue construct is relatively large, the cells in core are deprived of oxygen and nutrients and die.
Moreover, as the tissue matures upon time the encapsulated cells proliferate and fill the pores in the internal area of construct, which causes block of media transport channels. Therefore, a continuous vascular network is significant for the viability and maturation of fabricated tissue constructs. Sacrificial materials or coaxial nozzle-based 3D printing strategies may be the solution to develop vascular networks in skin tissue grafts (Abaci et al. 2016). The major limitation of printing with sacrificial materials is the channel diameter, which is larger than the actual diameter in native tissue. Furthermore, the endothelial lining is often not uniform and intact like the tunica intima of native blood vessels. Cell-based strategies could be the best approach to stimulate seeded endothelial cells to form the vascular network in the fabricated scaffold. The best strategy would be to create an optimum microenvironment for endothelial cells with supporting cells or growth factors, including a lumen, and further maturing into a vascular network. Zhu e al. (Zhu et al. 2017) introduced the prevascularization of skin tissue by digital light processing (DLP)-based bioprinting method. The endothelial and 10 T1/2 cell suspension with a photopolymer is exposed to hexagonally patterned UV light to produce cell-laden vascular channels. Then, the scaffold was transplanted to a dorsal region of immune-deficient mice that formed a lumen-like structure by integrating host blood vessels.
5.5 Validation of Bioprinted Functional Artificial Skin
The technology leading to a 3D reconstructed skin model was first introduced by James Rheinwald and Howard Green almost 40 years ago (Green et al. 1979; Connor et al. 1981). They cultivate human keratinocytes on a dermis tissue and expose it to an air–liquid interface culture to mature a fully differentiated epidermis to treat burn injury. Besides skin grafting in burn injuries, reconstructed skin tissue models are mainly used for research purposes. However, tissue-engineered skin models are being used for broader purposes, including the cosmetic industry, assessment of hazards by chemicals and pesticides, and preclinical testing of novel drugs. Following the fabrication of skin tissue grafts by researchers or by commercial developers, there is a need for international validation and acceptance for its use globally in both industrial and academic research (Fig. 5.6).
An essential feature of tissue-engineered skin is an effective barrier function that can resist the penetration of cytotoxic materials through skin. A well-established test to address skin’s barrier function is the assessment of ET-50 value (effective time at which fixed concentration of toxic materials penetrates tissue to 50%) or IC-50 (inhibition concentration of a toxic substance causes 50% reduction of cell viability). Usually, 1% Triton X-100 is used for ET-50, and a minimum of three concentrations of SDS is used for IC-50 for skin validation tests (Kandarova and Hayden 2021). As a structural validation of bioengineered skin products, the presence of different cells in a precise location with the secretion of specific extracellular matrix proteins is assessed by histology and immunohistochemistry techniques for further use. Immunofluorescent localization of epidermal markers like keratins 5, 10, and 14, involucrin, loricrin, filaggrin, and transglutaminase is required to confirm the maturation of keratinocytes. In the dermal–epidermal junction, there is a need to develop a functional basement membrane that could hold and separate epidermis from the dermis with the presence of expression markers such as laminin 5, collagen IV, and collagen VII. In the dermis, fibroblasts cell maturation is required, and papillary and reticular compartmentalization is required for the elasticity and toughness of skin tissue. The expression of different markers such as decorin, versican, alpha-SMA, and collagen I to III ratios is also essential to validate the skin model for dermal maturation. A further critical parameter for barrier function and evaporation is the lipid profile of the available skin model, which needs to be assessed with standard quality control (QC).
5.6 Challenges and Future Directions of Skin Tissue Fabrication
Although the primary goal of tissue engineering and regenerative medicine focuses on achieving closure and healing of wounds at a reasonable rate, further additional considerations are to be made from the perspective of patients and evaluators. By traditional manufacturing, 3D tissue analogs are often made by molding. Later, the fabricated tissues are curved out or removed by a sharp cutting tool till the desired shape is formed. However, there are still challenges associated with these methods, especially when creating or controlling the internal structure with organ-specific microarchitecture of a specific organ.
To date, the generation of laboratory-grown skin substitutes only partially addressed the requirement for stable wound closure, lacking several appendages and vasculature deemed to be accepted equally by industry, regulatory bodies, clinicians, and patients. The formation of blood vessels and neural growth in native tissues generally occurs on the interface between the hypodermis and dermis, which matured to a deep vascular plexus; capillaries spread into the dermis layer provide a gaseous exchange; however, the epidermis get oxygen and nutrients through diffusion only. A lack of organized synergistic layer makes unviable the growth of nerve and basement membrane formation, impairing sensing, protecting, and thermoregulatory functions. As mentioned earlier, the inclusion of skin appendages has the additional complication to fabricating by current strategies as the microenvironment needed for each of the appendages depends upon growing other tissue architecture (Moroni et al. 2018). One further consideration for the fabrication of skin tissue is industrial-scale mass production with consistency between different lots having optimum properties. This includes the procedures used in processing, manufacturing, and characterizing the products with repeatable and reliable results. Finally, if the cells used in therapy are autologous, consistent methods for harvesting cells are required for mass-scale production, as the phenotypic properties may change, and effectiveness may get reduced when those cells grow with extensive passage.
The current biofabrication technology and advances in tissue engineering can reduce and to some extent replace the need for skin autograft in clinical translation. Patients with life-threatening burns or major accidental cases would no longer suffer from painful morbidity if bioprinted skin grafts were available. It can also reduce the total time in ICU and hospitalization and would further reduce the need for reconstructive surgery and overall cost (Yu et al. 2019). The tissue-engineered construct also has implications in congenital defect surgery, certain surgical reconstruction, and epidermolysis bullosa treatment contributing to the importance and requirement of fabricated skin tissue. The requirement of epidermal appendages having naturally matched skin pigmentation, sweat glands, vascular plexus, and sensory nerves further opens new challenges to investigate homeostasis and abnormality (Dearman et al. 2021). The advancement of tissue engineering and regenerative medicine has a high degree of confidence that many of the appendages can be incorporated into a further skin substitute model as a full-thickness skin graft for clinical application.
5.7 Conclusion
Ultimate goal of skin tissue engineering by biofabrication is to meet the requirement or replace the current gold standard autologous skin graft. With respect to clinical application, there is a need for additional requirement of minimizing or eliminating scar formation for broad range of patients, and wound types are reliable. The regenerated skin is not only functionally or looks like native tissue but also could match patients’ specific pigmentation for a wide range of pigmentation across the population. Despite some initial successful transplantation of 3D bioprinted skin tissue grafts, there is an unfulfilled need for further incorporation of different skin appendages to treat full-thickness skin wounds. However, the market’s current biofabricated skin substitute options remain limited by too often a trade-off between efficacy and too high cost. Further efforts are needed to achieve an ideal skin substitute by continuous collaboration, exchange of opinion among researchers, regulatory bodies, and clinicians to ensure the final product attains a wide range of perspectives to use both in clinics and for drug and toxicity testing.
References
Abaci HE, Guo Z, Coffman A, Gillette B, Lee WH, Sia SK et al (2016) Human skin constructs with spatially controlled vasculature using primary and iPSC-derived endothelial cells. Adv Healthc Mater 5(14):1800–1807
Abaci HE, Coffman A, Doucet Y, Chen J, Jacków J, Wang E et al (2018) Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat Commun 9(1):1–11
Barros NR, Kim HK et al (2021) Biofabrication of endothelial cell, dermal fibroblast, and multilayered keratinocyte layers for skin tissue engineering. Biofabrication 13(3). https://doi.org/10.1016/j.snb.2014.04.005
Bera AK, Sriya Y, Pati F (2022) Formulation of dermal tissue matrix bioink by a facile Decellularization method and process optimization for 3D bioprinting toward translation research. Macromol Biosci 22(8):1–15
Bikle DD (2014) Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 21(3):319–329
Bikle DD, Xie Z, Tu CL (2012) Calcium regulation of keratinocyte differentiation. Expert Rev Endocrinol Metab 7(4):461–472
Cannon CL, Neal PJ, Southee JA, Kubilus J, Klausner M (1994) New epidermal model for dermal irritancy testing. Toxicol in Vitro 8(4):889–891. https://www.sciencedirect.com/science/article/pii/0887233394900957
Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D et al (2002) Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA 99(20):12877–12882
Chen X, Yue Z, Winberg PC, Lou YR, Beirne S, Wallace GG (2021) 3D bioprinting dermal-like structures using species-specific ulvan. Biomater Sci 9(7):2424–2438
Connor NEO, Mulliken JB, Banks-Sciilegel S, Kehinde O, Green H (1981) Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet 1:75–78
Cross VL, Zheng Y, Won Choi N, Verbridge SS, Sutermaster BA, Bonassar LJ et al (2010) Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 31(33):8596–8607
Dearman BL, Boyce ST, Greenwood JE (2021) Advances in skin tissue bioengineering and the challenges of clinical translation. Front Surg 8:640879
Derr K, Zou J, Luo K, Song MJ, Sittampalam GS, Zhou C et al (2019) Fully three-dimensional bioprinted skin equivalent constructs with validated morphology and barrier function. Tissue Eng Part C Methods 25(6):334–343
Diani J, Gall K (2006) Finite strain 3D Thermoviscoelastic constitutive model. Society 1–10:486
Duan L, Li J, Li C, Li G (2013) Effects of NaCl on the rheological behavior of collagen solution. Korea Aust Rheol J 25(3):137–144
Eaglstein WH, Falanga V (1998) Tissue engineering and the development of Apligraf, a human skin equivalent. Cutis 62(1 Suppl):1–8
Fries KM, Blieden T, Looney RJ, Sempowski GD, Silvera MR, Willis RA et al (1994) Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol 72(3):283–292
Green H, Kehinde O, Thomas J (1979) Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci USA 76(11):5665–5668
Hafezi F, Shorter S, Tabriz AG, Hurt A, Elmes V, Boateng J et al (2020) Bioprinting and preliminary testing of highly reproducible novel bioink for potential skin regeneration. Pharmaceutics 12(6):1–21
Hart CE, Loewen-Rodriguez A, Lessem J (2012) Dermagraft: use in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 1(3):138–141
Hospodiuk M, Dey M, Sosnoski D, Ozbolat IT (2017) The bioink: a comprehensive review on bioprintable materials. Biotechnol Adv 35(2):217–239
Intini AC, Elviri L, Cabral J, Bergonzi C, Bianchera A, Flammini L et al (2018) 3D-printed chitosan-based scaffolds: an in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats. Carbohydr Polym 199:593
Jahoda CAB (2003) Cell movement in the hair follicle dermis - more than a two-way street? J Invest Dermatol 121(6):ix–xi
Kandarova H, Hayden PJ (2021) Standardised reconstructed skin models in toxicology and pharmacology: state of the art and future development. Handb Exp Pharmacol 265:57–71
Kim G, Ahn S, Yoon H, Kim Y, Chun W (2009) A cryogenic direct-plotting system for fabrication of 3D collagen scaffolds for tissue engineering. J Mater Chem 19(46):8817–8823
Kim G, Ahn S, Kim Y, Cho Y, Chun W (2011) Coaxial structured collagen–alginate scaffolds: fabrication{,} physical properties{,} and biomedical application for skin tissue regeneration. J Mater Chem 21(17):6165–6172. https://doi.org/10.1039/C0JM03452E
Kim BS, Lee JS, Gao G, Cho DW (2017) Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 9(2):025034
Koch L, Kuhn S, Sorg H, Gruene M, Schlie S, Gaebel R et al (2010) Laser printing of skin cells and human stem cells. Tissue Eng Part C Methods 16(5):847–854
Koch L, Deiwick A, Schlie S, Michael S, Gruene M, Coger V et al (2012) Skin tissue generation by laser cell printing. Biotechnol Bioeng 109(7):1855–1863
Lai G, Li Y, Li G (2008) Effect of concentration and temperature on the rheological behavior of collagen solution. Int J Biol Macromol 42(3):285–291
Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN et al (2014) Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C Methods 20(6):473–484
Lee HR, Park JA, Kim S, Jo Y, Kang D, Jung S (2021) 3D microextrusion-inkjet hybrid printing of structured human skin equivalents. Bioprinting 22:e00143. https://www.sciencedirect.com/science/article/pii/S2405886621000166
Liu N, Huang S, Yao B, Xie J, Wu X, Fu X (2016) 3D bioprinting matrices with controlled pore structure and release function guide in vitro self-organization of sweat gland. Sci Rep 6:34410
Min D, Lee W, Bae IH, Lee TR, Croce P, Yoo SS (2018) Bioprinting of biomimetic skin containing melanocytes. Exp Dermatol 27(5):453–459
Montagna W, Kligman AM, Carlisle KS (eds) (1992) Atlas of normal human skin. University of Texas Library
Moroni L, Burdick JA, Highley C, Lee SJ, Morimoto Y, Takeuchi S et al (2018) Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat Rev Mater 3:21
Ng WL, Yeong WY, Naing MW (2016) Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int J Bioprint 2(1):53–62
Pruniéras M, Régnier M, Woodley D (1983) Methods for cultivation of keratinocytes with an air-liquid interface. J Invest Dermatol 81(1 Suppl):28s–33s
Rhee S, Puetzer JL, Mason BN, Reinhart-King CA, Bonassar LJ (2016) 3D bioprinting of spatially heterogeneous collagen constructs for cartilage tissue engineering. ACS Biomater Sci Eng 2(10):1800–1805
Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY (2006) Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet 2(7):e119
Roguet R, Régnier M, Cohen C, Dossou KG, Rougier A (1994) The use of in vitro reconstituted human skin in dermotoxicity testing. Toxicol in Vitro 8(4):635–639. https://www.sciencedirect.com/science/article/pii/0887233394900337
Schneider MR, Schmidt-Ullrich R, Paus R (2009) The hair follicle as a dynamic Miniorgan. Curr Biol 19(3):132–142
Shpichka A, Butnaru D, Bezrukov EA, Sukhanov RB, Atala A, Burdukovskii V et al (2019) Skin tissue regeneration for burn injury. Stem Cell Res Ther 10(1):1–16
Sorrell JM, Caplan AI (2004) Fibroblast heterogeneity: more than skin deep. J Cell Sci 117(Pt 5):667–675
Sorrell JM, Baber MA, Caplan AI (2004) Site-matched papillary and reticular human dermal fibroblasts differ in their release of specific growth factors/cytokines and in their interaction with keratinocytes. J Cell Physiol 200(1):134–145
Sriram G, Bigliardi PL, Bigliardi-Qi M (2015) Fibroblast heterogeneity and its implications for engineering organotypic skin models in vitro. Eur J Cell Biol 94(11):483–512
Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM (2000) Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102(4):451–461
Todd L (2015) Accepted manuscript Development Accepted manuscript. (June): 1–33
Toyoshima KE, Asakawa K, Ishibashi N, Toki H, Ogawa M, Hasegawa T et al (2012) Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat Commun 3:784
Vaezi M, Yang S (2015) Extrusion-based additive manufacturing of PEEK for biomedical applications. Virtual Phys Prototyp 10(3):123–135
Wang Y, Beekman J, Hew J, Jackson S, Issler-Fisher AC, Parungao R et al (2018) Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev 123:3–17
Xiong S, Zhang X, Lu P, Wu Y, Wang Q, Sun H et al (2017) A gelatin-sulfonated silk composite scaffold based on 3D printing technology enhances skin regeneration by stimulating epidermal growth and dermal neovascularization. Sci Rep 7(1):4288
Yoon H, Lee JS, Yim H, Kim G, Chun W (2016) Development of cell-laden 3D scaffolds for efficient engineered skin substitutes by collagen gelation. RSC Adv 6(26):21439–21447. https://doi.org/10.1039/C5RA19532B
Yoon J, Xing TL, Zhang HB, Yin RX, S.M. Yang JW and WJZ. (2018) Tyrosinase doped bioink for 3D bioprinting of living skin constructs. Sensors Actuators B Chem 199:369. https://doi.org/10.1088/1748-605X/aaa5b6
Yu JR, Navarro J, Coburn JC, Mahadik B, Molnar J, Holmes JH et al (2019) Current and future perspectives on skin tissue engineering: key features of biomedical research, translational assessment, and clinical application. Adv Healthc Mater 8(5):1–19
Zhu W, Qu X, Zhu J, Ma X, Patel S, Liu J et al (2017) Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 124:106–115
Zidarič T, Milojević M, Gradišnik L, Kleinschek KS, Maver U, Maver T (2020) Polysaccharide-based bioink formulation for 3D bioprinting of an in vitro model of the human dermis. Nano 10(4):1–19
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bera, A.K., Pati, F. (2023). 3D Bioprinting of Skin Tissue Model. In: Pant, A.B., Dwivedi, A., Ray, R.S., Tripathi, A., Upadhyay, A.K., Poojan, S. (eds) Skin 3-D Models and Cosmetics Toxicity. Springer, Singapore. https://doi.org/10.1007/978-981-99-2804-0_5
Download citation
DOI: https://doi.org/10.1007/978-981-99-2804-0_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2803-3
Online ISBN: 978-981-99-2804-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)