Abstract
The heterojunction composites (Ba0.85Ca0.15)(Zr0.1Ti0.9)TiO3(BCZT)/Bi2O3 with different weight ratios (75:25, 50:50 and 25:75) were successfully synthesized by solid-state route. As-synthesized composite powders were characterized by XRD, FESEM, EDX, UV–visible and photoluminescence spectroscopy. Photocatalytic activity evaluation was carried out by the degradation of rhodamine B (RhB) under UV and visible light exposure. The results show that the heterojunction composites BCZT/Bi2O3 display better photocatalytic activity than pure BCZT or Bi2O3. Among all the heterojunction composites, BCZT/Bi2O3 (50:50) composite exhibits a lower recombination rate of electron–hole pair and shows the highest photocatalytic activity. The rate constant of BCZT/Bi2O3 (50:50) composite for RhB degradation is 15.4 and 2.1 times higher than those of pure BCZT and Bi2O3 under visible light irradiation, respectively. Finally, a possible mechanism for enhanced charge separation and photodegradation is proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contamination of the water bodies is one of the major global problems, thus affecting the equilibrium of ecosystem which is caused by dumping all the waste from textile industries, chemical industries and other harmful organic pollutants due to rapid industrialization. There are many ways to treat these wastes such as filtration, ion exchange, biological treatment and adsorption on activated charcoal, but they have disadvantages such as high cost, low efficiency and high sludge production. Photocatalytic oxidation process has been extensively studied owing to its advantages such as low cost, non-toxic, stability under illumination and high reproducibility. Since the past decades, many semiconductor systems such as TiO2 [1], ZnO [2] and SnO2 [3] had been studied for photocatalytic activity. But the problems with this system are that they can utilize only 4% (UV) of the solar spectrum and high recombination rate of electrons and holes. There are many strategies to tackle this problem such as introduction of defect levels into the band gap by doping, metallization and heterojunction formation. Among all these strategies, heterojunction coupling of two or more semiconductors has been the most effective strategy to enhance the lifetime of electrons and holes, owing to its advantages including non-toxicity, non-selectivity, cost-effectiveness and total removal of pollutants [4].
Ferroelectric oxides such as PZT [3, 5, 6], BaTiO3 [7,8,9,10], KNbO3 [11] and SrTiO3 [12] are also studied for photocatalytic applications. But the problem with these systems is that they have high band gap (> 3 eV), thus utilizing only UV portion of solar spectrum. The advantage of these systems is the presence of inbuilt electric filed which helps in separation of electrons and holes, thus enhancing their life span [13,14,15]. In order to enhance the photoabsorption in visible region of solar spectrum, heterojunction has been made with Bi2O3 [16,17,18,19], Cu2O [20], Ag2O [21] and Fe2O3 [22] due to their matching band potentials with ferroelectric systems. Among different narrowband gap oxides (Bi2O3, Cu2O, Ag2O and Fe2O3) semiconductors, Bi2O3 is preferred due to having a band gap of 2.2 eV, strong oxidation ability of the valence band (VB) and excellent visible-light-absorbing capacity. Despite the advantages of Bi2O3, it is seldom used as a photocatalyst due to the reduction capability of conduction band (CB) (0.63 eV) which is more positive than the potential of O2/\( ^{*} {\text{O}}_{2}^{ - } \) (− 0.33 eV). Therefore, the \( e^{ - } \) on the CB of Bi2O3 cannot reduce the adsorbed O2 into \( ^{*} {\text{O}}_{2}^{ - } \), which results in short life span of charge carriers [23]. During the past few years, many reports are available on the preparation of oxide ferroelectrics–narrowband gap oxide heterostructure due to polarization-dependent surface properties of heterogeneous photocatalyst. Among them, PZT/TiO2 [6], Bi2O3/BaTiO3 [17, 19], Fe2O3/BaTiO3 [22] and Bi2O3/KNbO3 [18] have been promising. The breakthrough made by Liu and Ren et al. in BaTiO3 ceramics with Ca and Zr co-doping [0.5Ba(Zr0.2Ti0.8)O3–0.5(Ba0.7Ca0.3)TiO3] has offered a significant impact on the development of lead-free piezoceramics. The high saturation polarization (17µC/cm2) and large piezoelectric properties (d33 > 500pC/N) of BCZT make it a potential material for ferroelectric and piezoelectric applications [24,25,26]. However, BCZT is a wide band gap material. The fabrication of heterojunction with narrow band gap oxide semiconductor with matching band potentials can facilitate the high visible light activity and interfacial charge transfers and enhance the life span of photoinduced charge carriers. Till now, to the best of our knowledge there is no literature available on photocatalysis of BCZT and there are also no reports available on formation of heterojunction with BCZT. The present paper aims to improve the photocatalytic activity of BCZT by making heterojunction with Bi2O3. So, in the present work heterojunction has been made between BCZT and Bi2O3 which has a band gap of 2.2 eV and matching band potentials. In the present work, the objective is to make the heterojunction between BCZT and Bi2O3 by varying weight ratios and to study the effect of composite on UV–visible absorption spectrum and photocatalysis. Finally, a mechanism behind the photocatalytic degradation of RhB has also been proposed.
Experimental procedure
Synthesis of BCZT
BCZT was synthesized by conventional solid-state reaction route. Barium carbonate (Sigma-Aldrich, 99%), calcium carbonate (Sigma-Aldrich, 99%), zirconium dioxide (Sigma-Aldrich, 99.9%) and titanium dioxide (Sigma-Aldrich, 99.0%) were used as precursor materials. A stoichiometric amount of powders was mixed by a planetary mill (Fritsch Pulverizer) for 10 h with zirconia ball using isopropyl alcohol as the media. The mixture was dried at 100 °C overnight in an oven. The mixture was placed in an alumina crucible, which was subsequently inserted into the furnace and calcined at 1300 °C/4 h, in air.
Fabrication of BCZT/Bi2O3 composite
BCZT/Bi2O3 composite was prepared by solid-state method. Nano-Bi2O3 (Sigma-Aldrich, 99%) and the prepared BCZT were weighed according to BCZT: Bi2O3 = 100:0%, 75:25%, 50:50%, 25:75% and 0:100% (mass ratio). After that the powders were wet milled in pot mill using isopropanol for 6 h. The mixture was dried under IR lamp and calcined at 500 °C for 1 h. The reason for calcination is to provide sufficient bonding between the two oxides, which could provide a special condition for charge transfer from one particle to another via interfaces.
Characterizations
XRD patterns of the composites were recorded by Rigaku Ultima-IV with CuKα (1.54178Ao). The particle morphology was characterized by using FESEM [Nova NanoSEM 450] and TEM (Tecnai G230ST-FEI). Photoluminescence analysis was obtained by using a WITec alpha 300R, using an excitation wavelength of 355 nm. The light absorption spectrums were measured using a UV–visible spectrophotometer (PerkinElmer, USA) equipped with an integration sphere. The photocatalytic reaction was carried out in a stirred photoreactor with a capacity of 400 mL with a medium-pressure mercury vapor lamp 250 W and high-pressure mercury vapor lamp 450 W (Lelesil) placed inside the photoreactor as the main UV and visible irradiation sources. The inner immersion well was fixed with a water inlet and outlet for cold water circulation during the photochemical process. For photocatalytic experiments, initially 100 mg of the sample was added to 100 ml of rhodamine B (RhB) solution (10 mg/L) and ultrasonicated for 5 min. Initial pH of the RhB solution was ~ 5. Later, the mixture was stirred in the dark condition for 30 min to reach the adsorption/desorption state of equilibrium. Periodically (15 min), a 10-ml sample was injected out of the reaction vessel and the extent of degradation was measured by using Eq. (1).
where C0 is the concentration at time ‘t0’ and C is the concentration at time ‘t’.
Experiments were also carried out under different pH conditions (1, 3, 9 and 11). pH of the RhB solution was varied by adding HCl or 1 M NaOH solutions. Finally, to check the reusability, the photocatalyst was separated by centrifugation after each cycle and used for the next cycle without any pre-treatment.
Results and discussion
XRD analysis
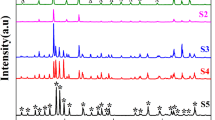
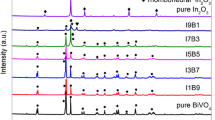
Figure 1 shows the XRD patterns of BCZT, Bi2O3 and BCZT/Bi2O3 composites with different weight ratios synthesized by solid-state route. For pure BCZT, all the diffraction peaks can be assigned to the tetragonal phase (JCPDS File No. 89-1428) and pure Bi2O3 was monoclinic (JCPDS File No. 71-2274). In the case of BCZT/Bi2O3 composite, there were two sets of diffraction peaks, which were correspondingly ascribed to tetragonal BCZT and monoclinic Bi2O3. No other characteristic peaks were detected, indicating that as-prepared composites consist of tetragonal BCZT and monoclinic Bi2O3 and no appreciable chemical reaction occurred between BCZT and Bi2O3 during the calcination process at 500 °C.
UV–visible spectroscopy
The UV–visible absorption spectra of prepared composites by solid-state method are shown in Fig. 2. The band gap of BCZT and Bi2O3 was found to be 3.1 eV and 2.2 eV, respectively. The optical absorption of BCZT/Bi2O3 composite prepared by solid-state route starts at about 550 nm. With increasing Bi2O3 content, absorbance intensity of composite increases under visible light. It can be observed that BCZT/Bi2O3 heterostructures show broad absorption bands, which shows that the composites have enhanced visible light absorption when compared with pure BCZT. In the present study, two absorption edges can be clearly observed when the content of Bi2O3 reaches 75% and 50% for the samples prepared by this synthesis technique. Xi et al. [17] reported that two absorption edges can be found when the content of Bi2O3 reaches 90%, and according to Fan et al. [19], single absorption edge can be observed for 75:25 and 50:50 Bi2O3/BaTiO3 composite. So BCZT/Bi2O3 composites can play a significant role in photocatalytic degradation of RhB under visible light irradiation.
FESEM analysis of BCZT/Bi2O3 composites
Figure 3 shows the FESEM micrograph of BCZT, Bi2O3 and calcined BCZT/Bi2O3 composite powders. From the micrograph, it can be observed that BCZT particles are agglomerated and submicron in size, while the Bi2O3 consists of spherical and plate like nature. BCZT/Bi2O3 composite contains both irregular and spherical kind of particles. The distribution of Bi2O3 on the surface of BCZT was further confirmed by EDS. Figure 4 shows the EDS color mapping of the BCZT/Bi2O3 composite samples. From the color mapping of the images, it can be found that the concentration of Bi2O3 increases on the surface of BCZT with an increase in BCZT/Bi2O3 molar ratio.
Photoluminescence spectroscopy of BCZT/Bi2O3 composites
The room-temperature PL spectra of BCZT and BCZT/Bi2O3 composites are shown in Fig. 5. The samples are excited at 427 , nm and all the samples show green emission (525 nm). The existence of visible light emission may be due to the inherent properties of perovskites structure [27]. The green emission band observed at 525 nm is frequently caused by the electron transition from the conduction band to oxygen antisites or by donor–acceptor recombination [28]. However, for BCZT/Bi2O3 composites, similar emission occurs with primary peak around 525 nm. In general, a high intensity in PL is attributed to the high recombination rate of electrons and holes. Therefore, lower intensity of PL reveals lower recombination probability of charge carriers and PL analysis can be regarded as an efficient approach to study the separation efficiency of photo-generated carriers [27, 28]. Due to formation of heterojunction, PL quenching is observed in BCZT/Bi2O3 composites, showing that heterojunctions possess lower recombination probability of photo-generated carriers compared to that of pure BCZT. Furthermore, lowest PL intensity observed for 50:50 composite among all the samples confirms the enhancement of charge separation capability in heterostructure catalyst between BCZT and Bi2O3.
Photocatalytic activity of BCZT/Bi2O3 composites
The photocatalytic activity of as-prepared BCZT/Bi2O3 composites was carried out under UV and visible light irradiation with time for degradation of RhB which has a significant peak at 554 nm. Figure 6 shows the degradation of the dye for all the samples as a function of time. From Fig. 6a, b, it can be observed that BCZT, Bi2O3 and BCZT/Bi2O3 (75:25, 50:50 and 25:75) composites can degrade only 23%, 33%, 66.5%, 85% and 53% of the dye after 60 min of UV light irradiation and 14%, 66%, 75.4%, 92% and 70% of the dye after 60 min of visible light irradiation. The experiment was also performed on RhB without adding any photocatalyst, and it has been observed that no degradation took place after illuminating for 1 h. It means that the degradation of dye took place only in the presence of photocatalyst. It was observed that 50:50 composite has yielded highest RhB degradation which was 3.69 and 2.5, under UV light irradiation and 6.5 and 2.5 times better than BCZT and Bi2O3 samples under visible light irradiation. For comparison, the commercial P25 was also studied under similar irradiation condition and it can degrade 87% and 60% of the RhB solution in 60 min under UV and visible irradiation conditions, respectively. It is clear that the photocatalytic activity of BCZT/Bi2O3(50:50) is comparable to the P25 under UV irradiation but almost 50% higher than the P25 under visible light irradiation. To quantitatively analyze the reaction kinetics of RhB degradation, the experimental data in Fig. 7 could be fitted by the Langmuir–Hinshelwood model, as expressed by the following equation [29]:
where Co and C are the concentrations of RhB at different irradiation times of to and t, respectively, and “k” is the pseudo-first-order rate constant of photodegradation (min−1). From the linear fitting curves of ln(Co/C) versus irradiation time ‘t’ in Fig. 7a, b, the RhB degradation rate constant k was found to be 0.0044, 0.0065, 0.0187, 0.030 and 0.012 under UV light irradiation for the BCZT, Bi2O3 and BCZT/Bi2O3 (75:25, 50:50 and 25:75) composites, respectively. Under visible light irradiation, the rate constants were 0.0025, 0.0188, 0.0237, 0.0395 and 0.0204 for the BCZT, Bi2O3 and BCZT/Bi2O3 (75:25, 50:50 and 25:75) composites, respectively. Among them, the 50:50 sample exhibited the highest k value which is 6.87 and 4.64, 15.4 and 2.1 times greater than that of BCZT and Bi2O3 samples under UV and visible light irradiation.
We have also studied the adsorption capacity of the catalyst with time before the light was turned on. The concentration of the dye decreases very marginally with time because of the adsorption of the dye molecules on the catalyst surface. In addition, the degradation of the dye due to the adsorption is very small [1.7% for BCZT and 2.8% for BCZT/Bi2O3 (50:50) after 30 min] as compared to the high degradation in the presence of light. So, we have not considered the contribution of the adsorption in the calculation of the rate constant.
During synthesis of BaTiO3/Bi2O3 composite by precipitation method, Fan et al. [19] found that with their best composition BaTiO3/Bi2O3 (40:60) degraded RhB solution within 80 min under UV exposure whereas 4 h was required for the same under visible light exposure. Also, Ye et al. [18] reported that while synthesis of KNbO3/Bi2O3 by solid-state method with their best composition KNbO3/Bi2O3 (30:70) degraded RhB and methyl orange (MO) solution within 50 min. Their rate constant of the composite was 3.7 and 6.9 times faster than that of pure KNbO3 and Bi2O3, respectively. In our case, 85% and 92% degradation of RhB solution happened after 60 min of UV and visible light irradiation, respectively, for 50:50 BCZT/Bi2O3 composition which is much better than the previous results.
The enhancement of photocatalytic activity for composite samples can mainly be attributed to the enhanced absorption in visible region and secondly due to the electric field-assisted charge transfer at the heterojunction interfaces between BCZT and Bi2O3 with matching band potentials, which consequently prevents the recombination of electrons and holes. The conduction band (CB) and valence band (VB) potentials of BCZT and Bi2O3 are calculated by using the following formulas [29]
where EVB is the valence band potentials of a semiconductor, χ is the electronegativity of a semiconductor which is the geometric mean of electronegativity of constituent atoms (Ba, Ti, Zr, Ca, Bi and O) and Ee is the energy of free electron on hydrogen scale (4.5 eV VS NHE). By using the above formula, the CB and VB potentials of BCZT are found to be − 0.94 and 2.16 eV and for Bi2O3 the values are found to be 0.63 and 2.83 eV, respectively. Under light irradiation, electrons present in the CB of Bi2O3 (0.63 eV) are more positive than the potential of O2/\( ^{*} {\text{O}}_{2}^{ - } \) (− 0.33 eV); therefore, the \( e^{ - } \) on the conduction band of Bi2O3 cannot reduce the adsorbed O2 into \( ^{*} {\text{O}}_{2}^{ - } \). Holes present in the VB of BCZT (2.16 eV) (considering the over potentials 0.2 eV) cannot oxidize H2O and \( {\text{OH}}^{ - } \) into \( {\text{OH}}^{ *} \) since the VB potential of BCZT was less than \( {\text{OH}}^{ *} \)/H2O (2.27 eV) and \( {\text{OH}}^{ *} \)/\( {\text{OH}}^{ - } \) (1.99 eV) potentials. So, in the present case photocatalytic degradation does not happen by a simple band-to-band transfer of electrons and holes. Here, a direct Z-scheme mechanism comes into play because the CB potential of BCZT has strong reduction ability and holes in the VB of Bi2O3 have strong oxidation ability [30]. When tight bonding occurs between BCZT and Bi2O3 due to their matching band potentials, band bending occurs at the interface which induces an inbuilt electric field across the interfaces. Thus, the internal electric field pushes the electrons and holes in the opposite direction, leading to a spatial separation of electrons and holes on different sides of heterojunction. The charge transfer mechanism is shown in Fig. 8.
Effect of pH on RhB degradation
The pH value has an important influence on the photodegradation of dye because it can influence the surface charge properties of a photocatalyst and ionic species in the solution [31,32,33]. Figure 9 displays the photocatalytic degradation of BCZT/Bi2O3 (50:50) composite as a function of pH. Initial pH of the RhB solution was ~ 5. The result shows that when the pH of the RhB solution was reduced to 3, the degradation efficiency increased to 90% in 45 min of visible light irradiation. With a further decrease in pH to 1, complete mineralization can be observed in 30 min. The higher degradation in low pH condition (pH < 5) occurs due to the electrostatic attraction between the negative charge of the BCZT/Bi2O3 catalyst and the positive charge of RhB [31, 32]. Moreover, at high initial pH values of 9 and 11, the percentage degradation was 54 and 23, respectively, which can be explained by the deprotonation of the carboxyl group of RhB and the transformation of the cationic form of RhB into zwitterionic form [31, 32].
Identification of active species in photocatalytic reactions
To understand the role of active species on RhB degradation, photocatalytic experiments were carried out under visible light irradiation with different scavengers. To do this, 1 mM of benzoquinone (as \( {\text{O}}_{2}^{ - } \) scavenger), 1 mM of IPA (isopropyl alcohol) (as.OH scavenger) and 1 mM of oxalic acid (as h+ scavenger) were added separately to RhB solution. Figure 10a shows the degradation of RhB for 50:50 BCZT/Bi2O3 sample with and without addition of scavengers as a function of irradiation time. From Fig. 10a, it can be observed that 50:50 BCZT/Bi2O3 can degrade 92% of the dye after 60 min of visible light irradiation. When benzoquinone was added into the system, only 60% of the dye was degraded. Degradation of RhB was also suppressed (38%) when IPA was added into the system. When oxalic acid was added into the system, 30% of the RhB was degraded in 60 min of photocatalytic experiment. RhB degradation was highly quenched when oxalic acid was added. Based on the above observation, it may be possible that \( {\text{O}}_{2}^{ - } \), \( {\text{OH}}^{ *} \) and h+ could play an important role in photocatalytic degradation of RhB under visible light. Based on the above results, the probable mechanism for the degradation of RhB by 50:50 BCZT/Bi2O3 composite is shown in Fig. 10b. Finally, the reusability of the photocatalyst was also examined and showed ~ 5% decrease in efficiency for RhB degradation under visible light irradiation, after five cycles of reuse as shown in Fig. 10c.
Conclusions
BCZT/Bi2O3 composite was successfully prepared by solid-state method at 500 °C to form a composite without any intermediate phase. Absorption edge shifts toward visible region of light for composites samples. FESEM micrographs show uniform distribution of BCZT and Bi2O3 in the composite powder. BCZT/Bi2O3 composite sample shows better photocatalytic activity under both UV and visible light irradiation than that of the pure BCZT and Bi2O3. BCZT/Bi2O3 (50:50) composite sample can degrade 92% of the RhB solution in 60 min under visible light irradiation. However, commercial P25 can degrade 60% of the RhB solution in 60 min under similar irradiation condition. The pH value has an important influence on the adsorption and the photocatalytic degradation of RhB upon BCZT/Bi2O3 composite. A large adsorption and degradation of the dye is observed at relatively low pH values (pH < 5). The photocatalytic degradation of RhB under visible light irradiation in the presence of different scavengers confirms that hole (h+) and superoxide radicals (\( {\text{O}}_{2}^{ - } \)) were found to be the dominant active species for BCZT/Bi2O3 composite sample. In this system, the electrons present in the conduction band of Bi2O3 cannot reduce the adsorbed O2 into \( ^{*} {\text{O}}_{2}^{ - } \) and holes present in the valence band of BCZT cannot oxidize H2O and \( {\text{OH}}^{ - } \) into \( {\text{OH}}^{ *} \). So, in the present case photocatalytic degradation does not happen by a simple band-to-band transfer of electrons and holes. In BCZT/Bi2O3 composites, the Z-scheme principle of photocatalyst is confirmed owing to its stronger oxidation and reduction capability.
References
Gaya UI, Abdullah AH (2008) Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J Photochem Photobiol C Photochem Rev 9:1–12
Boppella R, Anjaneyulu K, Basak P, Manorama SV (2013) Facile synthesis of face oriented ZnO crystals: tunable polar facets and shape induced enhanced photocatalytic performance. J Phys Chem C 117:4597–4605
Shakir I, Shahid M, Kang DJ (2013) Highly functional SnO2 coated PZT core-shell heterostructures as a visible light photocatalyst for efficient water remediation. Chem Eng J 225:650–655
Wang H, Zhang L, Chen Z et al (2014) Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chem Soc Rev 43:5234–5244
Wu Q, Li D, Wu L et al (2006) Unprecedented application of lead zirconate titanate in degradation of Rhodamine B under visible light irradiation. J Mater Chem 16:1116–1117
Huang H, Li D, Lin Q et al (2009) Efficient photocatalytic activity of PZT/TiO2 heterojunction under visible light irradiation. J Phys Chem C 113:14264–14269
Cui Y, Briscoe J, Dunn S (2013) Effect of ferroelectricity on solar-light-driven photocatalytic activity of BaTiO3—Influence on the carrier separation and stern layer formation. Chem Mater 25:4215–4223
Xiong X, Li S, Tian R et al (2015) Formation and photocatalytic activity of BaTiO3 nanocubes via hydrothermal process. J Nanomater 2015:1–6
Kappadan S, Woldu T, Thomas S, Kalarikkal N (2016) Materials science in semiconductor processing tetragonal BaTiO3 nanoparticles: an efficient photocatalyst for the degradation of organic pollutants. Mater Sci Semicond Process 51:42–47
Kaya C, Kalem V, Teber S (2018) Photocatalytic activity and dielectric properties of hydrothermally derived tetragonal BaTiO3 nanoparticles using TiO2 nano fibers. J Alloys Compd 765:82–91
Fu Q, Wang X, Li C et al (2016) Enhanced photocatalytic activity on polarized ferroelectric KNbO3. RSC Adv 6:108883–108887
Tonda S, Santosh Kumar OA, Shanker V (2014) Synthesis of Cr and La-codoped SrTiO 3 nanoparticles for enhanced photocatalytic performance under sunlight irradiation. Phys Chem Chem Phys 16:23819–23828
Yang SY, Seidel J, Byrnes SJ et al (2010) Above-bandgap voltages from ferroelectric photovoltaic devices. Nat Nanotechnol 5:143–147
Morris MR, Pendlebury SR, Hong J et al (2016) Effect of internal electric fields on charge carrier dynamics in a ferroelectric material for solar energy conversion. Adv Mater 28:7123–7128
Li L, Salvador PA, Rohrer GS (2014) Photocatalysts with internal electric fields. Nanoscale 6:24–42
Zhao X, Liu H, Qu J (2011) Photoelectrocatalytic degradation of organic contaminants at Bi2O3/TiO2 nanotube array electrode. Appl Surf Sci 257:4621–4624
Lin X, Xing J, Wang W et al (2007) Photocatalytic activities of heterojunction semiconductors Bi2O3/BaTiO3: a strategy for the design of efficient combined photocatalysts. J Phys Chem C 111:18288–18293
Ye X, Zhao S, Meng S et al (2017) Remarkable enhancement of photocatalytic performance via constructing a novel Z-scheme KNbO3/Bi2O3 hybrid material. Mater Res Bull 94:352–360
Fan H, Li H, Liu B et al (2012) Photoinduced charge transfer properties and photocatalytic activity in Bi2O3/BaTiO3 composite photocatalyst. ACS Appl Mater Interfaces 4:4853–4857
Chu S, Kong F, Wu G et al (2011) Architecture of Cu2O@TiO2 core-shell heterojunction and photodegradation for 4-nitrophenol under simulated sunlight irradiation. Mater Chem Phys 129:1184–1188
Zhou W, Liu H, Wang J et al (2010) Ag2O/TiO2 nanobelts heterostructure with enhanced ultraviolet and visible photocatalytic activity. ACS Appl Mater Interfaces 2:2385–2392
Cui Y, Briscoe J, Wang Y et al (2017) Enhanced photocatalytic activity of heterostructured ferroelectric BaTiO3/α-Fe2O3 and the significance of interface morphology control. ACS Appl Mater Interfaces 9:24518–24526
Jiang HY, Cheng K, Lin J (2012) Crystalline metallic Au nanoparticle-loaded α-Bi2O3 microrods for improved photocatalysis. Phys Chem Chem Phys 14:12114–12121
Liu W, Ren X (2009) Large piezoelectric effect in Pb-free ceramics. Phys Rev Lett 103:1–4
Abhinay S, Mazumder R, Seal A, Sen A (2016) Tape casting and electrical characterization of 0.5Ba(Zr0.2Ti0.8)O3–0.5(Ba0.7Ca0.3)TiO3 (BZT–0.5BCT) piezoelectric substrate. J Eur Ceram Soc 36:3125–3137
Adhikari P, Mazumder R, Abhinay S (2016) Electrical and mechanical properties of MgO added 0.5Ba(Zr0.2Ti0.8)O3–0.5(Ba0.7Ca0.3)TiO3 (BZT–0.5BCT) composite ceramics. J Electroceram 37:127–136
Raja S, Ramesh Babu R, Ramamurthi K, Moorthy Babu S (2018) Room temperature ferromagnetic behavior, linear and nonlinear optical properties of KNbO3 microrods. Ceram Int 44:3297–3306
Andrade GRS, Nascimento CC, Silva Júnior EC et al (2017) ZnO/Au nanocatalysts for enhanced decolorization of an azo dye under solar, UV-A and dark conditions. J Alloys Compd 710:557–566
Wei K, Wang B, Hu J et al (2019) Photocatalytic properties of a new Z-scheme system BaTiO3/In2S3 with a core-shell structure. RSC Adv 9:11377–11384
Xu Q, Zhang L, Yu J et al (2018) Direct Z-scheme photocatalysts: principles, synthesis, and applications. Mater Today 21:1042–1063
You-Ji L, Wei C (2011) Photocatalytic degradation of Rhodamine B using nanocrystalline TiO2-zeolite surface composite catalysts: effects of photocatalytic condition on degradation efficiency. Catal Sci Technol 1:802–809
Tabit R, Amadine O, Essamlali Y et al (2018) Magnetic CoFe2O4 nanoparticles supported on graphene oxide (CoFe2O4/GO) with high catalytic activity for peroxymonosulfate activation and degradation of rhodamine B. RSC Adv 8:1351–1360
Natarajan TS, Thomas M, Natarajan K et al (2011) Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chem Eng J 169:126–134
Acknowledgements
We are thankful to the Director, NIT Rourkela, Odisha, India, for providing experimental facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abhinay, S., Tarai, P. & Mazumder, R. Preparation and characterization of (Ba0.85Ca0.15)(Zr0.1Ti0.9)TiO3(BCZT)/Bi2O3 composites as efficient visible-light-responsive photocatalysts. J Mater Sci 55, 1904–1914 (2020). https://doi.org/10.1007/s10853-019-04175-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04175-3