Abstract
Heterojunction photocatalytic materials have received extensive attention by the researchers. In this work, g-C3N4/Bi2O3/TiO2 (TCB) composites with g-C3N4 as the main body were synthesized via microwave-assisted ball milling and the heat treatment method. The photocatalytic activity was evaluated by the degradation efficiency of rhodamine B (RhB) and methylene blue (MB) under visible or UV light irradiation. TCB with a mass ratio of melamine/TiO2/Bi(NO)3·5H2O of 30:1:0.25 has the best photocatalytic performance when fabricated at 450 °C in open air, and the degradation efficiency of the as-prepared material to RhB was 48.07% under visible light and 51.18% to MB under UV. The results of morphological structure analysis revealed that there was a flocculent structure on the edge of the g-C3N4 film and the phase of TiO2 and Bi2O3 changed. The presence of β-Bi2O3 enhanced photocatalytic efficiency of the as-prepared materials. The optical properties analysis confirmed that the structure of the hybrid samples can promote electron–hole pairs separately at multilayer heterogeneous interfaces and the electron can react with O2 to yield the ·\({O}_{2}^{-}\) radical. In summary, the ability of novel spatial structure materials to absorb visible light is enhanced, which are facilitated for photocatalytic activity and have excellent stability. Therefore, the study of ternary heterojunction photocatalytic materials provides a reference for environmental remediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heterojunction photocatalytic materials which can be classified into Type-I [1], Type-II [2], and Z-scheme type [3] according to the electron–hole pairs transmission mechanism are used in the field of environmental remediation. It not only can directly adsorb sunlight to generate a large number of electron–hole pairs but also can yield active radicals for the oxidation of refractory organic pollutants [4,5,6]. TiO2 has been used as a typical photocatalytic material for a long time [7, 8]. In recent years, g-C3N4 formed by the melamine thermal polymerization reaction has outstanding properties and has become a research focus in the field of photocatalysis [9]. Conventional photocatalytic materials have certain limitations and then launched the study of binary heterojunction. Related research has proven that TiO2-based heterojunction photocatalytic materials prepared by the sol–gel method have a better degradation effect on pollutants, such as methyl green and methyl orange, under visible light [10, 11], and others methods to synthesize TiO2/g-C3N4 composites for degradation organic pollutants have been reported [12,13,14]. Despite the binary heterojunction photocatalytic materials based on TiO2 or g-C3N4 have exhibited potential capabilities in environmental remediation, the responses of TiO2/g-C3N4 composites to sunlight were not as desirable, because the experiments were usually tested under visible light. [15,16,17,18].
To further improve the photocatalytic activity of the materials, the modification methods of ternary or multiple doped composite systems have attracted wide attention. Tahir et al. through hydrothermal method synthesized a novel ternary hybrid photocatalyst WO3/TiO2/g-C3N4 which have excellent structure and extend surface area were beneficial to the visible light absorption capacity and photocatalytic activity. The removal efficiency of acetylsalicylate and methyltheobromine could reach 98% and 97%, respectively [19]. 3D structure g-C3N4/TiO2/kaolinite composite exhibited degradation ability for the removal of ciprofloxacin (CIP) under visible light irradiation [20], but the ternary heterojunction material absorption range of light is not wide enough. Zhang et al. prepared g-C3N4/Bi2O3/TiO2 nanotube photocatalytic material electrodes by anodization [21], and the degradation efficiency to MB reached 77.5% after 3 h of photoelectron catalysis, demonstrating the ternary heterojunction materials have development potential in photocatalyst. There are few research reports on g-C3N4/Bi2O3/TiO2 heterojunction photocatalytic materials. Bismuth oxide (Bi2O3) as bismuth-based catalysts is a new type of nano-semiconductor material which has been widely studied in the field of photocatalysis due to its narrow band gap (2.8 eV), high surface area, and exposed active crystal plane [22,23,24,25,26]. Simultaneously, the responses of Bi2O3 to UV and visible light are excellent [27].
Previous studies have demonstrated that g-C3N4/TiO2 (TCN) hybrid photocatalytic materials which can be decided to Z-scheme electron–hole transfer model showed improved photocatalytic efficiency in degrading simulated pollutants under visible light irradiation [28]. Based on previous study, we plan to dope a certain around of Bi(NO)3·5H2O to prepare a novel spatial structure heterojunction photocatalytic material g-C3N4/Bi2O3/TiO2 (TCB) via microwave-assisted ball milling and the heat treatment method. In this work, the composite materials of doping Bi2O3 have potential to enhance the UV and visible light absorption ability, improve separation, and transfer efficiency of photogenerated charges, and yield more active radicals to degrade organic pollutants. The efficiency of TCB to degrade RhB and MB under visible or UV light was studied. A series of characterization methods were used to detect the structural properties and optical properties of composites, and combined with free radical capture experiment to the photocatalytic mechanism was further explored.
2 Materials and methods
2.1 Reagent
Titanium dioxide (TiO2) was provided by XuanCheng JingRui New Material Co. Ltd. Other chemical reagents melamine, bismuth nitrate pentahydrate (Bi(NO)3·5H2O), Rhodamine B (RhB), Methylene blue (MB), Benzoquinone (BQ), Isopropanol (IPA), and Ammonium oxalate (AO) were obtained from Aladdin Chemistry Co. Ltd. All of the reagents were of analytical purity and used without further purification. Deionized water was produced in an Ultra-pure water system for laboratory use (WP-UP-1810).
2.2 Experimental methods
2.2.1 Preparation of g-C3N4 and Bi2O3
An appropriate amount of melamine was placed in a muffle furnace (SX2-2.5-10, Zhengzhou, China) under atmospheric air and heated to 500 °C at a rate of 5 °C min−1, then steadied at 500 °C for 2 h. The resulting yellow solid was ground into powder in an agate mortar after cooling down to room temperature. The final product was g-C3N4. Bi2O3 was prepared at the same process under 450 °C, which was collected for further use.
2.2.2 Synthesis of g-C3N4/Bi2O3/TiO2
The synthesis of TCB involved four steps: (1) An appropriate amount of Bi(NO)3·5H2O was mixed with TNC (mass ratio of melamine/TiO2 30: 1), and the outstanding photocatalytic material in previous research was obtained [28]. The mixture was added into an agate ball milling tank (PM2L, Nanjing, China), with a ball:material ratio of 10:1, and deionized water was used as the dispersant. A uniform heterogeneous interface was obtained in the ball milling tank after 2–3 h at a speed of 400 rpm. (2) The suspension was heated in a microwave oven at 700 W for 20–30 min after milling to prepare the crystal structure and heterojunction material. (3) The faint yellow material was further milled in an agate mortar, and after the molecular structure reached thermal equilibrium it was placed in room temperature for 2–3 h. (4) The sample was placed in a muffle furnace under atmospheric air and heated up to 450 °C at a rate of 5 °C min−1, then steadied for 2 h. The as-synthesized material was obtained after regrinding in an agate mortar. The different hybrid composites with mass ratios of melamine/TiO2/Bi(NO)3·5H2O at 30:1:0.1, 30:1:0.25, 30:1:0.5, 30:1:1, 30:1:5, 30:1:10, 30:1:20, and 30:1:30 were labeled TCB-1, TCB-2, TCB-3, TCB-4, TCB-5, TCB-6, TCB-7, and TCB-8, respectively.
2.3 Characterizations
An X-ray diffractometer (XRD) (Empyrean, Shimadzu, Japan) with Cu-K radiation (λ = 0.15405 nm, 60 kV, 60 mA) was used to determine the sample crystal structure. X-ray photoelectron spectroscopy (XPS) was performed using Escalab 250Xi system (Thermo Fisher, America) analysis to determine the element composition of the sample. The sample surface morphology structure and element content were detected by a JSM-7500F (JEOL, Japan) scanning electron microscope (SEM) and energy dispersive spectrometer (EDS), respectively. The internal microstructure of the sample was observed by transmission electron microscopy (TEM). The optical performance was detected by UV–Vis diffuse reflectance spectra (DRS) equipped with a UV-3600 (Shimadzu, Japan) spectrophotometer. Photoluminescence (PL) emission spectra were detected on a Fluorescence Spectrometer (F-7000, Hitachi, Japan), and the excitation wavelength was 230–900 nm. The photoreactor is shown in Fig. 1
2.4 Photocatalytic performance tests
The photocatalytic performance of the as-obtained samples was determined by the degradation of RhB and MB, with an initial concentration of 20.0 mg L−1, under both UV and visible light irradiation. Reaction systems consisted of 50 mg catalysts (TCB, TiO2, g-C3N4, Bi2O3) and 50.0 mL of simulated dye wastewater in 100 mL beakers. The mixtures were dispersed by ultrasound for 5 min and stirred in a magnetic stirrer for 30 min with darkroom conditions. The first sample was taken when the adsorption–desorption reached dynamic equilibrium. Afterwards, a 5 mL sample was centrifuged at 10,000 rpm for 20 min after reacting in UV or visible light irradiation for 60 min each. The supernatant solution was analyzed by Alpha-1506 UV–vis spectroscopy (Shanghai Lab-Spectrum Instruments Co. Ltd., China) in which the visible light was 60 W 460 nm LED tube and UV was 60 W 254 nm tube at wavelengths of 554 nm and 664 nm for RhB and MB, respectively, and the average light intensity is 50 mW/cm2.
The degradation efficiency of simulated pollutants can be calculated using Eq. (1):
In the equation, \(\eta\) is the removal rate of the simulated pollutants; \({C}_{0}\) is the initial simulated pollutant concentration; and \({C}_{t}\) is the concentration of simulated pollutants after reacting \(t\) min.
In this experiment, the scavengers BQ, IPA, and AO were employed to capture superoxide anion radical (·\({O}_{2}^{-}\)), hydroxyl radicals (·\(OH\)), and photogenerated hole (\({h}^{+}\)) [29]. 50.0 mg TCB-2 and 50 mL RhB were added into 4 100 mL dry and transparent beakers and labeled 1–4, respectively. 2–4 sign beakers were added 1 mL (0.1 mM) scavenger of BQ, IPA, and AP, respectively [24, 30].
3 Results and discussion
3.1 XRD pattern
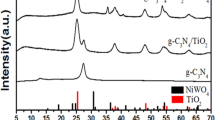
Figure 2 shows that Bi2O3 was the product of Bi(NO)3·5H2O direct thermal decomposition through comparing the XRD patterns of Bi2O3 with the PDF#71-0465 standard pattern. It was further found that the XRD peaks of g-C3N4 were in TCB-2 with no obvious XRD diffraction peaks of anatase phase TiO2. On the contrary, the rutile phase TiO2 can be observed when referred to the PDF#21–1272 standard pattern. It demonstrates that the anatase phase TiO2 is transformed into the rutile phase in the sample TCB-2. In addition, Bi2O3 has four crystallographic polymorphs with monoclinic-α, body-centered cubic-γ, tetragonal-β, and face-centered cubic-δ [31]. The α-Bi2O3, originally generated from Bi(NO)3·5H2O, is converted into β-Bi2O3 because the peaks belonging to β-Bi2O3 can be observed in TCB-2 [32]. The formation temperature of β-Bi2O3 and the rutile phase TiO2 are higher than α-Bi2O3 and the anatase phase TiO2[33], implying that the change of the crystal phase of TiO2 and Bi2O3 may be caused by exothermic reaction [34, 35]. On the other hand, the deamination and dehydrogenation of melamine impacted on the chemical bond of TiO2 and Bi2O3 and induced the change of the crystal phase. From the above comparative analysis, we can identify the sample of TCB-2 as containing the rutile phase TiO2, g-C3N4, and β-Bi2O3. In addition, the synthesis materials have good crystallinity due to the sharp shaped peaks observed.
3.2 XPS analysis
The XPS spectra were used to further probe the chemical compositions and chemical bonds in TCB-2. Figure 3 shows the XPS peak spectrum of five elements: carbon (C), nitrogen (N), titanium (Ti), bismuth (Bi), and oxygen (O) in TCB-2. Two peaks at 288.6 eV and 284.75 eV corresponding to sp2-bonded carbon (N–C=N) and C1s [36] are observed in Fig. 3a, respectively. As displayed in Fig. 3b, the peaks at 398.1 eV and 401.0 eV are ascribed to sp2-hybridized nitrogen (C–N=C) and N hydrogen bond (N–H) [37, 38], respectively. The XPS characteristic peaks of C and N elements indicate the presence of g-C3N in TCB-2. This is consistent with the XRD results. Meanwhile, the peaks attributed to Ti 2p3/2, Ti 2p1/2, Bi4d3, Bi4f7/2, and Bi4f5/2, respectively, can be determined from Fig. 3c and d [39, 40], respectively, suggesting the presence of TiO2 and Bi2O3 in TCB-2. Moreover, the peaks of Bi-O (528.3 eV), Ti–O (529.9 eV), and O–H (531.9 eV) in Fig. 3e [41], further confirm the existence of TiO2 and Bi2O3. In the whole process, no evidence was found of chemical bonding between TiO2, g-C3N4, and Bi2O3, indicating the synthesis of TCB-2 was not by chemical means but by van der Waals forces or electrostatic adsorption.
3.3 SEM and TEM analysis
The nanoparticles of TiO2 (Fig. 4a) and Bi2O3 (Fig. 4b) were densely and uniformly attached to the film formed by g-C3N4, and there was a flocculent structure on the edge of the g-C3N4 film, the results may have been caused by exothermic reaction releasing heat that promoted polymerization between substances when adding Bi(NO)3·5H2O in preparing hybrids during sintering of Bi2O3 which increased the actual temperature. Thus, the film edge of g-C3N4 produced by melamine changed into flocculent form. The as-prepared materials have formed the micro-nano structure, the size distribution was 0.4–3.0 μm. During the reaction, anatase phase TiO2 in the raw material was gradually converted into the rutile phase TiO2 which uniformly adhered to the g-C3N4 film together with the Bi2O3 formed by the decomposition of Bi(NO)3·5H2O. Consequently, the TCB composite heterojunction materials with g-C3N4 as the main body were formed. The EDS chart (Fig. 4c) revealed that the atomic ratio of C and N was close to 3: 4, demonstrating the g-C3N4 was existence in TCB-2. The nanoparticles of TiO2 and Bi2O3 were uniformly attached to the film of g-C3N4, as can be observed from the multilayer structure of TCB-2 in Fig. 4d, 3e, 3f. Furthermore, comparing to the PDF#27-0050 standard pattern, it can be seen in Fig. 4e, 3f that two different lattice fringes of 0.248 nm and 0.346 nm correspond to the [101] crystal plane of rutile TiO2[42] and [210] crystal plane of Bi2O3, respectively. In summary, this was in accordance with the analysis conclusions of XRD and XPS, further accounting for the rutile TiO2 and Bi2O3 nanoparticles uniformly attached to the surface of g-C3N4.
3.4 UV–vis diffuse reflection spectra
Figure 5 shows the UV–Vis diffuse reflectance spectra of TiO2, g-C3N4, Bi2O3, and TCB-2 at 200 to 800 nm and their corresponding Tauc plots. The light absorption range of TCB-2 was red-shifted relative to TiO2, Bi2O3, and g-C3N4 (Fig. 5a) and exhibited a certain absorption in the visible light range at 200 to 500 nm. Equation (2) can be used to calculate the band gap energy of the photocatalysis [43]:
In this equation, α, h, v, A, and Eg are absorption coefficient, Planck constant, light frequency, proportionality, and band gap energy, respectively; n represents the properties of the transition in a semiconductor (n = 1 for direct transition, and n = 4 for indirect transition). The α-Bi2O3 was an indirect transition, so the value of n was 4 [10]. From the tangents in Fig. 5b, Eg of Bi2O3 and TCB-2 were 2.80 eV and 2.83 eV, respectively. The Eg of Bi2O3 was similar to that of Cui et al. [41].
3.5 Photocatalytic activity
The results of the adsorption experiments performed in the dark are seen in Fig. 6. The adsorption capability of composites TCB to MB was better than RhB. TCB-7 had the best adsorption efficiency to MB reaching 8.82%, and the adsorption effect of samples for RhB was less than 2.5%. The adsorption capability of TCB was lower [28], demonstrating that the adding Bi2O3 affects adsorption performance, and it is possible that TCB also has certain selective adsorption.
Figure 7 shows the degradation of RhB and MB for the 8 different ratios of TCB hybrid samples under visible light and UV irradiation after 240 min. TCB-2 has a better visible light absorption capacity compared with traditional photocatalytic material. In the series of composites, TCB-2 has the best photocatalytic effect in degrading RhB whether in visible light or UV irradiation. For visible light irradiation, the degradation efficiency of TCB-2 to RhB was only 48.07%, and it is lower than the efficiency of g-C3N4 (99.36%). The degradation efficiency of TCB-2 to MB (40.10%) was lower than that of TiO2 (63.31%) and g-C3N4 (89.78%). Under UV light irradiation, the degradation effect of TCB-7 on RhB was the best (68.66%), which was higher than that of TiO2 (63.64%), and the degradation effect of TCB-2 on MB (51.18%) was not as good as TiO2 (91.39%). The above analysis clearly revealed that (1) the degradation effect of TCB under UV light was better than that of visible light; (2) the photocatalytic effect of TCB on RhB was slightly better than that of MB; (3) considering the irradiation, adsorption, and degradation three aspects, the comprehensive performance of TCB-2 was the best. Although the degradation effect of TCB-2 to 2 simulated pollutants were worse than the homogenous materials of TiO2 and g-C3N4. On the one hand, it may have been caused by the fact that β-Bi2O3 has the poor stability of thermodynamics in heterojunction interface [44], and the materials structure and photocatalytic system of TCB have been affected. On the other hand, it may also have been due to the selectivity and poor adsorption of TCB to pollutants resulting in a lower degradation efficiency. The DRS and other characterization analyses of TCB-2 showed that the hybrid material had a good response to visible light due to β-Bi2O3 having an excellent absorption to visible light, lower band gap, and higher energy compared with α-Bi2O3 [45, 46]. Therefore, this was further studied.
In order to investigate the reuse efficiency of hybrid materials, 50 mg TCB-2 samples were used to degrade simulated dye wastewater RhB and MB at a concentration of 20 mg L−1, and the number of times the photocatalytic material was recycled was investigated under visible light irradiation (Fig. 8). After 5 times of reuse, the degradation of RhB and MB by TCB-2 decreased from 51.91% and 40.63% to 45.46% and 36.04%, respectively. In general, the samples had a certain of photocatalytic stability.
3.6 Photocatalytic reaction mechanism
As we all know, the photoluminescence (PL) spectra had related to further reflect the electron–hole pairs separation and transfer efficiency of semiconductors in the photocatalytic reactions [6, 47]. As Fig. 9 shows, the PL intensity of the pure TiO2 was in the wavelength range between 360 and 540 nm, and has a strong emission peak at 426 nm. Obviously, there was a weaker PL peak than g-C3N4/TiO2 for TCB-2, indicating that the electron–hole pairs recombination is slower than g-C3N4/TiO2. It is not difficult to understand that the ternary heterojunction TCB-2 exhibits an enhanced photocatalytic performance which can efficiently separate the photogenerated carries.
In order to prove the influence of active radicals in the system of TCB-2 degrading RhB under visible light irradiation, we added three scavengers BQ, AO, and IPA to study the free radical capture experiment. The removal efficiency at 240 min can reach 51.8%, after adding 1 mL BQ, IPA, and AO, the RhB removal efficiency promptly decreased to 5.3%, 14.3%, and 20.1%, respectively. As Fig. 10 shows, the material’s photocatalytic efficiency was reduced and the degree of influence was BQ > IPA > AO, suggesting that ·\({O}_{2}^{-}\), ·\(OH\) and \({h}^{+}\) were generated in the system, and that ·\({O}_{2}^{-}\) is the main active radical in RhB degradation process.
According to four conditions: (a) the analysis of DRS, (b) Eq. (3): \({E}_{\text{VB}}=\chi -{E}_{\text{c}}+0.5{E}_{\text{g}}\), (c) Eq. (4): \({E}_{\text{CB}}={E}_{\text{VB}}-{E}_{\text{g}}\), and (d) the \(\chi\) values for g-C3N4, TiO2, and Bi2O3 were 4.73 eV, 5.81 eV, and 6.23 eV [6, 43, 47, 48]. We can calculate the conduction band and valence band of g-C3N4, TiO2, and Bi2O3, respectively, as shown in Table 1.
Based on the above results, we explored the composites g-C3N4/Bi2O3/TiO2 photocatalytic degradation reaction mechanism for RhB and MB in visible light or UV irradiation. For visible light irradiation (Fig. 11a), in the hybrid mainly composed of g-C3N4, the electrons in the VB of g-C3N4 and Bi2O3 transferred to the corresponding CB, respectively. On account of the CB of g-C3N4 has being more negative than that of Bi2O3 and TiO2. The electrons in the CB of g-C3N4 can cross the multilayer heterointerface and transfer to the CB of Bi2O3 and TiO2, then reacting with O2 to yield ·\({O}_{2}^{-}\) radical (− 0.33 eV) degrade RhB due to the ECB of g-C3N4 was estimated to be − 1.12 eV. ·\({O}_{2}^{-}\) radicals can further react with electrons to yield H2O2 and ·\(OH\). The VB of Bi2O3 (3.13) was more positive than that of g-C3N4 (1.58), so the holes can migrate to Bi2O3 owing to the different VB edge potentials, and participate in photocatalytic reactions to directly or indirectly degrade RhB or MB. TiO2 played an important role in electrons transfer in this system, though it cannot be induced by visible light. The entire photocatalytic reaction system realized the effective separation of the electron–hole pair to promote the photocatalytic ability. With UV irradiation (Fig. 11b), similarly, g-C3N4, Bi2O3 and TiO2 can be excited, respectively. Consequently, according to different band gap energy shown in Table 1, the separation of electron–hole pairs transferred better, but g-C3N4 as the major part of hybrid had a weak response to UV and only a certain improvement for photocatalytic efficiency was obtained.
4 Conclusions
The multilayered heterojunction photocatalytic material g-C3N4/Bi2O3/TiO2 was fabricated via microwave-assisted ball milling and the heat treatment method. This novel spatial structure facilitated electron transfer in the photocatalytic materials and the absorption ability in wide visible light range, and the entire photocatalytic system has achieved effectively separation of electron–hole pairs. It should be noted that in this study whether under visible or UV light to degrade RhB or MB, the photocatalytic effect of TCB is not satisfactory. This is probably a consequence of the photocatalytic selectivity of TCB and the change of the material crystal phase. The adsorption performance of composites to pollutants also affects the photocatalytic degradation ability. Notwithstanding its limitation, this study does suggest (1) The as-prepared material TCB boosts the separation and transfer of photogenerated charges at multilayer heterogeneous interfaces; (2) Scavenging experiments demonstrate that ·\({O}_{2}^{-}\) radicals played a major role in photocatalytic degradation process; (3) The composites still have outstanding stability after using 5 times to degrade organic pollutants; (4) The red shift of the light absorption range of TCB indicates that the ability of hybrid materials to absorb visible light is enhanced facilitating the degradation of RhB or MB. This paper focuses on TCB photocatalytic performance for organic pollutants under visible light or UV irradiation, and provides relevant information for preparing novel ternary heterojunction photocatalytic materials.
References
J. Yun, Y. Zhang, Y. Ren, P. Kang, J. Yan, W. Zhao, Z. Zhang, H. Guo, Sol. Energy Mater. Sol. Cells 210, 110516 (2020)
K.T. Wong, S.C. Kim, K. Yun, C.E. Choong, I.W. Nah, B.H. Jeon, Y. Yoon, M. Jang, Appl. Catal. B Environ. 273, 119034 (2020)
M. Jourshabani, B.K. Lee, Z. Shariatinia, Appl. Catal. B Environ. 276, 119157 (2020)
J. Su, L. Zhu, P. Geng, G. Chen, J. Hazard. Mater. 316, 159 (2016)
N. Guo, Y. Zeng, H. Li, X. Xu, H. Yu, X. Han, J. Hazard. Mater. 353, 80 (2018)
R. Hao, G. Wang, H. Tang, L. Sun, C. Xu, D. Han, Appl. Catal. B Environ. 187, 47 (2016)
A.L. Linsebigler, G. Lu, J.T. Yates, Chem. Rev. 95, 735 (1995)
S. Lakshmi, R. Renganathan, S. Fujita, J. Photochem. Photobiol. A Chem. 88, 163 (1995)
B. Jürgens, E. Irran, J. Senker, P. Kroll, H. Müller, W. Schnick, J. Am. Chem. Soc. 125, 10288 (2003)
D.A. Solís-Casados, L. Escobar-Alarcón, A. Arrieta-Castañeda, E. Haro-Poniatowski, Mater. Chem. Phys. 172, 11 (2016)
Y. Liu, F. Xin, F. Wang, S. Luo, X. Yin, J. Alloys Compd. 498, 179 (2010)
K. Hu, R. Li, C. Ye, A. Wang, W. Wei, D. Hu, R. Qiu, and K. Yan, J. Clean. Prod. 253, (2020).
Y. Sheng, Z. Wei, H. Miao, W. Yao, H. Li, Y. Zhu, Chem. Eng. J. 370, 287 (2019)
X. Chen, J. Wei, R. Hou, Y. Liang, Z. Xie, Y. Zhu, X. Zhang, H. Wang, Appl. Catal. B Environ. 188, 342 (2016)
F. Bairamis, I. Konstantinou, D. Petrakis, and T. Vaimakis, Catalysts (2019).
P. Mei, H. Wang, H. Guo, N. Zhang, S. Ji, Y. Ma, J. Xu, Y. Li, H. Alsulami, M.S. Alhodaly, T. Hayat, Y. Sun, Environ. Res. 182, 109090 (2020)
R. Zhang, Y. Yu, H. Wang, J. Du, Sci. Total Environ. 724, 138280 (2020)
R. Acharya, K. Parida, J. Environ. Chem. Eng. 8, 103896 (2020)
M.B. Tahir, M. Sagir, K. Shahzad, J. Hazard. Mater. 363, 205 (2019)
C. Li, Z. Sun, W. Zhang, C. Yu, S. Zheng, Appl. Catal. B Environ. 220, 272 (2018)
Y. Zhang, J. Lu, M.R. Hoffmann, Q. Wang, Y. Cong, Q. Wang, H. Jin, RSC Adv. 5, 48983 (2015)
L. Li, B. Yan, J. Alloys Compd. 476, 624 (2009)
S. Anandan, G.J. Lee, P.K. Chen, C. Fan, J.J. Wu, Ind. Eng. Chem. Res. 49, 9729 (2010)
W. Li, D. Li, Y. Lin, P. Wang, W. Chen, X. Fu, Y. Shao, J. Phys. Chem. C 116, 3552 (2012)
C. Pan, Y. Yan, H. Li, S. Hu, Adv. Mater. Res. 557–559, 615 (2012)
G. Ren, X. Ren, W. Ju, Y. Jiang, M. Han, Z. Dong, X. Yang, K. Dou, B. Xue, F. Li, J. Photochem. Photobiol. A Chem. 392, 112367 (2020)
J. Hu, H. Li, C. Huang, M. Liu, X. Qiu, Appl. Catal. B Environ. 142–143, 598 (2013)
Y. Wang, J. Yu, W. Peng, J. Tian, C. Yang, Sci. Rep. 9, 1 (2019)
S.H. Hsieh, A. Manivel, G.J. Lee, J.J. Wu, Mater. Res. Bull. 48, 4174 (2013)
X. Yao, X. Liu, J. Hazard. Mater. 280, 260 (2014)
Q. Hao, R. Wang, H. Lu, C. Xie, W. Ao, D. Chen, C. Ma, W. Yao, Y. Zhu, Appl. Catal. B Environ. 219, 63 (2017)
H. Cheng, B. Huang, J. Lu, Z. Wang, B. Xu, X. Qin, X. Zhang, Y. Dai, Phys. Chem. Chem. Phys. 12, 15468 (2010)
P. Kumar, P. Kar, A.P. Manuel, S. Zeng, U.K. Thakur, K.M. Alam, Y. Zhang, R. Kisslinger, K. Cui, G.M. Bernard, V.K. Michaelis, Adv (Opt, Mater, 2020).
J. Matos, R. Montaña, E. Rivero, A. Escudero, D. Uzcategui, Water Sci. Technol. 69, 2184 (2014)
W. Xiaohong, Q. Wei, H. Weidong, J. Mol. Catal. A Chem. 261, 167 (2007)
X. Li, G. Hartley, A.J. Ward, P.A. Young, A.F. Masters, T. Maschmeyer, J. Phys. Chem. C 119, 14938 (2015)
P.Y. Kuang, Y.Z. Su, G.F. Chen, Z. Luo, S.Y. Xing, N. Li, Z.Q. Liu, Appl. Surf. Sci. 358, 296 (2015)
X. Dong, Z. Sun, X. Zhang, C. Li, S. Zheng, J. Taiwan Inst. Chem. Eng. 84, 203 (2018)
P. Zhang, C. Shao, M. Zhang, Z. Guo, J. Mu, Z. Zhang, X. Zhang, Y. Liu, J. Hazard. Mater. 217–218, 422 (2012)
K. Su, Z. Ai, L. Zhang, J. Phys. Chem. C 116, 17118 (2012)
Y. Cui, H. Zhang, R. Guo, Q. Ma, X. Deng, X. Cheng, X. Li, M. Xie, Q. Cheng, C. Zou, Electrochim. Acta 246, 1075 (2017)
Y. Gun, G.Y. Song, V.H.V. Quy, J. Heo, H. Lee, K.S. Ahn, S.H. Kang, A.C.S. Appl, Mater. Interfaces 7, 20292 (2015)
J. Zhang, Y. Hu, X. Jiang, S. Chen, S. Meng, X. Fu, J. Hazard. Mater. 280, 713 (2014)
T. Chen, Q. Hao, W. Yang, C. Xie, D. Chen, C. Ma, W. Yao, Y. Zhu, Appl. Catal. B Environ. 237, 442 (2018)
C. Wang, C. Shao, L. Wang, L. Zhang, X. Li, Y. Liu, J. Colloid Interface Sci. 333, 242 (2009)
Y. Wang, Y. Wen, H. Ding, Y. Shan, J. Mater. Sci. 45, 1385 (2010)
S. Chen, Y. Hu, S. Meng, X. Fu, Appl. Catal. B Environ. 150–151, 564 (2014)
W.K. Jo, T.S. Natarajan, Chem. Eng. J. 281, 549 (2015)
Acknowledgements
The work was supported by National Key Research and Development Program (No. 2018YFC1802605), Sichuan Provincial Major Science and Technology Project (No. 19ZDZX011), Nature Science Foundation of Sichuan Province (No. 2017SZ0181), International Cooperation Project of Sichuan Province (No. 2019YFH1027), Sichuan University-Yibin City school and City Strategic Cooperation Project (N0.2019 CDYB-26).
Author information
Authors and Affiliations
Contributions
CZ: Wrote the main manuscript text. WW: Performed the experiments. HH: Performed the experiments. ZH: Analyzed the data. YW: Analyzed the data. JY: Review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhong, C., Wei, W., He, H. et al. The photocatalytic performance of ternary g-C3N4/Bi2O3/TiO2 heterojunction composite for degradation of organic pollutants under visible and ultraviolet light. J Mater Sci: Mater Electron 32, 2146–2157 (2021). https://doi.org/10.1007/s10854-020-04980-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04980-6