Abstract
Steroid hormones play a crucial role in the body by acting as chemical messengers. They are, however, poorly soluble in water, and cyclodextrins can increase their solubility thus leading to increased bioavailability when used in drug formulations. Accuracy in the prediction of the free energy of binding of cyclodextrin/steroid inclusion complexes with simulation is important because of the potential value it brings by providing low-cost predictions of the real-life behavior of the cyclodextrin/steroid inclusion complex and the potential for high-through-put screening. Many computational methods exist, and it is therefore important to understand the ability of current theoretical models to accurately predict the free energy of binding for these inclusion complexes. We focused specifically on the estimation of the free energy of binding of inclusion complexes of four steroids: Hydrocortisone, dexamethasone, prednisolone, and 6α-methylprednisolone with native α-CD, β-CD, γ-CD, (2-hydroxy)propyl-β-CD, and sulfobutylether-β-CD by phase solubility as well as with α, β, and γ-CD by simulations. The simulations were assessed with both docking and the molecular mechanics combined with the generalized Born and surface area (MM/GBSA) continuum solvation approach. Considering the phase solubility diagram, (2-hydroxy)propyl-β-CD and sulfobutylether-β-CD dissolved more steroids in the higher concentration range as expected. The assessment of the free energy of binding obtained from the phase solubility and theory showed that the MM/GBSA method has shown promise in reliably generating accurate predictions in the field of calculating the free energy of binding of steroids/cyclodextrins with a correlation coefficient (R2) = 0.94.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Computational modeling has emerged as a powerful and cost-effective strategy for predicting properties of guest–host inclusion complexes [1] such as structures and the free energy of binding. Although computational techniques have the potential to reduce laboratory costs by predicting experimental outcomes, careful and critical evaluation of such models must be carried out. Accordingly, finding better and more reliable experimental sources of data as well as setting more realistic expectations for computational modeling should increase confidence in such predictions and ultimately accelerate research.
One important guest–host system is the steroid hormone-cyclodextrin system, which have found important applications due to the ability of cyclodextrin (CD) to change the apparent behavior of the included guest molecule. Steroid hormones are a group of organic compounds that act as chemical messengers and play an essential role in the body [2, 3]. Corticosteroids are a group of steroid hormones containing four cycloalkane rings that are linked together [4]. Hydrocortisone, dexamethasone, prednisolone, and 6α-methylprednisolone belongs to the glucocorticoid group of corticosteroids (Fig. 1). They are very important for protecting against stress, shock, and inflammation and these recent years they have also been used for treatment of COVID-19 patients [5, 6].
Steroids are poorly soluble in water which limit their use as efficient drugs. CDs are relatively small cyclic molecules, which can participate in the host (\(\text{H}\))-guest (\(\text{G}\)) interactions to form 1:1 complex (\(\text{HG}\)) in water (Eq. 1).
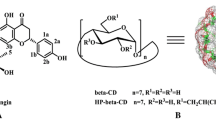
CDs are typically used to bring more drug, including steroid hormones, into solutions or increase the dissolution rate of solid formulations leading to increased bioavailability [7]. They are obtained from starch and are considered for a large number of administration routes [8]. CDs have been shown to have good chemical stability and are in general non-toxic to humans in food and medicines [9, 10]. α-, β- and γ-CD consist of 6, 7, or 8 glucopyranose units, respectively, connected through α(1 → 4)-D-glucosidic [11] as shown in Fig. 2. The truncated cone-like structure of CDs presents a hydrophilic exterior formed by hydroxyl groups while the interior is relatively hydrophobic due to the structure of the pyranose rings. This allows the CDs to act as host molecules for smaller guests or parts of larger molecules.
CDs can be chemically modified by substitution of some (or all) of the hydroxyl groups with various side chains as shown in Fig. 2. Known derivatives that are already used for drug delivery in approved and marketed products are hydroxypropyl CDs (HP-CD) and sulfobutylether CDs (SBE-CD) where the β-CD versions of the two derivatives ((2-hydroxy)propyl-β-CD (HP-β-CD) and sulfobutylether β-cyclodextrin (SBE-β-CD)) are leading in the market [7].
Studies of the equilibrium (Eq. 1) are important for understanding the effects CDs exert on different drugs. Various experimental methods can be used for this purpose where the most common include phase solubility [12,13,14,15] or titration based methods relying on UV–Vis absorption, fluorescence [16], or NMR [14, 16]. In general, a straightforward model assuming a simple stoichiometry (e.g. 1:1) is fitted to the obtained data to predict key parameters of the equilibrium, including the stability constant (\(K\)) and the free energies of binding (\(\Delta G)\). In some studies, theoretical calculations from molecular dynamic (MD) or molecular mechanics (MM) are included to back up the experimental data, i.e., investigate structure and dynamics of the inclusion complex and validate the free energies of binding [17,18,19].
The explosive increase of data-based techniques raises an important question: to what extend are current theoretical models able to reproduce experimental the free energies of binding? We will in this study address this question by computing the free energy of binding by a range of computational modeling methods and comparing them to values determined experimentally (by phase solubility method) using steroid-CD equilibria as models.
Methods
Materials
α-, β- and γ-CD (pharmaceutical grade) were a gift from Wacker Chemie AG (Burghausen, Germany). Hydrocortisone 98.0%, dexamethasone 98.0%, prednisolone 99% and 6α-methylprednisolone 98.0% were purchased from TCI, Combi-Blocks, Sigma-Aldrich and TCI, respectively. (2-hydroxy)propyl-β-CD (KLEPTOSE® HPB Oral grade) DS 4.38 (average MW 1389.37 Da) was a gift from Roquette (Lestrem, France). SBE-β-CD (Captisol) DS 6.3 (average MW 2131.46 Da) was kindly donated by Ligand Pharmaceutical (San Diego, United States), Milli-Q water (Millipore, Billerica, MA, USA) was used to prepare CD solutions and high-performance liquid chromatography (HPLC) mobile phases. Acetonitrile used to prepare the HPLC mobile phases was purchased from Sigma-Aldrich.
Phase-solubility studies
Phase-solubility studies were performed in accordance with the Higuchi and Connors method [20]. All CDs were dried under vacuum at 110 °C prior to use. An excessive amount of hydrocortisone, dexamethasone, prednisolone, and 6α-methylprednisolone [approximately 5–10 times the amount of CDs (in units of mol)] were added to aqueous solutions containing various concentrations of CDs (0–0.009 M for β-CD, 0–0.1 M for α-, γ-, HP-β-CD and SBE-β-CD). The suspensions were stirred for 24 and 48 h to reach equilibrium at 23 °C. The mixture was centrifuged [Sigma model (1-15PK) with 12,124 rotor, Taufkirchen, Germany] at 14.000 rpm for 20 min at room temperature to separate the soluble and insoluble fractions. The resulting supernatant was collected with syringe and needle and was filtered with a 0.45 µm Phenex™-RC membrane 15mm Syringe Filters (Phenomenex, Aschaffenburg, Germany) before HPLC analysis. 1000 µL of the filtered solution was diluted with 250 µL of the same starting solution without steroid to prevent precipitation.
The amount of steroids in solution was quantified using HPLC. A Dionex (Germering, Germany) setup equipped with P 680A LPG-4 HPLC Pump and ASI-100 Automated Sample Injector with Dionex UVD 170U UV/VIS Detector and Kinetex C18 column (150 × 4.6 mm, 5 μm; Alltech, Værløse, Denmark). The isocratic mobile phase was acetonitrile/water (10/90, 20/80, 10/90 and 10/90 for hydrocortisone, dexamethasone, prednisolone, 6α-methylprednisolone, respectively) with a flow rate of 1.0 ml/min. The eluent was monitored at 210 nm for hydrocortisone and 245 nm for the other steroids. The data were recorded and treated using Chromeleon™ 6.8 SR15 program from Thermo Scientific (Göteborg, Sweden).
As you can see in Fig. 3, Linear external calibration curves in the concentration range from 0.01 to 0.20 mg/mL for hydrocortisone, 0.01 to 0.10 mg/mL for dexamethasone and 0.01 to 0.07 mg/mL for both prednisolone and 6α-methylprednisolone were used.
The resulting phase-solubility profiles are typically divided into two major groups called A- and B-type. A-type profiles describes systems where the apparent solubility of the guest/drug molecule increases as the concentration of cyclodextrin is increased. This can happen linearly (AL) or non-linearly (AP or AN). B-type profiles, however, describe systems where solubility is not necessarily increasing for all added concentrations for cyclodextrin (BS) or even produce insoluble complexes (BL) [7].
The apparent stability constant (\({K}_{1:1}\)) was in all experiments determined from the linear part of the phase-solubility diagrams using the linear model described in Eq. 2: [20, 21]
Here, the slope is obtained from the linear relationship between the concentration of steroids and the concentration of CDs, and the intrinsic solubility \({S}_{0}\) of the steroid was assessed in pure water in the absence of CD [22]. All phase-solubility results are reported as means with a standard error based on four experiments \(\left(n=4\right)\). The change in the free energies of binding (\({\Delta G}_{bind, exp}\)) of the steroid/CD equilibria obtained from the phase solubility experiments was derived from Eq. 3:
Here, \(R\) is the ideal gas constant (8.314 J K−1 mol−1) and \(T\) is the temperature used in the experiments (296 K).
Molecular simulations
Preparation of molecular structures
The starting structures of native α-, β-, and γ-CD were generated with the tleap program in the AMBER 16 software [23]. Structures of hydrocortisone, dexamethasone, prednisolone and 6α-methylprednisolone (Fig. 1) were prepared with the 2D sketcher in Maestro 12.7 [24] in Schrödinger 2021-3 software [24] and minimized without solvent using the OPLS4 force field [25].
Molecular docking
Molecular docking simulations were performed with Glide [26,27,28] to obtain \(\Delta {G}_{bind}\) for the inclusion complexes of hydrocortisone, dexamethasone, prednisolone, and 6α-methylprednisolone with α-, β- and γ-CD, respectively. Stable conformations of each steroid were found using a conformational search with enhanced torsional sampling enabled [29]. The docking simulations were performed by choosing the centroid of the CDs as the center for the docking grid. Both standard precision (SP) and extra precision (XP) [28] docking simulations were carried out. Mean and standard error of the simulated the free energy of binding were obtained from 5 independent docking simulations assuming 1:1 interaction to assess the free energy of binding for each steroid/CDs.
Molecular mechanics with generalized born and surface area method
We also employed the molecular mechanics energies in combination with the Generalized Born (GB) and Surface Area (SA) method (MM/GBSA) [30, 31] on inclusion complexes to obtain the free energies of binding of the four ligands and the three native CDs. This was done with the AMBER 16 software using the one-trajectory approach where a single molecular dynamics (MD) simulation is carried out on the inclusion complex (\(HG\), Eq. 1) and used to compute \(\Delta G\): [30]
Here, the free energies (\({G}_{HG}\), \({G}_{H}\) and \({G}_{G}\)) are all computed according to
The free energy of \(X\) in Eq. 5 is calculated from bonded \(\left({E}_{bnd}\right),\) electrostatic \(\left({E}_{el}\right)\) and van der Waals \(\left({E}_{vdw}\right)\) terms of the respective part of the system. The polar solvation energy, \({G}_{pol},\) is obtained solving the GB model (igb = 5) [32] and non-polar contribution to the free energy of binding, \({G}_{np},\) was estimated from the solvent accessible surface area [33]. The entropy, \(S\), was computed using a normal mode analysis [34] for all included snapshots.
For each steroid, we performed two simulations with the polar groups of each steroid pointing either down through the primary side (orientation-b) or up through the secondary side (orientation-a). The ligands were parameterized with the GAFF forcefield using the tleap program in AMBER. All CDs were parameterized with the GLYCAM06j carbohydrate force field [35]. The final inclusion complex was solvated in a cubic box of TIP3P [36] water with at least 14 Å of boundary between the CD and the periodic walls. To equilibrate the systems in AMBER, we initially minimized each structure with steepest descent for a maximum of 1000 steps or until the root mean square deviation on the gradient was below 10–3 kcal mol−1 Å−1. Due to observed instabilities in preliminary tests of the molecular dynamic simulations, we chose a conservative equilibration protocol. Unless otherwise noted, we harmonically restrained the atoms of both steroid and CD with a force constant of 100 kcal mol−1 Å−2 to their initial positions. We subsequently performed 100 ps simulation in the NVT at 10 K ensemble with a step size of 1 fs followed by 2 ns of NPT simulation where the temperature was increased linearly to 300 K while keeping the pressure constant at 1 bar and a step size of 2 fs. In the final part of the equilibration, we performed 10 ns of equilibration in the NPT ensemble where the force constant for the restraints were lowered to 10 kcal mol−1 Å−2. We finally performed the production with no restraints for a total of 100 ns simulation in the NPT ensemble. A total of 1000 snapshots were saved for postprocessing with MM/GBSA for each orientation for each ligand.
The analysis on the convergence of the free energy of binding \(\Delta {G}_{bind}\) from the MM/GBSA simulations is based on the cumulative average, \({\text{CA}}_{n}\), of the free energy of binding obtained from the first \(n\) snapshots from the simulation consisting of \(N=1000\) snapshots in total. The cumulative average is defined as
where the sum runs from the start of the simulation \(\left(i=1\right)\) to a later snapshot \(n\). The values \(\Delta {G}_{i}\) refers to free energies obtained for snapshot \(i\) with the MM/GBSA method. This is repeated for all snapshots sequentially until \(n=N\) which is the total average. We also compute the standard error of the mean, \({\text{SEM}}\), from the cumulative average to study the convergence behavior. It is defined as
Here \({s}_{n}\) is the sample standard deviation computed up to snapshot \(n\). \({t}_{95}\) is the value of the confidence limit at 95% and has a value of \({t}_{95}=1.96\). We use the autocorrelation function \({\rho }_{\tau }\) of the computed free energies which is defined as
The autocorrelation function is a measure of how much the data is correlated. For uncorrelated data the autocorrelation function oscillates around \({\rho }_{\tau }=0\) [37].
Results and discussion
Phase-solubility studies
Both AL and BS-type phase-solubility diagrams display a linear initial slope (Figs. 3 and 4), and we use this to estimate K1:1 by fitting the data to Eq. 2. Comparison of samples harvested at 24 and 48 h showed no significant change in amount of drug in solution, signifying that the equilibrium was established already after 24 h of incubation. In Fig. 3, we present our phase-solubility results of the four steroids in complex with various hosts in concentrations up to 0.10 M. We initially used hydrocortisone to explore the experimental behavior. For example, a BS-type plot is observed for γ-CD/hydrocortisone, as shown in Fig. 3a. γ-CD concentration up towards 0.012 M shown an initial increasing linear trend whereafter we observe a plateau in the concentration of hydrocortisone brought into solution. Therefore, K1:1 for γ-CD/hydrocortisone was calculated from the data in the concentration interval from 0 to 0.012 M. Since the initial linear slope on the BS diagrams and AL-type diagrams all had slopes less than unity, formation of 1:1 complex within the range of concentrations studied is likely [38, 39] and consistent with previous results [14, 40]. Based on these preliminary results for hydrocortisone the concentration ranges for all subsequent experiments were changed to 0–0.009 M for β- and γ-CD and 0–0.100 M for α-, HP-β-CD, and SBE-β-CD. The number of data points used in the experiments were decreased from 11 to 6 for the remaining phase solubility studies.
Assuming a 1:1 complex stoichiometry, the stability constants for the inclusion complex of hydrocortisone, dexamethasone, prednisolone, and 6α-methylprednisolone/CDs were calculated (Table 1). The stability constants of hydrocortisone with all five CDs varied from 125 ± 6 to 3509 ± 206 M−1. The complex between hydrocortisone and β-CD exhibited the strongest stability constants compared to the other CDs (Table 1). The solubility of β- and γ-CD are relatively limited and typically makes poorly soluble complexes resulting in Bs type diagrams [41]. The HP-β-CD and SBE-β-CD phase solubility diagrams with the various steroids were linear in a larger concentration range as expected for heterogeneous CD derivatives (Fig. 3). The amorphous nature of HP-β-CD and SBE-β-CD, i.e., their inability to form crystal structures, leads to higher solubility of both the CD derivative itself and its inclusion complexes [42]. As shown in Table 1, the stability constants found between the steroids and β-CD is relatively higher than those found for HP-β-CD and SBE-β-CD. This is probably due to the presence of side chains in HP-β-CD and SBE-β-CD causing a narrowing of the β-CD cavity opening and steric hindrance. SBE-β-CD have a slightly higher stability constant compared to HP-β-CD, possibly due to the longer side chain or the polar head group or both [40, 43, 44].
The change in the free energies of binding of the steroids/CD inclusion complexes (phase solubility ΔG in kJ mol−1) at 23 °C was calculated and reported in Table 1. The free energy of binding for the hydrocortisone/β-CD inclusion complex was estimated to − 20.09 ± 0.15 kJ mol−1. This was − 8.21, − 0.95, − 2.83, and − 2.74 kJ mol−1 more favorable than for hydrocortisone in complex with α-CD, γ-CD, HP-β-CD, and SBE-β-CD, respectively. Similarly, the free energy of binding of prednisolone/β-CD was − 6.00, − 1.07, − 2.27, and − 2.09 kJ mol−1 more favorable than for α-CD, γ-CD, HP-β-CD, and SBE-β-CD, respectively. We found that dexamethasone/γ-CD displayed the most stable inclusion complex with the free energy of binding of − 23.43 ± 0.05 kJ mol−1. 6α-methylprednisolone exhibited a similar pattern for the free energy of binding as for dexamethasone with strongest binding to γ-CD. The increased free energy of binding of both 6α-methylprednisolone and dexamethasone towards γ-CD is rationalized below. For 6α-methylprednisolone the added methyl group (Fig. 1) increases steric hindrance and is most likely the main reason for the less favorable free energy of binding when compared to prednisolone in complex with α-CD, β-CD, HP-β-CD, and SBE-β-CD. On the other hand, as γ-CD has a wider cavity and steric hindrance from the additional the methyl group may not play such a large role in inclusion complex formation. Therefore, we conclude that the presence of a methyl group in 6α-methylprednisolone significantly affects the interaction of the steroid/CD as observed both in Fig. 3 and as presented in Table 1. Similar arguments can be made for dexamethasone which has a fluorine atom that also increases steric hindrance. The large difference in the free energy of binding between some steroids/β-CD and other CDs is most likely related to the fact that the size of steroids and the cavity size of β-CD is particularly favorable. For all steroid/CD inclusion complexes we found that α-CD had the least favorable free energy of binding. This is not unexpected due to the size of the steroids and since α-CD in general favor smaller ligands (e.g., linear alkanes) due to the small size of the cavity.
The stability constants found in the literature were also converted to the free energies of binding for comparison (Table 1). Most of the free energy of binding obtained from literature were estimated from phase-solubility diagrams, except a few that was obtained using ITC [50] and UV/Vis-spectroscopy [49, 53]. We found only few studies on the free energy of binding of α-CD with hydrocortisone, dexamethasone, prednisolone, and 6α-methylprednisolone inclusion complexes.
We found four different the free energies of binding for hydrocortisone/β-CD inclusion complex in the literature obtained using similar phase solubility protocols with the free energy of binding values ranging from − 17.76 to − 20.29 kJ mol−1 [38, 40, 45, 46]. For Dexamethasone in complex with HP-β-CD and SBE-β-CD our obtained the free energy of binding of − 18.92 and − 19.60 kJ mol−1 are in good agreement with the free energy of binding reported by Usayapant et al. (− 18.93 to − 18.96 kJ mol−1) [53] and Jansook et al. (− 19.60 kJ mol−1) [12], respectively. Previous studies have examined the dexamethasone/γ-CD inclusion complex with a broad distribution of obtained the free energies of binding. Compared to our results, previous results were − 23.29 [51], − 17.47 [12], − 16.95 [52], and − 16.79 [52] kJ mol−1, deviate by 0.14, 5.59, 6.48 and 6.64 kJ mol−1 to our result. The free energy of binding of dexamethasone/β-CD in the literature was found to vary between − 21.12 [12] and − 16.23 [49] kJ mol−1 with the first value being in very good agreement with our − 21.23 ± 0.18 kJ mol−1 and the latter is underestimated by 5.00 kJ mol−1. We do note that this free energy of binding was obtained with ITC [48].
Taken together, some of the obtained free energies of binding were comparable to previously reported results (Table 1), but different methods may have led to differences between the free energy of binding obtained in this study and the literature. These differences can be due to deviations in the temperature, the specific CD derivative used with various substitution degrees (Captisol [14, 49, 54] and SBE4.6-β-CD [54]), the use of buffer and other additives or the use of different protocols and experimental methods such as UV/Vis-spectroscopy and ITC [49, 50, 53]. We observe that reproducing experimental work can be challenging due to the differences noted. Hence, to ensure a uniform dataset for comparison with simulations, we adhered to a single protocol throughout all experiments.
Simulation methods
We have assessed to what extend the free energy of binding of the complexes could be reproduced with two end-point methods: docking [27] and MM/GBSA [30, 31]. We have compared the results with experimental data obtained using classical phase solubility studies as presented above. Docking is easily accessible and has seen a resurgence in use in high-throughput virtual screening of large libraries for drug discovery [56] although the accuracy can vary across investigated systems [57] as it omits the hosts ability to adjust itself to each ligand. The MM/GBSA approach is based on molecular dynamics simulations and thus both host and ligand are able to adjust themselves during complexation to optimize binding. Due to the use of molecular dynamics, the computational cost is however vastly increased over the simpler docking approach.
Docking with standard precision
Docking poses obtained with Glide SP are illustrated in Fig. 4 for the four steroids when docked in β-CD. In Table 2, we present the free energies of binding obtained with Glide SP \(\left(\Delta {G}_{bind}^{SP}\right)\). For hydrocortisone, we obtain \(\Delta {G}_{bind}^{SP}\) values of − 15.21 ± 0.80 kJ mol−1, − 16.01 ± 1.04 kJ mol−1 and − 16.76 ± 0.11 kJ mol−1 for α-, β-, and γ-CD, respectively. Compared with the phase solubility experiments presented above these results overestimate the free energy of binding of hydrocortisone and α-CD by 3.3 kJ mol−1. The results are underestimated by roughly 4 kJ mol−1 for β-CD and by 2.4 kJ mol−1 for γ-CD. Due to the structural similarity of all the tested ligands, it is not unexpected that we obtain similar results for dexamethasone, prednisolone, and 6α-methylprednisolone where results for α-CD are typically overestimated by 2–4 kJ mol−1 and both β- and γ-CD are underestimated by 1–5 kJ mol−1.
Docking with extra precision
The results of the Glide XP score (\({\Delta G}_{bind}^{XP}\)) are listed in Table 2 and shows that \({\Delta G}_{bind}^{XP}\) for hydrocortisone are 11.41 ± 0.21 kJ mol−1, − 15.49 ± 0.82 kJ mol−1 and − 14.93 ± 0.80 kJ mol−1 α-, β- and γ-CD, respectively. The free energies of binding obtained with Glide XP are slightly less overestimated for α-CD with for example hydrocortisone/α-CD is deviating only − 0.47 kJ mol−1 compared with \({\Delta G}_{bind, exp}\). For β- and γ-CD in complex with hydrocortisone the computed free energies of binding (\(\Delta {G}_{bind}^{XP})\) are underestimated by 4.60 and 4.21 kJ mol−1, respectively. With Glide XP, β-CD was predicted to form the strongest inclusion complexes with the free energies of binding of − 15.49, − 14.24, − 15.87, and − 16.77 kJ mol−1 for hydrocortisone, dexamethasone, prednisolone, and 6α-methylprednisolone, respectively.
MM/GBSA
Prior to detailing the calculated binding free energies via the MM/GBSA method, we examine the convergence behavior to ensure a meaningful average to compute. In Fig. 5 we present the cumulative averages (Eq. 6) of the free energy of binding of hydrocortisone bound to \(\alpha\)-, \(\beta\)- and \(\gamma\)-CD for both orientation-a (red) and orientation-b (blue). The results for the three remaining ligands are presented in the Supporting Information where we also present example snapshots from the MD simulations.
For α-CD and hydrocortisone (Fig. 5 left panel) the cumulative average appears to converge fast towards a constant value. Similarly for hydrocortisone in orientation-a for β-CD (Fig. 5 center panel). For orientation-b in β-CD and both simulations with γ-CD, it is not directly apparent when, or if, convergence happens due to the fluctuations in the cumulative averages. These fluctuations most likely stem from not being in equilibrium or the presence of multiple stable minima on the potential energy surface. Thus, using the total average to compute \({\Delta \text{G}}_{\text{bind}}^{\text{MM}/\text{GBSA}}\) of the entire simulation (indicated by black dashed lines) would in this case only be correct for the first two simulations, but incorrect for the remaining three. Thus remains the question of how much of the simulation should be discarded to use only relevant data. To quantify this, we used the autocorrelation function (Eq. 8) and measured how much of the simulation was required to be discarded from the initial part of the 100 ns simulation in order to obtain non-correlated data. We found that discarding the initial parts of the simulation until \({\rho }_{\tau }\le 0.15\) (Figure S1 in the Supporting Information) is a suitable requirement to obtain converged free energies of binding. Using this threshold, we have indicated in Fig. 5 with vertical-colored lines how much data should be discarded for all simulations. This information is also presented in Figures S2 through S4 for the remaining steroids and all values are listed in Table S2 in the Supporting Information. We also found that discarding 90 ns or more of the simulation yielded numerical instabilities and those free energies have been discarded and not included in the presented averages. Based on this, we present the obtained \({\Delta \text{G}}_{\text{bind}}^{\text{MM}/\text{GBSA}}\) for α-, β-, and γ-CD/steroids pairs in Table 2.
In all MD simulations, the steroids penetrated the CDs cavity completely except for α-CD which is also corroborated by the low the free energies of binding obtained from both simulations and phase-solubility experiments.
Comparison of the free energies of binding obtained using experiments and simulations
In Fig. 6, a scatter plot of experimental the free energies of binding (\({\Delta G}_{bind, exp}\)) versus the free energies of binding obtained from simulations (\({\Delta G}_{bind, sim}\)) for native CDs in complex with steroids is presented. For standard precision docking (\({\Delta G}_{bind}^{SP}\)), the curve is observed to show some correlation to experimental the free energies of binding with an \({R}^{2}\) value of 0.70. However, as discussed above in detail, the overestimation of binding with α-CD and underestimation for β-CD and γ-CD yields little predictive power of the model as shown in Fig. 5. Also, the free energy of binding for a set of complexes between β-CD and 57 small organic molecules have been explored in previous studies with MM/GBSA, which showed a very weak correlation (R2 = 0.24) [58]. For extra precision docking (\(\Delta {G}_{bind}^{XP}\)) we observe no clear trend (\({R}^{2}\) value of 0.33) and although some the free energies of binding agree more with experiments, others agree less. However, For the MM/GBSA simulation results (\(\Delta {G}_{bind}^{MM/GBSA}\)), we observe a fair correlation with experimental values across the wide range of native CDs, concluding with an \({R}^{2}\) value of 0.64. Although the mean signed error for MM/GBSA is low, the root mean square error is quite large and comparable to the results obtained from docking. It is dominated by two outlying binding affinities: dexamethasone in \(\alpha\)-CD which is underestimated by close to 6 kJ mol−1 and the methylprednisolone bound to \(\beta\)-CD which is overestimated by almost 10 kJ mol−1. It is ongoing work to understand the cause of such large discrepancies and whether it can be ameliorated with alternative simulation protocols or a careful reevaluation of the non-polar solvation term [30].
Scatter plot of experimental \({\Delta G}_{bind, exp}\) (kJ mol−1) versus simulated \({\Delta G}_{bind, sim}\) (kJ mol−1) [\(\Delta {G}_{bind}^{SP}\) (SP): ■, \(\Delta {G}_{bind}^{XP}\) (XP): ●, and \({\Delta G}_{bind}^{MM/GBSA}\): ▲] for α-CD, β-CD and γ-CD with hydrocortisone, dexamethasone, prednisolone, and 6α-methylprednisolone with standard errors
Conclusion
The stability constants from the phase solubility studies obtained for steroids in complex with various CDs showed that complex stability was highly dependent on CD cavity size and type of substitution. HP-β-CD and SBE-β-CD formed weaker inclusion complexes compared to native β-CD, but they were capable of bringing more steroid in solution due to their higher intrinsic solubility and amorphous nature. All the data obtained from the simulations support the formation of steroid/CDs inclusion complexes in solution. The range of obtained free energies of binding varies depending on the simulation method used. We found that docking in general is not reliable when reproducing experimental the free energies of binding to CDs because it either overestimated the free energies of binding greatly for small cavities (α-CD), underestimated binding for larger cavities (β-, and γ-CD) or showed no systematic behaviour at all. We did, however, find that the MM/GBSA method based on molecular dynamics simulations was able to reproduce experimental the free energies of binding with good accuracy. The observed correlation between the computed free energies of binding and those obtained from phase solubility experiments leads us to conclude that simulations based on the MM/GBSA method may be a useful tool to study CD/steroid inclusion complexes before attempting to produce them in the laboratory.
References
Mazurek, A.H., Szeleszczuk, Ł, Gubica, T.: Application of molecular dynamics simulations in the analysis of cyclodextrin complexes. Int. J. Mol. Sci. 22, 1–35 (2021). https://doi.org/10.3390/ijms22179422
Whirledge, S., Cidlowski, J.A.: Steroid hormone action. In: Strauss, J.F., Barbieri, R.L. (Eighth eds.) Yen and Jaffe's Reproductive Endocrinology, pp. 115–131.e4. Elsevier (2019). https://doi.org/10.1016/C2015-0-05642-8
Beato, M., Klug, J.: Steroid hormone receptors: an update. Hum. Reprod. Update 6, 225–236 (2000). https://doi.org/10.1093/humupd/6.3.225
Moss, G.P.: Nomenclature of steroids. Pure Appl. Chern. 61, 1783–1822 (1989). https://doi.org/10.1515/iupac.61.0063
Nozaki, O.: Steroid analysis for medical diagnosis. J. Chromatogr. A 935, 267–278 (2001). https://doi.org/10.1016/S0021-9673(01)01104-9
Sen, S., Singh, B., Biswas, G.: Corticosteroids: a boon or bane for COVID-19 patients? Steroids 188, 109102 (2022). https://doi.org/10.1016/j.steroids.2022.109102
Brewster, M.E., Loftsson, T.: Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 59, 645–666 (2007). https://doi.org/10.1016/j.addr.2007.05.012
Trotta, F., Loftsson, T., Gaud, R.S., Trivedi, R., Shende, P.: Integration of cyclodextrins and associated toxicities: a roadmap for high quality biomedical applications. Carbohydr. Polym. 295, 119880 (2022). https://doi.org/10.1016/j.carbpol.2022.119880
Chen, X.M., Chen, Y., Hou, X.F., Wu, X., Gu, B.H., Liu, Y.: Sulfonato-β-cyclodextrin mediated supramolecular nanoparticle for controlled release of berberine. ACS Appl. Mater. Interfaces 10, 24987–24992 (2018). https://doi.org/10.1021/acsami.8b08651
Saker, B., Das, K., Saha, T., Prasad, E., Gardas, R.L.: Insights into the formations of host-guest complexes based on the benzimidazolium based ionic liquids−β-cyclodextrin systems. ACS Phys. Chem. Au 2, 3–15 (2022). https://doi.org/10.1021/acsphyschemau.1c00016
Crini, G.: Review: a history of cyclodextrins. Chem. Rev. 114, 10940–10975 (2014). https://doi.org/10.1021/cr500081p
Jansook, P., Loftsson, T.: γCD/HPγCD: synergistic solubilization. Int. J. Pharm. 363, 217–219 (2008). https://doi.org/10.1016/j.ijpharm.2008.07.011
Kfoury, M., Landy, D., Fourmentin, S.: Characterization of cyclodextrin/volatile inclusion complexes: a review. Molecules 23, 1–23 (2018). https://doi.org/10.3390/molecules23051204
Larsen, K.L., Aachmann, F.L., Wimmer, R., Stella, V.J., Kjølner, U.M.: Phase solubility and structure of the inclusion complexes of prednisolone and 6α-methyl prednisolone with various cyclodextrins. J. Pharm. Sci. 94, 507–515 (2005). https://doi.org/10.1002/jps.20192
Bíró, T., et al.: Development of prednisolone-containing eye drop formulations by cyclodextrin complexation and antimicrobial, mucoadhesive biopolymer. Drug Des. Dev. Ther. 12, 2529–2537 (2018). https://doi.org/10.2147/DDDT.S165693
Chen, H., Ji, H., Zhou, X., Wang, L.: Green synthesis of natural benzaldehyde from cinnamon oil catalyzed by hydroxypropyl-β-cyclodextrin. Tetrahedron 66, 9888–9893 (2010). https://doi.org/10.1016/j.tet.2010.10.063
Roy, N., Ghosh, B., Roy, D., Bhaumik, B., Roy, M.N.: Exploring the inclusion complex of a drug (umbelliferone) with α-cyclodextrin optimized by molecular docking and increasing bioavailability with minimizing the doses in human body. ACS Omega 5, 30243–30251 (2020). https://doi.org/10.1021/acsomega.0c04716
Chen, M., et al.: Molecular dynamics simulations and theoretical calculations of cyclodextrin-polydatin inclusion complexes. J. Mol. Struct. 1230, 129840 (2021). https://doi.org/10.1016/j.molstruc.2020.129840
Ali, S.M., Khan, S., Crowyn, G.: Structure determination of fexofenadine-α-cyclodextrin complex by quantitative 2D ROESY analysis and molecular mechanics studies. Magn. Reson. Chem. 50, 299–304 (2012). https://doi.org/10.1002/mrc.3807
Higuchi, T., Connors, K.A.: Phase solubility techniques. Adv. Anal. Chem. Instrum. 4, 117–212 (1965)
Schönbeck, C., Madsen, T.L., Peters, G.H., Holm, R., Loftsson, T.: Soluble 1:1 complexes and insoluble 3:2 complexes—understanding the phase-solubility diagram of hydrocortisone and γ-cyclodextrin. Int. J. Pharm. 531, 504–511 (2017). https://doi.org/10.1016/j.ijpharm.2017.05.024
Loftsson, T., Hreinsdóttir, D., Másson, M.: Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 302, 18–28 (2005). https://doi.org/10.1016/j.ijpharm.2005.05.042
Case, D.A., Betz, R.M., Cerutti, D.S., Cheatham, T.E., Darden, T.A., Duke, R.E., Giese, T.J., Gohlke, H., Goetz, A.W., Homeyer, N., Izadi, S., Janowski, P., Kaus, J., Kovalenko, A., Lee, T.S., LeGrand, S., Li, P., Lin, C., Luchko, T., Luo, R., Madej, B., Mermelstein, D., Merz, K.M., Monard, G., Nguyen, H., Nguyen, H.T., Omelyan, I., Onufriev, A., Roe, D.R., Roitberg, A., Sagui, C., Simmerling, C.L., Botello-Smith, W.M., Swails, J., Walker, R.C., Wang, J., Wolf, R.M., Wu, X., Xiao L., Kollman, P.A.: AMBER 2016.0, University of California, San Francisco (2016). https://ambermd.org/
Schrödinger Release 2021-3: Maestro, Schrödinger, LLC, New York, NY (2021). https://schrodinger.com
Lu, C., et al.: OPLS4: improving force field accuracy on challenging regimes of chemical space. J. Chem. Theory Comput. 17, 4291–4300 (2021). https://doi.org/10.1021/acs.jctc.1c00302
Halgren, T.A., et al.: Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 47, 1750–1759 (2004). https://doi.org/10.1021/jm030644s
Friesner R.A., et al.: Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 47, 1739–1749 (2004). https://doi.org/10.1021/jm0306430
Friesner, R.A., et al.: Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 49, 6177–6196 (2006). https://doi.org/10.1021/jm051256o
Watts, K.S., Dalal, P., Murphy, R.B., Sherman, W., Friesner, R.A., Shelley, J.C.: ConfGen: A conformational search method for efficient generation of bioactive conformers. 50, 534–546 (2010). https://doi.org/10.1021/ci100015j
Genheden, S., Ryde, U.: The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 10, 449–461 (2015). https://doi.org/10.1517/17460441.2015.1032936
Wang, E., et al.: End-point binding free energy calculation with MM/PBSA and MM/GBSA: strategies and applications in drug design. Chem. Rev. 119, 9478–9508 (2019). https://doi.org/10.1021/acs.chemrev.9b00055
Onufriev, A., Bashford, D., Case, D.A.: Exploring protein native states and large-scale conformational changes with a modified generalized born model. PROTEINS: Struct. Funct. Bioinform. 55, 383–394 (2004). https://doi.org/10.1002/prot.20033
Still, W.C., Tempczyk, A., Hawley, R.C., Hendrickson, T.: Semianalytical treatment of solvation for molecular mechanics and dynamics. J. Am. Chem. Soc. 112, 6127–6129 (1990). https://doi.org/10.1021/ja00172a038
Kuhn, B., Kollman, P.A.: Binding of a diverse set of ligands to avidin and streptavidin: an accurate quantitative prediction of their relative affinities by a combination of molecular mechanics and continuum solvent models. J. Med. Chem. 43, 3786–3791 (2000). https://doi.org/10.1021/jm000241h
Kirschner, K.N., Yongye, A.B., Tschampel, S.M., González-Outeiriño, J., Daniels, C.R., Foley, B.L., Woods, R.J.: Glycam06: A generalizable biomolecular force field. Carbohydr. J. Comput. Chem. 29, 622–655 (2008). https://doi.org/10.1002/jcc.20820
Jorgensen, W.L., Chandrasekhar, J., Madura, J.D., Impey, R.W., Klein, M.L.: Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983). https://doi.org/10.1063/1.445869
Bishop, M., Frinks, S.: Error analysis in computer simulations. J. Chem. Phys. 87, 3675–3676 (1987). https://doi.org/10.1063/1.452965
Al-Sou’od, K.A.: Investigation of the hydrocortisone-β-cyclodextrin complex by phase solubility method: some theoretical and practical considerations. J. Solut. Chem. 37, 119–133 (2008). https://doi.org/10.1007/s10953-007-9216-4
Beig, A., Agbaria, R., Dahan, A.: Oral delivery of lipophilic drugs: the tradeoff between solubility increase and permeability decrease when using cyclodextrin-based formulations. PLoS ONE 8, e68237 (2013). https://doi.org/10.1371/journal.pone.0068237
Messner, M., Kurkov, S.V., Brewster, M.E., Jansook, P., Loftsson, T.: Self-assembly of cyclodextrin complexes: aggregation of hydrocortisone/cyclodextrin complexes. Int. J. Pharm. 407, 174–183 (2011). https://doi.org/10.1016/j.ijpharm.2011.01.011
Loftsson, T.: Self-assembled cyclodextrin nanoparticles and drug delivery. J. Incl. Phenom. Macrocycl. Chem. 80, 1–7 (2014). https://doi.org/10.1007/s10847-013-0375-1
Mosher, G., Machatha, S.: Complexation: cyclodextrins. In: Swarbrick, J. (ed.) Encyclopedia of Pharmaceutical Science and Technology, Six Volume Set (Print), pp. 563–584. CRC Press (2013)
Yoshida, A., Yamamoto, M., Irie, T., Hirayama, F., Uekama, K.: Some pharmaceutical properties of 3-hydroxypropyl- and 2, 3-dihydroxypropyl-β-cyclodextrins and their solubilizing and stabilizing abilities. Chem. Pharm. Bull. 37, 1059–1063 (1989). https://doi.org/10.1248/cpb.37.1059
Ribeiro. L., Veiga, F.: Complexation of vinpocetine with cyclodextrins in the presence or absence of polymers. Binary and ternary complexes preparation and characterization. J. Incl. Phenom. Macrocycl. Chem. 44, 251–256 (2002). https://doi.org/10.1023/A:1023069609303
Uekama, K., Fujinaga, T., Hirayama, F.: Inclusion complexations of steroid hormones with cyclodextrins in water and in solid phase. Int. J. Pharm. 10, 1–15 (1982). https://doi.org/10.1016/0378-5173(82)90057-6
Liu, F.Y., Kildsig. D.O., Mitra A.K.: Beta-cyclodextrin/steroid complexation. Beta-cyclodextrin/steroid complexation: effect of steroid structure on association equilibria. Pharm. Res. 7, 869–873 (1990). https://doi.org/10.1023/A:1015973218303
Saokham, P., Loftsson, T.: A new approach for quantitative determination of γ-cyclodextrin in aqueous solutions: application in aggregate determinations and solubility in hydrocortisone/γ-cyclodextrin inclusion complex. J. Pharm. Sci. 104, 3925–3933 (2015). https://doi.org/10.1002/jps.24608
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins. J. Pharm. Pharmacol. 62, 1607–1621 (2010). https://doi.org/10.1111/j.2042-7158.2010.01030.x
Zia, V., Rajewski, R.A., Stella, V.J.: Effect of cyclodextrin charge on complexation of neutral and charged substrates: comparison of (SBE)7M-β-CD to HP-β-CD. 18, 667–673 (2001). https://doi.org/10.1023/A:1011041628797
Mateen, R., Hoare, T.: Carboxymethyl and hydrazide functionalized b-cyclodextrin derivatives: a systematic investigation of complexation behaviours with the model hydrophobic drug dexamethasone. Int. J. Pharm. 472, 315–326 (2014). https://doi.org/10.1016/j.ijpharm.2014.06.046
Sripetch, S., Jansook, P., Loftsson, T.: Effect of porcine pancreatic α-amylase on dexamethasone release from aqueous solution containing natural γ-cyclodextrin. Int. J. Pharm. 585, 119452 (2020). https://doi.org/10.1016/j.ijpharm.2020.119452
Moya-ortega, M.D., et al.: Dexamethasone eye drops containing γ-cyclodextrin-based nanogels. Int. J. Pharm. 441, 507–515 (2013). https://doi.org/10.1016/j.ijpharm.2012.11.002
Usayapant, A., Karara, A.H., Narurkar, M.M.: Effect of 2-hydroxypropyl-β-cyclodextrin on the ocular absorption of dexamethasone and dexamethasone. Pharm. Res. 8, 1495–1499 (1991). https://doi.org/10.1023/A:1015838215268
Okimoto, K., et al.: Design and evaluation of an osmotic pump tablet (OPT) for prednisolone, a poorly water soluble drug, using (SBE)7m-β-CD. Pharm. Res. 15, 1562–1568 (1998). https://doi.org/10.1023/A:1011955117026
Tongiani, S., Velde, D.V., Ozeki, T., Stella, V.J.: Sulfoalkyl ether-alkyl ether cyclodextrin derivatives, their synthesis, NMR characterization, and binding of 6α-methylprednisolone. 94, 2380–2392 (2005). https://doi.org/10.1002/jps.20367
Lyu, J., et al.: Ultra-large library docking for discovering new chemotypes. Nature 566, 224–229 (2019). https://doi.org/10.1038/s41586-019-0917-9
Fischer, A., Smieško, M., Sellner, M., Lill, M.A.: Decision making in structure-based drug discovery: visual inspection of docking results. J. Med. Chem. 64, 2489–2500 (2021). https://doi.org/10.1021/acs.jmedchem.0c02227
Suárez, D., Díaz, N.: Affinity calculations of cyclodextrin host-guest complexes: assessment of strengths and weaknesses of end-point free energy methods. J. Chem. Inf. Model. 59, 421–440 (2019). https://doi.org/10.1021/acs.jcim.8b00805
Funding
Open access funding provided by Aalborg University.
Author information
Authors and Affiliations
Contributions
P.F., C.S. and K.L.L. designed the experiments. P.F. was responsible for carrying out the experiments. P.F. drafted the initial manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fereidounpour, P., Steinmann, C. & Larsen, K.L. Prediction of the free energy of binding for cyclodextrin-steroid complexes: phase solubility and molecular dynamics studies. J Incl Phenom Macrocycl Chem 104, 535–546 (2024). https://doi.org/10.1007/s10847-024-01255-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-024-01255-z