Abstract
In the Colombian Caribbean, the Tropical Dry Forest (TDF) is among the most important ecosystems for conservation providing both economic and regulatory services to local communities and it is the habitat of many dung beetles. Particularly, the species Deltochilum guildingii is notable for its large size and greater capacity to bury organic matter, thus facilitating the recycling of soil nutrients and seed germination. The current degradation of the TDF, however, may have adverse effects on its population genetics. In this study, based on 2,587 single nucleotide polymorphisms (SNPs) generated by restriction-site associated DNA sequencing in 60 individuals of D. guildingii in six TDF fragments in the Colombian Caribbean (departments of Guajira, Magdalena, Cesar, Atlántico, Bolívar, and Sucre), we aimed to estimate genetic diversity and its relationship with fragmentation. Moreover, we evaluated spatial patterns of genetic structure, and inferred D. guildingii demography history. Our results showed reduced heterozygosity in all populations with the lowest for the Magdalena fragment. There was no relationship between genetic diversity and fragment area, perimeter, or degree of insularization. Multivariate and Bayesian clustering analyses of genetic structure identified two genetic groups of biogeographic affinity: one pertains to the northern departments (Guajira, Magdalena, and Cesar) and the other to the south (Montes de María and Piojó). In addition, a population expansion was estimated 2.5 million years ago during the Pleistocene, which coincided with the time of TDF diversification in Colombia and of the genus Alouatta, the primates that provide the principal source of excrement to D. guildingii.
Implications for insect conservation
It would be important to increase environmental vigilance because four out of six TDF fragments were insularized, where all populations showed low genetic diversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the most relevant ecosystems for conservation in the Colombian Caribbean is the Tropical Dry Forest (TDF), which provides both economic and regulatory services to local communities, such as the regulation of climate, the fertility of soils, and watershed protection (Gillespie et al. 2013; Quijas et al. 2019; Andrade et al. 2020). Its deciduous vegetation and marked climatic seasonality, coupled with large levels of water stress, contribute to its high turnover of plants and animals, making it highly diverse biologically (Kattan et al. 2019; Romero-Duque et al. 2019). For instance, 3,395 plants, 145 birds, 60 mammals, and 68 dung beetle species have been recorded for the TDF of Colombia (Pizano and García 2014). Unfortunately, it is at risk due to anthropogenic changes in land use and climate vulnerability (Torres-Romero and Acosta-Prado 2022), and currently less than 8% of the TDF’s original extent remains (García et al. 2014). Accordingly, the Colombian government considers the TDF to be a critically endangered ecosystem (Etter et al. 2017).
Dung beetle species are key to ecosystem health due to their dung removal activity that cleans the surface of pastures, favors soil structures, and reduces the presence of parasitic agents (Gómez-Cifuentes et al. 2018). They are sensitive to changes in vegetation cover, variations in microclimate conditions, and the availability of excrement as food sources and for breeding chambers, primarily from primates. Moreover, the density of native vertebrates providing sources of excrement for dung beetles has decreased due to the exploitation of forests (Bicknell et al. 2014). Evidence shows that the species richness of dung beetle species in tropical ecosystems has declined (Scarabaeinae subfamily), resulting in the loss of ecological niches and ecosystem services, especially of the specialist species which are more likely to decrease in abundance (Carrión-Paladines et al. 2021; Noriega et al. 2021).
The Colombian Caribbean region, including the departments of Guajira and Sucre, is experiencing habitat deterioration due to urban, mining, and agricultural activities, which have resulted in the loss of forest cover and soil degradation (Ballesteros-Correa and Pérez-Torres 2022). The TDF in this region provides habitat for Deltochilum guildingii (Westwood 1835), one of the largest roller dung beetle species, which has a limited flight range and a preference for conserved forests. This species plays an active role in recycling nutrients and in removing excrement of native mammals, such as primates of the genus Alouatta whose abundance has decreased locally in the TDF in recent years (e.g., Colosó in Sucre; Martínez-Hernández et al. 2012; De la Ossa et al. 2013; Estrada 2015; Rangel-Acosta and Martínez-Hernández 2017; Noriega et al. 2020). As a large roller, D. guildingii provides a greater burial of organic matter that contributes to soil fertility (Correa-Cuadros et al. 2022), favoring the germination viability of tropical plant species (Andresen 2003; Barragán et al. 2011).
The loss of vegetation cover may restrict D. guildingii dispersal between increasingly isolated habitat fragments (Cox et al. 2020), while the size reduction of habitat fragments coupled with the reduction of excrement sources (Rondón et al. 2017; Medina-Hernández et al. 2020) may have adverse impacts on population size and reproductive success (Leroy et al. 2017). According to the population genetics theory, small and highly fragmented populations will lose genetic variation and adaptive potential because of genetic drift and inbreeding (Hooftman et al. 2016; Dillon and Lozier 2019). Moreover, it has been shown that the largest telecoprids and paracoprids beetles are the most vulnerable to extinction (Tonelli et al. 2020).
Integral conservation plans are required to protect TDF species. Combining ecology and genetics enables a better understanding of the influence of humans on landscapes and the designation of genetically and ecologically representative areas (Etter et al. 2008; Narum et al. 2013; Schierenbeck 2017; Matos-Maraví et al. 2019; Maxwell et al. 2020). Furthermore, we can better understand climate adaptation by examining how the demography of populations has changed historically and how these changes can be attributed to previous climate events (Ho and Shapiro 2011). This understanding is relevant for the Caribbean region, which has three biogeographic regions with heterogeneous climates and topographies: (1) the Sierra Nevada de Santa Marta (SNSM), (2) the mountainous system of Montes de María y Piojó (DMMP), and (3) the Guajira (GUAJ) (Fig. 1) (Arenas 2012; Fremout et al. 2021). For these regions, few genetic studies have been conducted on key species.
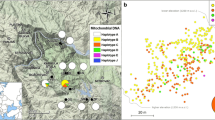
Studied fragments of the Tropical Dry Forest (TDF) in the Colombian Caribbean. Three biogeographic regions are denoted with a different grey-coloring background in the map: Sierra Nevada de Santa Marta (SNSM) (fragments PNNT and TG); Montes de María and Piojó (DMMP) (fragments COL, RCM and SFFC); Guajira (GUA) (fragment BRUN). Red dots denote the fragment location
In this study, based on double digest Restriction-site Associated DNA sequencing (ddRadSeq) analysis for 60 individuals of D. guildingii sampled in six TDF fragments of these three biogeographic regions in the Colombian Caribbean, each with varying degrees of anthropogenic habitat disturbance, we aimed to: (i) estimate levels of genetic diversity and their relationship with habitat fragmentation, (ii) evaluate patterns of spatial genetic structure, and (iii) infer the demography history of the species and its potential associations with past climatic events. This study will provide valuable information for future conservation decisions for this roller dung beetle species and others that could have similar genetic responses to the changing conditions of the TDF in the Colombian Caribbean.
Materials and methods
Study area and species
In the Colombian Caribbean region, we sampled the following TDF fragments: (1) Bruno (BRUN) in the Guajira region (GUAJ); (2) The Tayrona National Natural Park (PNNT in Magdalena), and (3) Tierra Grata (TG in César) in the Sierra Nevada de Santa Martha (SNSM); (4) the Flora and Fauna Los Colorados Sanctuary (SFFC in Bolívar), (5) the Reserva Campesina “La Montaña” (RCM in Atlántico), and (6) Estación Biológica Primatológica de Colosó (COL in Sucre) in the Montes de María y Piojó region (DMMP) (Fig. 1). All fragments have the typical TDF rainfall regime (900–1200 mm) and vegetation cover (Galván-Guevara et al. 2009). These fragments have different levels of environmental vigilance (Table 1).
Deltochilum guildingii is a large species, with limited flight range and nocturnal activity. For the genus, it has been observed that the egg balls are buried in the ground in a cup-shaped depression (Howden and Ritcher 1952; Pessôa et al. 2017). It has a wide distribution in Brazil, Colombia, Trinidad and Tobago, Venezuela, and Suriname (González-Alvarado and Vaz-de-Mello 2014).
Sampling and fragmentation index
Deltochilum guildingii individuals were collected during 2018 and 2019 (Colombian sampling permits Resolution 00949, and No. CITES permit 1607). For each fragment, four transects were located 500 m apart and for each transect, five pitfall traps were placed separated by 100 m each following da Silva and Medina-Hernández (2016) for a total of 20 traps per fragment. Each pitfall consisted of a 32-ounce plastic container buried in the ground without lethal liquid and a plastic funnel was placed at the aperture of the container. A rod in the shape of an inverted L was placed above the container, upon which was hung a bait consisting of human excrement and chicken viscera wrapped in gauze (Martínez-Hernández et al. 2022). The traps were revised every 24 h for two days and the material was stored in whirlpack bags with 96% ethanol (on average ~ 52 individuals were collected per fragment). The material was identified taxonomically and with the extraction of genitalia using the keys of González-Alvarado and Vaz-De-Mello (2014). Ten individuals were selected for each fragment to extract the two middle and hind legs, which were stored in cryovials with ethanol 96% until genomic DNA extraction.
For each fragment, we calculated the fragmentation F Index (IF) according to Lozano-Botache et al. (2011), whose value ranges from 0 to 1. The values correspond to the following categories: insularized fragment (IF < 0.5), highly fragmented (IF = 0.5–0.7), moderately fragmented (IF = 0.7–0.99), and no fragmented (IF = 1) (Galván-Guevara et al. 2015). The IF was calculated using the TDF cover map created by the Institute Alexander von Humboldt (González et al. 2014). Moreover, we calculated the area (\(\:{m}^{2}\)) and perimeter (\(\:{m}^{2}\)) of each fragment in ArcGIS pro v.2.9 (Esri Inc 2020).
DNA extraction and ddRadseq protocol
Genomic DNA was extracted using the NucleoSpinTissue (R) tissue kit (Macherey-Nagel 2017) according to the manufacturer’s instructions. Approximately 200 ng of high-molecular-weight DNA was digested with two restriction endonucleases enzymes (EcoRI and HpyCH4IV) following the dd-RadSeq library preparation of Peterson et al. (2012). DNA fragments were searched in a target range of 280 to 375 bp to obtain the genomic libraries. The DNA extraction and ddRadSeq protocol were carried out by the Australian Genomics Research Facility (AGRF).
Bioinformatic analysis
Raw Illumina reads were demultiplexed, cleaned, and quality checked using the module process_radtags in STACKs v.2.53 (Rochette et al. 2019), setting a Phred quality score of 20 (Mastretta-Yanes et al. 2015; Rochette and Catchen 2017). A reference sequence for the species D. guildingii was constructed from an initial mapping of the available dung beetle genome Onthophagus taurus (NCBI: JHOM00000000.2) using NextGenmap v. 0.6 (Sedlazeck et al. 2013) to inspect 3,200,000 reads per individual against the O. taurus reference. The resulting files were then organized and merged with Samtools v.1.17 (Li et al. 2009) and a final reference fasta file was generated using mpileup and seqtk v.1.3 (Li 2012). All read samples were then aligned against the new reference genome with NextGenMap.
The read depth was evaluated using ref_map.pl in STACKs (Rivera-Colón and Catchen 2022), and populations programs parameters to filter SNPs loci were: minimum percentage of populations of the presence of the locus (3%), minimum percentage of individuals with the presence of a locus (70%) and maximum heterozygosity observed per locus (90%). The average read depth was 37.3x. Potential loci under selection were detected using the R package pcadapt v.4.3 (Luu et al. 2016). For this, the Manhattan plot of SNPs with a min MAF > 0.05 was plotted and a frequency histogram was constructed based on the p-values (Luu et al. 2016). Twelve SNPs potentially under selection were removed to obtain a data set of 2,587 SNPs under neutrality for subsequent analyses.
Genetic diversity and genetic structure
We estimated genetic diversity parameters, such as allelic richness (Ar), observed (Ho) and expected (He) heterozygosity using the R package hierfstat v0.5-11 (Goudet 2005). The number of private alleles (PrA) per fragment was calculated in poppr v.2.9.3 (Kamvar et al. 2014), while FIS per fragment and nucleotide diversity (\(\:\pi\:\)) was obtained in STACKs v.2.53 (Rochette et al. 2019).
Genetic structure was evaluated by implementing a sparse non-negative matrix factorization (snmf) from LEA package v.2.8 (Frichot and François 2015) to identify the best number of genetic clusters, and setting K = 1 to 6, 10 repetitions and 1000 iterations. Moreover, we applied Principal Component Discriminant Analysis (DAPC) and a Spatial Principal Component Analysis (sPCA) in the R package adegenet v.2.1.1 (Jombart et al. 2008). For the DAPC, the fragments were used a priori for population grouping, and the optimal PCs to retain were optimized using xval cross-validation criteria (Jombart 2008). For the sPCA we used the Delaunay triangulation to obtain the connection network between fragments. Furthermore, an analysis of molecular genetic variance (AMOVA) was carried out in poppr v.2.9.3 testing a priori the amount of genetic variation explained by the three biogeographic regions, and by the six TDF fragments.
We performed a Mantel correlation to test the effect of isolation by distance (IBD) using Nei FST pairwise genetic distances and Euclidean distances using the R package ade4 v.1.7 with 999 permutations (Dray and Dufour 2007). Moreover, Pearson correlations between fragment area, perimeter, and fragmentation index with genetic diversity, and FIS estimates were tested using the R package past v.4.02 (Hammer et al. 2001).
Historical demography
To infer historical demographic changes, we estimated the Tajima’s D estimator for each fragment in pegas v.1.0 (Paradis 2010). Tajima’s D with significant negative values suggests rejection from neutrality and can be interpreted as population expansion. Also, we estimated Bayesian skylineplots in BEAST v.2.7.1 (Drummond and Bouckaert 2015) to assess effective population changes (Ne) over time. We used the substitution model GTG + R as selected from the Akaike Information Criterion (AIC) in JModeltest v.2.1.10 (Darriba et al. 2012). A strict molecular clock with 20 million generations, a clock rate of 0.012 mutations per year (Gunter et al. 2016), and trees and parameters sampled every 2000 iterations were chosen. The log file was viewed in TRACER v1.7.1 (Rambaut et al. 2018) after analysis to ensure that effective sample sizes (ESS) for all priors were greater than 100.
Results
Estimates of genetic diversity and patterns of genetic structure
Genetic diversity for the species level was low (Ho = 0.07, He = 0.09). Genetic diversity estimates per fragment were similar and, with the highest values for TG (Ho = 0.083, He = 0.09) and SFFC (Ho = 0.084, He = 0.09), and the lowest for COL (Ho = 0.072, He = 0.086) (Table 2). The fragment with the most exclusive alleles was SFFC with 325 and the lowest with 74 in PNNT. Inbreeding FIS values ranged from 0.076 to 0.122.
Results of genetic structure in LEA showed that the most likely number of genetic clusters was K = 2, of which we observed high admixture (Fig. 2A). The most differentiated fragments were BRUN at the northeast and SFFC to the southwest, each belonging to one of the two groups identified. Results from the DAPC along axis 1 also showed the separation of fragments into two groups: one formed by the northernmost fragments of the Colombian Caribbean coast, BRUN, TG, and PNNT, and the second group by the south fragments, SFFC, COL, and RCM (Fig. 2B). The sPCA retrieved the same spatial pattern as the DAPC along axis 1, with the separation of BRUN, TG, and PNNT in one group, and SFFC, COL, and RCM in a second group (Fig. 2C).
Results of genetic structure in D. guildingii: (A) LEA analysis as piecharts and a barplot, (B) Discriminant Analysis of Principal Components (DAPC), and (C) Spatial Analysis of Principal Components (sPCA). The two different colors denote the two genetic groups identified. Abbreviations: Sierra Nevada de Santa Marta (SNSM) (fragments PNNT and TG); Distrito Montes de María and Piojó (DMMP) (fragments COL, RCM and SFFC); Guajira (GUAJ) (fragment BRUN)
The pairwise FST indices showed moderate genetic differentiation between PNNT and COL (FST = 0.070) and the lowest between RCM and SFFC (FST = 0.031) (Supplementary material). The AMOVA showed that the genetic proportion of genetic variance explained between the three biogeographic regions (1.5%) was similar to the proportion between fragments (1.7%). For both analyses, most of the genetic variation was within individuals (71%) (Table 3).
Relationship between habitat fragmentation and genetic diversity
Four fragments (COL, RCM, TG, and BRUN) were insularized (IF < 0.5), one was highly fragmented (PNNT, IF = 0.53) and the other was moderately fragmented (SFFC, IF = 0.7). The most critical situation was for RCM in Atlántico, which was the most insularized (IF = 0.17) (Table 1). None of the Pearson correlations between fragment area, perimeter, and the F index with genetic diversity parameters were significant (P > 0.05). Moreover, we found no significant effect of isolation by distance on genetic differentiation (r = 0.052, P = 0.36).
Demography history
Results from the historical demography analyses using the complete neutral SNPs data set showed that the best nucleotide substitution model was GTG + R (-Ln = 52260.66) and stability was achieved for the reconstruction parameters (posterior-ESS = 380; likelihood-ESS = 180). The Bayesian skyline plot of Ne through time showed a population expansion 2.5 million years ago (Fig. 3). This result was in agreement with the ones of the Tajima’s D estimator, which values were negative and significant suggesting population expansions for all TDF fragments: PNNT (D = -4.16, P < 0.05), COL (D = -4.14, P < 0.05), BRUN (D = -4.07, P < 0.05), RCM (D = -3.56, P < 0.05) and TG together with SFFC (D = -2.37, P = 0.018).
Discussion
Genetic diversity and its relationship with habitat fragmentation
Overall, it was observed low genetic diversity for D. guildingii compared to the values observed in other members of the Scarabaeidae family, such as Trypoxylus dichotomus (Ho = 0.097–0.17) (Yang et al. 2021). Inbreeding values were also within the range reported for other Coleoptera using SNPs and microsatellites for the genus Eucryptorrhynchus (Zhang et al. 2021).
The lowest value of expected heterozygosity and the highest level of inbreeding were observed for PNNT, which was the most fragmented site. This observation agrees with another study that observed that anthropogenic pressures, such as ecotourism and other activities affected the quality of the habitat and the abundance of D. guildingii (Noriega et al. 2020). Other fragments, such as BRUN suffer from mining activities with unfavorable implications for the ecosystem (Gómez-Betancur and Vilardy 2022), while RCM is small and where agricultural activities are practiced. Both fragments are insularized (IF = 0.27 and 0.17 respectively) but with similar levels of genetic diversity to other sites. Moreover, livestock, agriculture, and mining are well recognized in Montes de María (Sampedro-Marín et al. 2011), where the COL fragment occurs. The IF in COL, denoting isolation (IF = 0.35) was consistent with those of other localities in Sucre, such as Tolú Viejo and Colosó (IF = 0.36) (Galván-Guevara et al. 2015). COL showed lower genetic diversity and a lower number of private alleles compared to other insularized fragments, which suggests that this fragment is more prone to the erosion of genetic diversity due to land-use changes.
The surrounding areas in TG are highly modified by anthropogenic activities, such as cattle grazing, pig farming, and agriculture, which isolate the population of D. guildingii. Due to the vulnerability of the species to move between areas with low forest cover, these land-use modifications may change matrix permeability, restricting dispersal and gene flow between populations (IAVH 2016). Interestingly, TG has the second highest number of unique alleles, which may be due to the influence of the Serranía de Perijá, a region of high biological diversity and endemism (López-O et al. 2014).
In contrast to the rest of the fragments, the highest degree of genetic diversity and number of private alleles were found in the fragment SFFC, which agreed with its highest conservation status (IF = 0.71). This fragment is within the Serranía de San Jacinto and the primary conservation area for San Juan Nepomuceno, which falls within the Local System of Protected Areas (Galván et al. 2015; Jiménez et al. 2018). Additionally, in this region, socio-ecosystemic connectivity initiatives have been implemented that integrate multiple social and governmental actors to ensure the conservation of vertebrates such as the monkey Alouatta seniculus and Saguinus oedipus, important sources of excrements for dung beetles and the Malibú Biological Corridor for big cats such as Panthera onca (Ange et al. 2020). These initiatives help to maintain viable populations and would indirectly benefit D. guildingii at the genetic level, and for this reason, this protected area in the department of Bolívar is an important natural heritage of the Caribbean region (Bermúdez-Wilches 2012).
Patterns of spatial genetic structure
The reported values of FST for D. guildingii agree with those described for species of the same genus, such as D. speciosissimum (FST = 0.029) (Linck et al. 2020). Interestingly, the absence of isolation by geographic distance suggests that the patterns of genetic differentiation are due to other processes such as genetic drift, geologic history, landscape resistance, or environmental differences (Slatkin 1993; Peterson and Denno 1998; Taylor et al. 2020).
Specifically, patterns of genetic variation were not explained by the three biogeographic regions as initially thought. Instead, from clustering analyses we found consistent patterns in two genetic groups in D. guildingii, but that also can be related to the different relief and geological processes forming the mountain systems in the region. The first group included BRUN, TG, and PNNT, and is defined by the Oca fault in the Guajira, the Santa Marta fault in Magdalena, and the Serranía de Perijá in Cesar, in which the geotectonic history of northern Colombia shows similarities between the Sierra Nevada de Santa Marta (SNSM), the Ranchería river basin, and the Serranía de Perijá (Rodríguez and Londoño 2002; Chicangana et al. 2011). Based on our data, BRUN, situated north of the Colombian Caribbean coast, does not function as an isolated biogeographic region as previously assumed. Instead, it is closely related to other prominent mountain massifs with a shared history, such as PNNT and TG in the SNSM region.
The differentiation of COL, SFFC, and RCM between PNNT, TG, and BRUN can be explained by different biogeographic influences (Jiménez et al. 2018). Both biogeographic regions experienced different paleoclimatic events, causing changes in the distribution of genetic variation and other biotic responses (Riddle et al. 2008). Likewise, the lower differentiation between the SFFC and the RCM can be associated with the shared geomorphological history between the Luruaco and San Jacinto anticlines (Banco de Occidente 1999), and the fact that there is still a forest belt between Bolívar and Atlántico which would promote gene flow.
Historical demography and climatic events
The negative Tajima D values for all fragments suggest demographic expansions after bottleneck events (Dogan and Dogan 2017). With the complete data set for the species level, the estimated population expansion event of 2.5 million years ago corresponds to the transition between the Pliocene and Pleistocene, a period of orogenic and paleoclimatic relevance that marked the evolutionary history of many biological groups, such as vertebrates and insects. For instance, it has been documented that the Canthonini tribe, where Deltochilum was formerly included, appeared in the Pliocene. During this period the reduction in rainfall and temperature up to 5 °C (Hooghiemstra and Ran 1994) were important for the development of seasonally dry areas that marked the trajectory of the TDF and allowed the diversification of Alouatta primates with the expansion of families such as Fabaceae. Thus, the increase in effective population size of D. guildingii coincides with the phylogeny of Alouatta in South America, during which the primate clades expanded and diversified between 3.3 and 2.4 million years ago (Cuervo-Díaz et al. 1986; Cortés-Ortiz et al. 2003; Padilla-Gil and Halffter 2007; Gattepaille et al. 2013; Mercado-Gómez et al. 2019).
Conservation implications
For the first time, a population genetic study for D. guildingii was conducted, contributing to our understanding of the genetic status of dung beetles in the Colombian Caribbean. Large telecoprids such as D. guildingii, which are normally associated with primate excrement and dense forest cover, and with low dispersal capacity may be affected by the reduction in excrement availability and limited connectivity under the current TDF situation with accentuated differences between conserved and not protected areas (Díaz et al. 2010). According to our results, D. guildingii populations are vulnerable to the effects of genetic drift and inbreeding as populations become smaller under increasingly isolated fragments and land-use changes.
Specific conservation efforts to protect viable populations of D. guildingii are necessary given the dynamics of each TDF fragment and their human threats. For example, increasing environmental vigilance and genetic monitoring in areas that have a high number of exclusive alleles but that are not included as protected areas and that have many anthropogenic pressures such as RCM and TG are critical, which, without proper intervention can be detrimental to the gene pool.
COL and PNNT have lower genetic diversity than other areas without permanent environmental monitoring and demonstrate that even in protected areas, dung beetles can be affected by human disturbance at different levels. Specifically, it would be important to increase the genetic monitoring in COL and surrounding reserves because the high frequency of local extractive pressures in Sucre may affect the population densities of vertebrates such as A. seniculus on which D. guildingii relies as sources of excrement (Ochoa et al. 2011; Martínez-Hernández et al. 2012; Fajardo-Patiño and De la Ossa 2014).
Anthropogenic disturbances in the TDF of the Colombian Caribbean cause changes in the ecological requirements and microhabitat conditions for dung beetles due to the generation of relict areas with variable excrement conditions (Martínez-Hernández et al. 2012). These modifications in habitat connectivity increase the occurrence of small and isolated patches and affect the composition and abundance of dung beetles (Pryke et al. 2013; Pablo-Cea et al. 2020). Future studies are needed to expand the number of fragments sampled for genetic analysis and to evaluate if the landscape matrix or the environment influences patterns of gene flow in D. guildingii, which can inform the implementation of dispersal corridors among isolated fragments and the effect of climate adaptation for the long term viability of populations (Ho and Shapiro 2011).
Data availability
The neutral SNP data used for analyses can be found in Zenodo: https://zenodo.org/records/13306672
References
Andrade E, Guerreiro M, Palácio H, Campos D (2020) Ecohydrology in a Brazilian tropical dry forest: thinned vegetation impact on hydrological functions and ecosystem services. J Hydrol Reg Stud 27:100649. https://doi.org/10.1016/j.ejrh.2019.100649
Andresen E (2003) Effect of forest fragmentation on dung beetle communities and functional consequences for plant regeneration. Ecography 26:87–97. http://www.jstor.org/stable/3683529
Ange C, Peña M, Ferrer Sotelo J (eds) (2020) El Proyecto De Conectividades Socio-Ecosistémicas en Los Montes De María, 2013–2020. Fundación Herencia Ambiental Caribe. Bogotá, Colombia
Arenas J (2012) Una montaña bañada por El Mar. La Sierra Nevada De Santa Marta en El Caribe Colombiano. Rev Bras do Caribe São Luis-MA, Bras. XIII, pp 73–102
Ballesteros-Correa J, Pérez-Torres J (2022) Silvopastoral and conventional management of extensive livestock and the diversity of bats in fragments of tropical dry forest in Córdoba. Colombia Agrofor Syst 96:589–601. https://doi.org/10.1007/s10457-021-00698-4
Banco de Occidente (1999) Sierras y serranías de Colombia: Banco de Occidente, Bogotá. http://www.imeditores.com/banocc/sierras/cap8. html. Accessed 17 February 2023
Barragán F, Moreno C, Escobar F, Halffter G, Navarrete D (2011) Negative impacts of Human Land Use on Dung Beetle Functional Diversity. PLoS ONE 6(3):e17976. https://doi.org/10.1371/journal.pone.0017976
Bermúdez-Wilches L (2012) Capital social y gobernanza en mosaicos de áreas protegidas: El caso del Santuario de Flora y Fauna Los Colorados. Dissertation, Pontificia Universidad Javeriana
Bicknell J, Phelps S, Davies R, Mann D, Struebig M, Davies Z (2014) Dung beetles as indicators for rapid impact assessments: evaluating best practice forestry in the neotropics. Ecol Indic 43:154–161. https://doi.org/10.1016/j.ecolind.2014.02.030
Carrión-Paladines V, Fries A, Muñoz A, Castillo E, García-Ruiz R, Marín-Armijos D (2021) Effects of land-use change on the community structure of the dung beetle (Scarabaeinae) in an altered ecosystem in southern Ecuador. Insects 12. https://doi.org/10.3390/insects12040306
Chicangana G, Kammer A, Alberto C et al (2011) El Posible Origen De La Sismicidad somera que se presenta en la región que corresponde a la Sierra Nevada De Santa Marta, La Serranía De Perijá Y La Península De La Guajira, noreste de Colombia. Cap&Cua 6:1–33
Correa-Cuadros J, Gómez-Cifuentes A, Noriega J (2022) Comparative effect of forest cutting and mammal hunting on dung beetle assemblages in Chocó Biogeographic forests in Colombia. Int J Trop Insect Sci 42:3045–3055. https://doi.org/10.1007/s42690-022-00839-x
Cortés-Ortiz L, Bermingham E, Rico C et al (2003) Molecular systematics and biogeography of the neotropical monkey genus. Alouatta Mol Phylogenet Evol 26:64–81. https://doi.org/10.1016/s1055-7903(02)00308-1
Cox K, McKeown N, Vanden Broeck A, Van Breusegem A, Cammaerts R, Thomaes A (2020) Genetic structure of recently fragmented suburban populations of European stag beetle. Ecol Evol 10:12290–12306. https://doi.org/10.1002/ece3.6858
Cuervo-Díaz A, Barbosa CE, De la Ossa J (1986) Aspectos ecológicos y etológicos de primates con énfasis en Alouatta seniculus (Cebidae), de la región de Colosó, Serranía De San Jacinto (Sucre), costa norte de Colombia. Caldasia 14:709–741
da Silva P, Hernández M (2016) Spatial variation of dung beetle assemblages associated with forest structure in remnants of southern Brazilian Atlantic Forest. Rev Bras Entomol 60:73–81. https://doi.org/10.1016/j.rbe.2015.11.001
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9(8):772. https://doi.org/10.1038/nmeth.2109
De La Ossa J, Galván-Guevara S, Fajardo-Patiño A (2013) Densidad, composición de grupo y distribución vertical de primates simpátricos en un bosque de galería fragmentado, Colosó, Sucre-Colombia. Rev UDCA Act Div Cient 16(1):185–192
Díaz A, Galante E, Favila M (2010) The effect of the landscape matrix on the distribution of dung and carrion beetles in a fragmented tropical rain forest. J Insect Sci 10:1–16. https://doi.org/10.1673/031.010.8101
Dillon M, Lozier J (2019) Adaptation to the abiotic environment in insects: the influence of variability on ecophysiology and evolutionary genomics. Curr Opin Insect Sci 36:131–139. https://doi.org/10.1016/j.cois.2019.09.003
Dogan I, Dogan N (2017) Statistical tests for Neutrality: a Review Statistical tests for Neutrality: Review Nötralite İçin İstatistiksel Testler. Türkiye Klin Biyoistatistik 9(2):167–174. https://doi.org/10.5336/biostatic.2016-53446
Dray S, Dufour A (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22(4):1–20. https://doi.org/10.18637/jss.v022.i04
Drummond A, Bouckaert RR (2015) Bayesian evolutionary analysis with BEAST. Cambridge University Press
Esri Inc (2020) ArcGIS Pro (Version 2.5) Esri Inc. https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview Accessed 17 February 2023
Estrada A (2015) Conservation of Alouatta: social and economic drivers of habitat loss, information vacuum, and mitigating population declines. Howler monkeys: Behavior, ecology, and conservation. Springer Science + Business Media, New York, pp 383–409
Etter A, McAlpine C, Possingham H (2008) Historical patterns and drivers of landscape change in Colombia since 1500: a regionalized spatial approach. Ann Assoc Am Geogr 98:2–23. https://doi.org/10.1080/00045600701733911
Etter A, Andrade A, Saavedra K, Amaya P, Arévalo P (2017) Estado De Los Ecosistemas colombianos: Una aplicación De La metodología De La Lista Roja De Ecosistemas Ver. 2.0. Informe Final. Pontificia Universidad Javeriana, Bogotá, Colombia
Fajardo-Patiño A, De la Ossa J (2014) Prueba De una dieta concentrada y suplementada con elementos naturales para primates neotropicales cautivos. Rev Colomb Cienc Anim - RECIA 6(1):70–85. https://doi.org/10.24188/recia.v6.n1.2014.205
Fremout T, Thomas E, Bocanegra-González KT et al (2021) Forest Ecology and Management Dynamic seed zones to guide climate-smart seed sourcing for tropical dry forest restoration in Colombia. Ecol Manag 490. https://doi.org/10.1016/j.foreco.2021.119127
Frichot E, François O (2015) LEA: an R package for landscape and ecological association studies. Methods Ecol Evol 6:925–929. https://doi.org/10.1111/2041-210X.12382
Galván-Guevara S, Sierra I, Gómez F et al (2009) Biodiversidad en El área de influencia de la estación primates de Colosó, Sucre, Colombia. Rev Colomb Cienc Anim- RECIA 1:98
Galván-Guevara S, Ballut-Dajud G, De La Ossa J (2015) Determinación de la fragmentación del bosque seco del arroyo Pechelín, Montes De María, Caribe, Colombia. Biota Colomb 16:149–157
García H, Corzo G, Isaacs P, Etter A (2014) In: Pizano C, García H (eds) Distribución Y Estado actual de Los remanentes del bioma de Bosque Seco Tropical en Colombia: Insumos para su gestión. El Bosque Seco Tropical en Colombia Instituto Alexander von Humboldt, Bogotá, pp 228–251
Gattepaille L, Jakobsson M, Blum M (2013) Inferring population size changes with sequence and SNP data: lessons from human bottlenecks. Heredity (Edinb) 110:409–419. https://doi.org/10.1038/hdy.2012.120
Gillespie T, Keppel G, Pau S et al (2013) Scaling species richness and endemism of tropical dry forests on oceanic islands. Divers Distrib 1–11. https://doi.org/10.1111/ddi.12036
Gómez-Betancur L, Vilardy S, Torres D (2022) Ecosystem Services as a Promising paradigm to Protect Environmental Rights of Indigenous Peoples in Latin America: the Constitutional Court Landmark decision to protect Arroyo Bruno in Colombia. Environ Manage 69:768–780. https://doi.org/10.1007/s00267-021-01483-w
Gómez-Cifuentes A, Giménez Gómez V, Moreno C, Zurita GA (2018) Tree retention in cattle ranching systems partially preserves dung beetle diversity and functional groups in the semideciduous Atlantic forest: the role of microclimate and soil conditions. Basic Appl Ecol 34:64–74. https://doi.org/10.1016/j.baae.2018.10.002
González-Alvarado A, Vaz-De-Mello F (2014) Taxonomic review of the subgenus Hybomidium Shipp 1897 (Coleoptera: Scarabaeidae: Scarabaeinae: Deltochilum) Annales De La Société. Entomologique De France 50(3–4):431–476. https://doi.org/10.1080/00379271.2014.989178
Goudet J (2005) HIERFSTAT, a package for R to compute and test hierarchical F-statistics. Mol Ecol Notes 5:184–186. https://doi.org/10.1111/j.1471-8278.2004.00828.x
Gunter NL, Weir TA, Slipinksi A et al (2016) If Dung Beetles (Scarabaeidae: Scarabaeinae) Arose in Association with dinosaurs, did they also suffer a Mass Co-extinction at the KPg Boundary? PLoS ONE 11(5):e0153570. https://doi.org/10.1371/journal.pone.0153570
Hammer Ø, Harper D, Ryan P (2001) PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol Electron 4:1–9
Ho S, Shapiro B (2011) Skyline-plot methods for estimating demographic history from nucleotide sequences. Mol Ecol Resour 11:423–434. https://doi.org/10.1111/j.1755-0998.2011.02988.x
Hooftman D, Edwards B, Bullock JM (2016) Reductions in connectivity and habitat quality drive local extinctions in a plant diversity hotspot. Ecography 39:583–592. https://doi.org/10.1111/ecog.01503
Hooghiemstra H, Ran E (1994) Late pliocene-pleistocene high resolution pollen sequence of Colombia: an overview of climatic change. Quat Int 21:63–80. https://doi.org/10.1016/1040-6182(94)90021-3
Howden H, Ritcher P (1952) Biology of Deltochilum gibbosum (Fab.) with a description of the larva. Coleopt Bull: 53–57
IAVH (Instituto de Investigación en Recursos Biológicos Alexander von Humboldt) (2016) Hito 7 3 Propuesta de Monitoreo. Programa de paisajes de conservación. Reporte del Convenio No. CLP-044-G-LO-044
Jiménez B, De la Rosa N, Naranjo D (2018) Plan de Manejo del Santuario de Flora y Fauna Los Colorados. Parques Nacionales Naturales de Colombia. https://www.parquesnacionales.gov.co/portal/wp-content/uploads/2020/10/plan-de-manejo-sff-los-colorados.pdf. Accessed 5 February 2024
Jombart T (2008) Adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24(11):1403–1405. https://doi.org/10.1093/bioinformatics/btn129
Kamvar Z, Tabima J, Grünwald N (2014) Poppr: an R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. https://doi.org/10.7717/peerj.281. PeerJ
Kattan G, Sánchez C, Vélez C, Ramírez L, Celis M (2019) Beta diversity and knowledge gaps of Colombia’s dry forests: implications for their conservation. Caldasia 41:5–11. https://doi.org/10.15446/caldasia.v41n1.76229
Leroy G, Carroll E, Bruford M et al (2017) Next-generation metrics for monitoring genetic erosion within populations of conservation concern. Evol Appl 11:1–18. https://doi.org/10.1111/eva.12564
Li H (2012) seqtk Toolkit for processing sequences in FASTA/Q formats. GitHub: 767, 69. https://github.com/lh3/seqtk. Accessed 29 January 2022
Li H, Handsaker B, Wysoker A, Fennell T et al (2009) The sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. https://doi.org/10.1093/bioinformatics/btp352
Linck E, Celi J, Sheldon K (2020) Panmixia across elevation in thermally sensitive Andean dung beetles. Ecol Evol 10:4143–4155. https://doi.org/10.1002/ece3.6185
López-O J, Avendaño JE, Gutiérrez-Pinto N, Cuervo A (2014) The birds of the Serranía De Perijá: the northernmost avifauna of the Andes. Ornitol Colomb 14:62–93
Lozano-Botache L, Gómez Aguilar F, Valderrama Chaves S (2011) Estado De fragmentación De Los bosques naturales en El norte del departamento del Tolima-Colombia. Tumbaga 1:125–140
Luu K, Bazin E, Blum MGB (2016) Pcadapt: an R package to perform genome scans for selection based on principal component analysis. Mol Ecol Resour 17:67–77. https://doi.org/10.1111/1755-0998.12592
Macherey-Nagel (2017) Genomic DNA from Tissue, User manual, NucleoSpin®Tissue. https://www.bioke.com/blobs/manuals/MN/UM_gDNATissue_2017.pdf Accesed 17 February 2023
Martínez-Hernández N, Muñoz G, Quintero K, Méndez J (2012) Escarabajos coprófagos (Coleoptera: Scarabaeinae) asociados a excrementos de mamíferos en un fragmento de bosque seco tropical en El Departamento Del Atlántico, Colombia. Ecol Austral 22:203–210
Martínez-Hernández N, Rangel-Acosta J et al (2022) ¡El tamaño sí importa! Incidencia Del tamaño del cebo en la captura de escarabajos coprófagos en El bosque seco tropical. Rev Biol Trop 70:1–19. https://doi.org/10.15517/rev.biol.trop.v70i1.46712
Mastretta-Yanes A, Arrigo N, Alvarez N et al (2015) Restriction site-associated DNA sequencing, genotyping error estimation and de novo assembly optimization for population genetic inference. Mol Ecol Resour 15:28–41. https://doi.org/10.1111/1755-0998.12291
Matos-Maraví P, Duarte Ritter C, Barnes CJ et al (2019) Biodiversity seen through the perspective of insects: 10 simple rules on methodological choices and experimental design for genomic studies. PeerJ 7:e6727. https://doi.org/10.7717/peerj.6727
Maxwell S, Cazalis V, Dudley N et al (2020) Area-based conservation in the twenty-first century. Nature 586:217–227. https://doi.org/10.1038/s41586-020-2773-z
Medina-Hernández M, Niero MM, Schumacher F, Wuerges M (2020) Feeding and reproductive behavior of the dung beetle Canthon rutilans cyanescens (Coleoptera: Scarabaeinae). Rev Bras Entomol 64:1–10. https://doi.org/10.1590/1806-9665-RBENT-2019-0007
Mercado-Gómez J, Herazo-Vitola F, Morales-Puentes M (2019) Phytogeography and Floristic Affinities of Woody Plants in Los Montes De María, a Tropical Dry Forest Fragment in the Colombian caribbean. Bot Rev 1–19. https://doi.org/10.1007/s12229-019-09212-z
Narum S, Buerkle C, Davey J, Miller M, Hohenlohe P (2013) Genotyping-by-sequencing in ecological and conservation genomics. Mol Ecol 22:2841–2847. https://doi.org/10.1111/mec.12350
Noriega J, Zapata-Prisco C, García H, Hernández E et al (2020) Does ecotourism impact biodiversity? An assessment using dung beetles (Coleoptera: Scarabaeinae) as bioindicators in a tropical dry forest natural park. Ecol Indic 117:106580. https://doi.org/10.1016/j.ecolind.2020.106580
Noriega JA, March-Salas M, Castillo S et al (2021) Human perturbations reduce dung beetle diversity and dung removal ecosystem function. Biotropica 53:1–14. https://doi.org/10.1111/btp.12953
Ochoa D, Martínez E, De la Ossa J (2011) Densidad poblacional y estructura de grupo de Alouatta seniculus (Primates: Atelidae) en Colosó. Sucre Rev UDCA Actual Divulg Científica 14:101–108. https://doi.org/10.31910/rudca.v14.n2.2011.780
Pablo-Cea J, Velado-Cano MA, Noriega JA (2020) A first step to evaluate the impact of ecotourism on biodiversity in El Salvador: a case study using dung beetles in a National Park. J Ecotourism 20:51–69. https://doi.org/10.1080/14724049.2020.1772798
Padilla-Gil D, Halffter G (2007) Biogeography of the areas and Canthonini (Coleoptera: Scarabaeidae) of dry tropical forest in Mesoamerica and Colombia. Acta Zoológica Mex 23:73–108. https://doi.org/10.21829/azm.2007.231559
Paradis E (2010) Pegas: an R package for population genetics with an integrated-modular approach. Bioinformatics 26:419–420. https://doi.org/10.1093/bioinformatics/btp696
Pessôa M, Izzo T, Vaz-de-Mello FZ (2017) Assemblage and functional categorization of dung beetles (Coleoptera: Scarabaeinae) from the Pantanal. PeerJ 2017:1–19. https://doi.org/10.7717/peerj.3978
Peterson M, Denno R (1998) The influence of dispersal and diet breadth on patterns of genetic isolation by distance in phytophagous insects. Am Nat 152:428–446. https://doi.org/10.1086/286180
Peterson B, Weber J, Kay EH et al (2012) Double Digest RADseq: an Inexpensive Method for De Novo SNP Discovery and genotyping in Model and Non-model Species. PLoS ONE 7(5):e37135. https://doi.org/10.1371/journal.pone.0037135
Pizano C, García H (2014) El Bosque Seco Tropical en Colombia. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt. Bogotá, Colombia
Pryke J, Roets F, Samways M (2013) Importance of habitat heterogeneity in remnant patches for conserving dung beetles. Biodivers Conserv 22:2857–2873. https://doi.org/10.1007/s10531-013-0559-4
Quijas S, Romero-Duque L, Trilleras J et al (2019) Linking biodiversity, ecosystem services, and beneficiaries of tropical dry forests of Latin America: review and new perspectives. Ecosyst Serv 36. https://doi.org/10.1016/j.ecoser.2019.100909
Rambaut A, Drummond A, Xie D et al (2018) Posterior summarization in bayesian phylogenetics using Tracer 1.7. Syst Biol 67:901–904. https://doi.org/10.1093/sysbio/syy032
Rangel-Acosta J, Martínez-Hernández N (2017) Comparación De Los ensamblajes de escarabajos copronecrófagos (Scarabaeidae: Scarabaeinae) entre fragmentos de bosque seco tropical Y La Matriz Adyacente en El Departamento Del Atlántico-Colombia. Rev Mex Biodivers 88:389–401. https://doi.org/10.1016/j.rmb.2017.03.012
Riddle B, Dawson M, Hadly E et al (2008) The role of molecular genetics in sculpting the future of integrative biogeography. Prog Phys Geogr 32:173–202. https://doi.org/10.1177/0309133308093822
Rivera-Colón A, Catchen J (2022) Population Genomics Analysis with RAD, reprised: stacks 2. In: Verde C, Giordano D (eds) Marine Genomics. Methods in Molecular Biology, vol 2498. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2313-8_7
Rochette N, Catchen J (2017) Deriving genotypes from RAD-seq short-read data using Stacks. Nat Protoc 12:2640–2659. https://doi.org/10.1038/nprot.2017.123
Rochette N, Rivera-Colón A, Catchen J (2019) Stacks 2: Analytical methods for paired-end sequencing improve RADseq-based population genomics. Mol Ecol 28:4737–4754. https://doi.org/10.1111/mec.15253
Rodríguez G, Londoño A (2002) Mapa geológico del departamento de La Guajira. Geología, recursos minerales y amenazas potenciales, Escala, 1(250.000). Ministerio De Minas y Energía. Instituto De Investigación E Información Geocientífica, Minero-Ambiental y Nuclear, Medellín. https://www.researchgate.net/publication/270882602_MAPA_GEOLOGICO_DEL_DEPARTAMENTO_DE_LA_GUAJIRA Accessed 17 February 2023
Romero-Duque L, Rosero-Toro JH, Fernández-Lucero M et al (2019) Trees and shrubs of the tropical dry forest of the Magdalena river upper watershed (Colombia). Biodivers Data J 7:1–21. https://doi.org/10.3897/BDJ.7.e36191
Rondón S, Ortiz M, León C et al (2017) Seasonality, richness and prevalence of intestinal parasites of three neotropical primates (Alouatta seniculus, Ateles hybridus and Cebus versicolor) in a fragmented forest in Colombia. Int J Parasitol Parasites Wildl 6:202–208. https://doi.org/10.1016/j.ijppaw.2017.07.006
Sampedro-Marín A, Aguas-Montes K, Jiménez-Pineda D (2011) Estado De conservación y caracterización del hábitat de Bradypus variegatus Schinz 1825 (Mammalia: Xenarthra) Durante La época Seca, en El Departamento De Sucre, Colombia. Rev Colomb Cienc Anim - RECIA 3:15–34. https://doi.org/10.24188/recia.v3.n1.2011.247
Schierenbeck K (2017) Population-level genetic variation and climate change in a biodiversity hotspot. Ann Bot 119:215–228. https://doi.org/10.1093/aob/mcw214
Sedlazeck F, Rescheneder P, Von Haeseler A (2013) NextGenMap: fast and accurate read mapping in highly polymorphic genomes. Bioinformatics 29:2790–2791. https://doi.org/10.1093/bioinformatics/btt468
Slatkin M (1993) Isolation by distance in equilibrium and non-equilibrium populations. Evolution 47:264–279. https://doi.org/10.1111/j.1558-5646.1993.tb01215.x
Taylor C, Barker N, Barber-James HM et al (2020) Habitat requirements affect genetic variation in three species of mayfly (Ephemeroptera, baetidae) from South Africa. Zookeys: 1–24. https://doi.org/10.3897/zookeys.936.38587
Tonelli M, Verdú J, Morelli F, Zunino M (2020) Dung beetles: functional identity, not functional diversity, accounts for ecological process disruption caused by the use of veterinary medical products. J Insect Conserv 24:643–654. https://doi.org/10.1007/s10841-020-00240-4
Torres-Romero F, Acosta-Prado J (2022) Knowledge Management practices and Ecological Restoration of the Tropical Dry Forest in Colombia. Land 11(3):330–331. https://doi.org/10.3390/land11030330
Westwood J (1835) Beetles. In: Partington CF (ed) British cyclopaedia of natural history: combining a scientific classification of animals, plants, and minerals. Orr & Smith, London, pp 372.
Yang H, You C, Tsui C, Tembrock L, Wu Z, Yang D (2021) Phylogeny and biogeography of the Japanese rhinoceros’ beetle, Trypoxylus dichotomus (Coleoptera: Scarabaeidae) based on SNP markers. Ecol Evol 153–173. https://doi.org/10.1002/ece3.6982
Zhang Y, Song W, Chen J, Cao L et al (2021) Genome-wide characterization of microsatellites and development of polymorphic markers shared between two weevils of Eucryptorrhynchus (Coleoptera: Curculionidae). Zool Syst 46:273–280. https://doi.org/10.1016/j.ejbt.2020.01.00
Acknowledgements
Thanks to Universidad del Atlántico, Ministry of Science and Technology of Colombia, National Natural Parks of Colombia, Corporación Autónoma Regional de Sucre, and Autoridad Nacional de Licencias Ambientales (ANLA). We thanks to PhD Rafik Neme and his team at Universidad del Norte, to Ricardo Ortega an expert in geographic information systems, to Salvador Guzmán and Roberto Pestana for their advice on bioinformatics and Miguel A. Leon-Tapia and Carlos Prieto for their comments to early versions of the work. Also, we thanks to the IDEAWILD Foundation for the support with technological equipment and the Australian Genomic Research Facility (AGRF).
Funding
Partial financial support was received for the call 727 COLCIENCIAS 2015 Colombia for doctoral grant and to the first internal call to support the development of degree research projects 2018 by the Universidad del Atlántico to NMH.
Author information
Authors and Affiliations
Contributions
Planned and conceived the study: NMH, MGM. Conducted field work: NMH, MGM. Conducted laboratory procedures: MGM. Data Analysis: MGM, YR. Manuscript writing: MGM, YR. All authors revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
González-Molina, M., Martínez-Hernández, N. & Rico, Y. Genetic structure and demographic history of the dung beetle Deltochilum guildingii (Scarabaeinae): implications for conservation of the Tropical Dry Forest in the Colombian caribbean. J Insect Conserv (2024). https://doi.org/10.1007/s10841-024-00618-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10841-024-00618-8