Abstract
Landscape heterogeneity affects the spatial distribution of species. This makes it an important consideration for conservation planning, particularly when designing sustainable production landscapes. We determine whether conserving landscape elements within a transformed landscape is adequate for conserving dung beetle biodiversity. Dung beetles are excellent indicators for landscape biodiversity studies as they are ecologically sensitive. Here we measure dung beetle alpha-diversity, as well as beta-diversity within landscape elements and across different landscape elements. In doing so, we assess the value of landscape elements, as well as variation within landscape elements, in determining the spatial distribution of dung beetles across a production landscape. The study was conducted in the commercial timber production area of the KwaZulu-Natal Midlands, South Africa. In this system, the different landscape elements are a mosaic of natural indigenous forests, grasslands and alien pine plantation blocks. Our results show that the only response for dung beetle alpha-diversity was higher species richness in grasslands and pine blocks compared to natural forests. The highest beta-diversity for a landscape element was the grassland, for elevational category was low elevational areas and grassland type was the Midlands Mistbelt Grassland. The compositional diversity (beta-diversity between elements) was significantly different for all pairwise variations between landscape elements, the elevational categories and grassland types. Natural forests embedded in the two different grassland types had greater differences in compositional diversity than those embedded in natural (grassland) or transformed (pine blocks) matrices. This highlights the need to conserve a range of similar remnant patches of natural vegetation regionally, in addition to conserving broad landscape elements (i.e. grasslands or natural forests) as conservation targets. Furthermore, our results are encouraging for the potential benefits from the ecosystem service provided by dung beetles across the whole landscape, even in the transformed elements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patches which are similar to each other in terms of their structure and vegetation components are often grouped together as “landscape elements” in landscape ecology and conservation planning (Forman 1995). How species respond to these various landscape elements affects spatial dispersion of species, which in turn, affects local species richness and species turnover across entire landscapes (Romero-Alcaraz and Ávila 2000; Tscharntke et al. 2002; Fischer et al. 2004). Understanding how species use these different landscape elements enables conservation practitioners to maximise biodiversity conservation by identifying significant features to conserve (Svoray et al. 2005). However, landscape elements alone do not necessarily predetermine where specific species occur, with other variables such as landscape structure (Cook 2002; Louzada et al. 2010), patch matrix (Muriel and Kattan 2009), soil type (Li et al. 2007), also playing an important role. Across agroforestry landscapes this is important, as transformed areas often isolate natural patches (Kirkman and Pott 2002; Samways 2007).
Although ecologically robust species may survive well in transformed areas (Pineda et al. 2005; Schlaepfer et al. 2011), it is the most environmentally sensitive species (specialist species) which are often of greatest conservation concern (Rodewald 2012). This means for conservation planning, significant natural patches must be left for these sensitive species. However, remnant natural patches are often small in production landscapes, and do not have the required conditions for many species (Saunders et al. 1991). To overcome this short-coming and to improve connectivity across the landscape, ecological networks (ENs) have been introduced to reduce isolation of the patches (Beier and Noss 1998; Yu et al. 2006; Jongman et al. 2011; Samways et al. 2010).
Dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae) are an excellent indicator taxon for landscape diversity studies, as they are ecologically sensitive and can compositionally respond to slight changes in the local environment (Nichols et al. 2008). This has been shown in many different areas of the world from the rain forests of Borneo (Davis et al. 2001), to the scrublands of the Mediterranean (Numa et al. 2009) and even in the Kalahari desert (Davis et al. 2008b). Dung beetles have high alpha-diversity rates along ecological gradients (Davis et al. 1999; Spector and Ayzama 2003; Louzada et al. 2010; Filgueiras et al. 2011). Dung beetles are also sensitive to habitat change (Nielsen 2007; Gardner et al. 2007) and even to subtle changes in land use (Almeida et al. 2011). Fragmentation and isolation are also important determinates of dung beetle species local distribution (Klein 1989; Andresen 2003; Nichols et al. 2007; Escobar et al. 2008). Numa et al. (2012) found that Spanish dung beetles are responsive to many environmental variables, although spatial configuration and habitat connectivity had the greatest influence on their composition and abundance.
The natural remnant ENs within the South African timber industry are ideal for testing conservation concepts within transformed landscapes, as they are extensive (500,000 ha of natural grassland and forest among 1.8 million ha of commercial timber plantations) (Kirkman and Pott 2002; Samways 2007). Plantation forestry using alien trees is a serious risk to local biodiversity, as blocks of exotic trees contain impoverished indigenous biodiversity (Pryke and Samways 2009; Bremer and Farley 2010). In turn, there are indigenous forest patches within the grassland/plantation system, although they are naturally small and isolated (Kotze and Lawes 2007). Certainly, ENs in the South African timber production landscape largely conserve grassland arthropod diversity (Pryke and Samways 2012a). However, one component of this diversity, dung beetles, is poorly known. However, they are known to be more abundant on the edge of natural forest and exotic plantations (Pryke and Samways 2012b), possibly an artefact of mammal species resting in the shade provided by these wooded areas during the heat of the day.
The measurement of alpha-diversity (measures of species richness) and beta-diversity (species turnover) in different landscape elements, as well as between landscape elements, is essential to our understanding of which landscape elements are required to best conserve biodiversity (Kessler et al. 2009). Here we aim to test the concept of whether conserving discrete landscape elements within a transformed landscape satisfactorily conserves dung beetle biodiversity. We achieve this by assessing the alpha-diversity and beta-diversity in and between landscape elements, as well as assessing how these diversity indices change in landscape elements along elevational and vegetational gradients. We expect to see changes in dung beetle diversity between the landscape elements (specifically the natural forests, pine forests and grasslands), although if there are major changes within these landscape elements across various environmental gradients, then we need to conserve a range of these individual landscapes elements and not just representatives of the landscape elements themselves. The matrix can also be a major influence on the natural forest patch in which it is embedded. Here we also determine whether the natural matrix (grasslands) in which indigenous forest patches are embedded, is necessarily better than the transformed matrix (pine blocks) for dung beetle diversity within the patches. We discuss the consequences of our results for both local and global conservation actions.
Methods
Study area and design
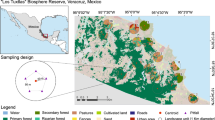
This study was undertaken in the commercial timber plantations of the KwaZulu-Natal Midlands, South Africa. This provided us with a system which has natural indigenous forests and grasslands, as well as transformed habitat (commercial pine plantation blocks) close to each other. This study was spatially extensive, with the maximum distance between plantations over 160 km and the minimum ca. 32 km (Fig. 1). The grassland areas consisted of the threatened Midlands Mistbelt Grassland and Drakensberg Foothill Moist Grassland types, with small patches of Southern Mistbelt Forest dispersed within the grassland matrix (Fig. 2a–c) (Mucina and Rutherford 2006). Although structurally similar, these grassland types differ in their grass species composition and have different soil types. Both grassland types grow on soils that have high clay content, although Midlands Mistbelt Grassland soils have poor water infiltration, resulting in a wet soil dominated by shale and with some sandstone, while the Drakensberg Foothill Moist Grasslands are on well drained soils, which are drier and dominated by mudstone and sandstone (Mucina and Rutherford 2006). Nevertheless, both soil types occur in areas with similar climatic conditions, although the Midlands Mistbelt Grasslands receive additional precipitation from summer mists (Mucina and Rutherford 2006). The Weza (30°37S; 29°41E) and Maybole (29°44S; 30°15E) timber plantations are in Midlands Mistbelt Grasslands while the Gilboa (29°16S; 30°18E) and Good Hope (29°40S; 29°58E) timber plantations are in both Midlands Mistbelt Grasslands and Drakensberg Foothill Moist Grassland and range in elevation for all sites is ca 1,000–1,700 m a.s.l. (Supplementary Table 1; Fig. 1).
Site map showing the relative position of each of the four plantations sampled. Top map shows the vegetation types sampled from Midlands Mistbelt Grassland (light grey), Drakensberg Foothill Moist Grassland (dark grey) and the Southern Mistbelt Forest (black). The bottom map shows the position of the 50 paired sites; black shows the location of the natural forests, grey shows pine blocks and clear shows grasslands; the G-NF indicates grassland paired with a natural forest and P-NF indicates pine plantation block paired with natural forest
a A panoramic view of the area around the Gilboa commercial forestry plantation with the landscape matrix of pine blocks, natural forests and grasslands in summer, b a grassland corridor in the Weza commercial forestry plantation during winter, c inside a natural forest and d the trapping technique with a wire harp holding the dung bait above a buried bucket
Sampling sites were paired between an indigenous forest patch (n = 50 paired sites) and an adjacent grassland (n = 25 paired) or pine block (n = 25 paired sites) (Supplementary Table 1). So in total we had 50 paired sites, or a 100 separate sites with 25 sites in natural forest surrounded by grassland, 25 in natural forest surrounded by pine blocks, 25 in grassland, 25 in pine blocks (Supplementary Table 1). Two baited pitfall traps were placed at each site, giving a total of 200 baited pitfall traps laid out. The two traps per site were >15 m apart and their catches were pooled for statistical analyses. The pitfall traps were placed greater than 30 m from the boundary edges for grasslands and pine blocks (Pryke and Samways 2012b). Due to the smaller sizes of the indigenous forests, traps were placed at least 10 m from their boundary edges. Field work was carried out in a 2 week intensive sampling regime in the summer of 2011 (February 2011) to minimise seasonal effects on sampling (Edwards 1991; de Andrade et al. 2011).
Each pitfall trap consisted of a 2.5 L plastic bucket that was buried so that its rim was flush with the ground. Each trap had a top cover with a hole in the top to allow the beetles to enter the trap, while preventing them from escaping (Fig. 2d). On a wire harp above each trap was a small cloth bag containing ca. 100 ml pig dung and chicken liver mix (8:1 ratio; Fig. 2d). Pig dung has been shown to capture most species of dung beetles present (Davis 1994) and the chicken liver to capture specialist carrion feeders. As we have targeted generalist feeders, many herbivore dung and fungi specialists would have not responded to these baits and so are missing from our results. Each bait bag was made using the same batch of pig dung collected on a single day. All bait bags were frozen and only defrosted on the day of use. This allowed us to standardise the dung and carrion volatiles. Traps were left open for 2 days, after which dung beetle specimens were collected, preserved and identified to genus level (Davis et al. 2008a), afterwards they were assigned to morphospecies. Voucher specimens are in the Entomology Museum, Department of Conservation Ecology and Entomology, Stellenbosch University.
Statistical analysis
Beta-diversity is generally poorly defined (Tuomisto 2010; Jurasinski and Koch 2011), so we have chosen to use two beta-diversity terms to differentiate between beta-diversity within a factor and beta-diversity between factors. Thus, we have defined the diversity classifications of: (1) alpha-diversity (α): species richness at a site, (2) beta-diversity 1 (β1): species turnover among similar sites (e.g. among forest patches; Anderson 2006) and (3) beta-diversity 2 (β2): assemblage compositional changes between factors (e.g. between grasslands and forests). These three measures of diversity were compared for the four landscape elements (grassland; pine block; natural forest in a grassland matrix; natural forest in a pine matrix), the three elevational categories (<1,199; 1,200–1,499; >1,500) and the two grassland vegetation types (Midlands Mistbelt Grasslands; Drakensberg Foothill Moist Grassland) were treated as fixed effects, while the four plantations sampled was treated as a random effect.
To test the influence of each of the factors had on alpha-diversity Generalized Linear Mixed Models (GLMM) were calculated. These GLMMs were performed in R (R Development Core Team 2007) using the lme4 package (Bates and Sarkar 2007). The overall model incorporated all the fixed effects as well as interactions between landscape elements and elevation and landscape elements and grassland type. This model followed the formula of: alpha-diversity ~ landscape elements + elevation + grassland type + landscape element*elevation + landscape element*grassland type + (1/plantation (randomised)). These data were non-normal, although fitted a Poisson curve when a Likelihood Ratio Test was performed, thus a GLMM fit by a Laplace approximation and with a Poisson distribution was used (Bolker et al. 2008). As these analyses showed no overdispersion of variances compared to the models (Pearson residuals = 1.17), a χ2 statistic and p-value were calculated (Bolker et al. 2008). Posthoc analyses were performed on significant factors using a Tukey posthoc test in the R package multcomp (Hothorn et al. 2008).
PERMDISP analysis tests the homogeneity of multivariate dispersion to determine the variability in species composition within a predetermined grouping of sites and thus can be used as a measure of beta-diversity (β1; Anderson 2006). These analyses were performed in PRIMER 6 (PRIMER-E 2008) using the PERMDISP software with 9,999 permutations (Anderson 2006). This analysis firstly determines the distance of samples to the centroid of each predefined group. For example, the software plots all the grassland sites and then determines the geometric centre (i.e. the centroid) for the shape that the grasslands plot creates (Anderson 2006). Then the software calculates distances between the centroid and each of the sites. This then allows the program to calculate the mean distances and perform ANOVAs to determine F- and p-values between various groupings of sites (e.g. between the grasslands and pine blocks) (Anderson 2006). It also allows for pairwise testing (PRIMER-E 2008). Thus, PERMDISP measures beta-diversity by measuring the dispersal of each independent group. These analyses were performed for the four landscape elements, the three elevational categories and the two grassland vegetation types. Analyses were performed using Jaccard similarity measures, as these data needed to be transformed to presence/absence data (Anderson 2006).
To determine the differences in beta-diversity between elements (β2) we determined the assemblage composition similarity between sampling units using Permutational multivariate analyses of variance (PERMANOVA) in PRIMER 6 (PRIMER-E 2008). These were performed to determine F- and p-values, as well as pairwise difference within tests, using 9,999 permutations to assess changes between the four landscape elements, the three elevational categories and the two grassland vegetation types. Analyses were performed using Bray-Curtis similarity measures assessing the specific species and their abundances, with these data square-root transformed to reduce the weight of common species (Anderson 2001). The landscape elements data were further explored using canonical analysis of principal coordinates (CAP) (Anderson and Willis 2003). CAP analysis is effective in delineating particular gradients of interest within a multivariate dataset, despite the presence of other potentially important factors which were not measured (Anderson 2008). The non-parametric species estimators of Chao2 and Jacknife2 (Hortal et al. 2006) were calculated using PRIMER 6 (PRIMER-E 2008) to determine how close sampling got to the estimated species richness.
Results
Overall, 4,165 individuals from 64 species were sampled. Chao2 estimated alpha-diversity of 73.4 (±6.8) and Jacknife2 estimated 80.9 species the species accumulation curve for these data nearly flatten. Dung beetle alpha-diversity was significantly higher in grassland and pine blocks than in natural forest (Table 1; Fig. 3). Species estimates were also higher in the grasslands (Chao2 = 69.0 ± 10.6; Jacknife2 = 76.5) than natural forests (Chao2 = 54.0 ± 24.2; Jacknife2 = 46.8) or pine blocks (Chao2 = 35.8 ± 2.07; Jacknife2 = 36.26). Dung beetle alpha-diversity was similar in natural forests surrounded by pine and those surrounded by grasslands (Table 1; Fig. 3). There were no significant differences in alpha-diversity of the three landscape elements (grassland, natural forest and pine blocks) from different elevations or grassland types (Table 1).
Between landscape element, elevation and grassland type comparisons in alpha-diversity (top) and beta-diversity within factors (β1) (bottom). Mean (±1 SE); different letters above bars represent significantly different means (5 % level). Forest-P forest surrounded by pine, Forest-G forest surrounded by grassland, Mistbelt Midlands Mistbelt Grassland, Drakens Drakensberg Foothill Moist Grassland
Grasslands also had significantly higher β1 compared to natural forests and pine blocks (Table 1; Fig. 3). This was also true for low elevation sites compared to other elevational categories and for the Midlands Mistbelt Grassland compared to the Drakensberg Foothill Moist Grassland (Table 1; Fig. 3). There were no differences in the β1 between natural forest surrounded by pine compared to those surrounded by grasslands (Table 1; Figs. 3, 5). Pairwise comparisons using PERMDISP analyses between landscape elements embedded in the elevational categories, showed that low and medium grasslands had significantly higher levels of β1 than any other landscape element at any other elevation (Table 1). The landscape element with the highest β1 (for both grassland types) was the grasslands. All elements embedded in the Midlands Mistbelt Grasslands had higher β1 compared to the same elements in Drakensberg Foothill Moist Grassland (Fig. 4). Additionally, elevation played a significant role in the β1 within grasslands (Table 1).
Between landscape element within grassland type comparisons in alpha-diversity (top) and beta-diversity within landscape element (β1) (bottom). Mean (±1 SE); different letters above bars represent significantly different means (5 % level). Forest-P forest surrounded by pine, Forest-G forest surrounded by grassland, Mistbelt Midlands Mistbelt Grassland, Drakens Drakensberg Foothill Moist Grassland
When the β2 of the landscape elements were compared to each other, all comparisons were significantly different with the exception of the natural forest surrounded by pines compared to natural forest surrounded by grassland (Table 1; Fig. 5). When the landscape elements were compared to each other for each elevation category the two forest elements (those surrounded by pine and those surrounded by grassland) were always similar, while grasslands were consistently different to all other landscape elements (Table 2; Fig. 5). Low elevation pine blocks were significantly different to the low elevation natural forests and the medium level pine block were only significantly different to natural forest surrounded by grasslands (Table 2). Pairwise comparisons between landscape elements embedded in the two different grassland types showed the two natural forests and the grassland patches had significantly different β2 between the two grassland types (Table 3). Natural forests surrounded by pines were similar in dung beetle β2 to those surrounded by grasslands (Table 3).
From within the grasslands, we sampled 22 species that were unique to that habitat type (Fig. 6), with Onthophagus sp.1 having 19 individuals sampled, Aphodinae sp.1 with 15 individuals, Catharsius sp.2 with 12 and Epirinus validus and Drepanocerus sp.3 with 5 individuals (supplementary Table 2). There were five species that were only found in the natural forests (Fig. 6), these were: Nebulasilvius cf. insularis (38 individuals), Aphodinae sp.1 (3 individuals), Caccobius sp.8 and sp.7 (3 and 1 individuals respectively) and Trox sp.1 (2 individuals) (supplementary Table 2). Onthophagus sp. 5 and 6 were only found in the pine blocks although these were found at abundances of 1 and 3 respectively (Fig. 6; supplementary Table 2). The pine blocks were more related to the natural forests (Jaccard’s index = 0.67) than to the grasslands (index = 0.36) (Fig. 6).
Discussion
Our results show that dung beetle beta-diversity within landscape elements did not respond to the differences in these elements alone. In fact, there was a great deal of change due to the grassland type whether we sampled in natural indigenous forest or grassland. This is likely to be in response to differences in variables such as soil types, elevation and moisture levels between these vegetation types, rather than differences in grass species per se (Davis and Scholtz 2004). Dung beetle assemblage composition seemed to be highly sensitive to small changes in these habitat variables. This is emphasised by natural forests showing a greater change in beta-diversity (both within the element and between elements) compared to those embedded in either of the two grassland types compared to differences between natural forest surrounded by either grassland or pine blocks. This is despite all the natural forests sampled here being the same natural forest type (Southern Mistbelt Forest). As the natural indigenous forests are generally on the same soil types, and have the same elevation and precipitation as the surrounding grasslands, it appears that it is the soil characteristics, elevation and moisture levels which drive the differences in grassland type, and in turn, are also important for dung beetle assemblage structure.
There were also great changes in dung beetle beta-diversity in and between the landscape elements. The greatest of these differences was the higher beta-diversity within the grasslands compared to the other landscape elements. Dung beetle alpha-diversity was highest in the grasslands and pine blocks, while lowest in the natural indigenous forests. These blocks of exotic pines thus appear to house unique assemblages not found in the grassland and natural forest. This uniqueness in assemblage structure appears to be a mixture of grassland and natural forest species. The pine blocks also showed little variation between those embedded in the different grassland types and between different elevations, so it appears that this assemblage, although unique in the landscape, does not vary much across the region.
The pine plantations were more similar to the natural forests than to the grasslands. These two natural landscape elements (natural forest and grasslands) also had species that were unique to them, and which were sampled in large numbers. In comparison, the pine blocks only had two unique species (both Onthophagus species), which were both sampled in very low numbers. In fact, their occurrence in pine plantations may only be due to chance sampling events. It appears that the unique species assemblage that we found in the pine plantation is not due to alien dung beetle species moving in, but simply a mix of the grassland and natural forest generalists, with the natural forest adding the highest component in the mix. This highlights the biodiversity value of these natural elements in these production landscapes. Also, it indicates that the pine plantation may in fact be aiding some of the forest species in dispersal between naturally fragmented patches. This increase in dispersal seems to have been restricted to some of the species only, as five of the species here, despite being captured in high numbers, were never sampled outside the natural forests.
Numa et al. (2012) showed that dung beetle composition and abundance in Spain responded to many variables, although the greatest influence was from spatial configuration and habitat connectivity. In this study, landscape design variables (particular landscape elements) were highly influential, although the strongest influences were the background environmental variables (grassland type and elevation). The landscape elements are most likely landscape surrogates for other environmental variables (Billeter et al. 2007; Crous et al. 2013).
At the local scale, these results suggest that pine blocks are not necessarily detrimental to dung beetle species assemblages, provided the natural landscape elements are maintained in the landscape. Nevertheless, pine blocks do change the compositional diversity, as has been reported for other taxa (Kotze and Samways 2001; Pryke and Samways 2009, 2012b). Our results on alpha- and beta-diversity highlight the need to locally conserve areas with a range of various soil types, also across elevational and moisture gradients, and not to only focus on conserving landscape elements, or on the individual habitats of single species scale. For example, the natural forests were of the same forest type across the entire study area, yet showed differences in beta-diversity within and between patches embedded in different grassland types. This means that we need to conserve not just patches of natural forest across the KwaZulu-Natal Midlands but also a variety of patches embedded in various grassland types (as a surrogate for different soil types/moisture and elevation gradients). There appears to be few differences in the alpha- and beta-diversity in and between natural forest patches surrounded by natural grasslands compared to those surrounded by the transformed pine blocks. This would imply, as for birds (Wethered and Lawes 2003), small afromontane forests are effectively conserved even when surrounded by timber plantation. However, caution is required, as we have only assessed one taxonomic group here and other taxa may respond differently to the surrounding matrix.
Like most arthropod taxa (Bremer and Farley 2010; Pryke and Samways 2012b), these dung beetles responded strongly to plantation forestry. In fact many species seemed to respond positively to these transformed ecosystems, unlike most taxa. As in other studies (Nichols et al. 2007), this suggests that dung beetles may be good indicators of habitat change, particularly in this region. Furthermore, dung beetles showed strong responses to factors such as soil type, elevation and moisture gradients, highlighting the importance of these abiotic factors for conservation planning (Nichols et al. 2007). These factors are more than likely driving the changes in alpha- and beta-diversity seen here and should therefore be included in ecological studies along with multiple taxa (Kotze and Samways 1999; Tropek et al. 2008; Pryke and Samways 2010). A further interesting result here was the difference between the measures of α-diversity (species richness), β1-diversity (species turnover within an element) and β2-diversity (assemblage compositional changed between elements). The most important results from this study would have been omitted had we not included measures of β1-diversity, showing that a change in species composition is a better measure than just using species richness. This is probably due to subtle changes in the microhabitat creating a more complex matrix for the dung beetles to live in, creating a greater species turnover in the natural area. Results here show that it is imperative to include factors that may lead to changes in alpha-diversity within landscape elements in biodiversity conservation studies (Kessler et al. 2009).
This study underscores the importance of conserving as much habitat heterogeneity as possible. The conservation of habitat heterogeneity does not necessarily only apply to different landscape elements but can also relate to other variables, such as elevation, soil and moisture gradients (Romero-Alcaraz and Ávila 2000; Franklin et al. 2005). These results also indicate the need for conserving not just landscape elements but also variation within the landscape elements. In other words, and in terms of conservation planning, it cannot be assumed that all patches of the same landscape element are the same. This means that as many patches of a landscape element as possible should be included in the conservation network. Complicating this for managers is that at least two-thirds of these plantations are needed for timber production and so the correct selection of the remaining third for conservation is critical to ensure biodiversity is maintained across these landscapes (Samways et al. 2010). The results here, showing that a variety of grasslands and forests should be conserved, suggest that the conservation decisions need to be made at the area-wide level to ensure that a wide variety of natural elements are conserved across areas, rather than trying to conserve all these elements at a single plantation location.
Allowing ENs of connected grassland and natural forest patches within the timber production landscape seems to alleviate to some degree the effects of land transformation due to timber plantations, at least for dung beetles (Pryke and Samways 2012a). The ENs in the natural-agricultural system studied here seem to be hospitable to dung beetles, which is important, as it means that one ecosystem process at least, the burying of dung and recycling of nutrients, still takes place in this transformed production landscape.
References
Almeida S, Louzada J, Sperber C, Barlow J (2011) Subtle land-use change and tropical biodiversity: dung beetle communities in cerrado grasslands and exotic pastures. Biotropica 43:704–710
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253
Anderson MJ (2008) Animal-sediment relationships re-visited: characterising species’ distributions along an environmental gradient using canonical analysis and quantile regression splines. J Exp Mar Biol Ecol 366:16–27
Anderson MJ, Willis TJ (2003) Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84:511–525
Andresen E (2003) Effect of forest fragmentation on dung beetle communities and functional consequences for plant regeneration. Ecography 26:87–97
Bates DM, Sarkar D (2007) lme4: Linear mixed-effects models using S4 classes. R package version 0.99875-6
Beier P, Noss RF (1998) Do habitat corridors provide connectivity? Conserv Biol 12:1241–1252
Billeter R, Liira J, Bailey D, Bugter R, Arens P, Augenstein I, Aviron S, Baudry J, Bukacek R, Burel F, Cerny M, De Blust G, De Cock R, Diekötter T, Dietz H, Dirksen J, Dormann C, Durka W, Frenzel M, Hamersky R, Hendrickx F, Herzog F, Klotz S, Koolstra B, Lausch A, Le Coeur D, Maelfait JP, Opdam P, Roubalova M, Schermann A, Schermann N, Schmidt T, Schweiger O, Smulders MJM, Speelmans M, Simova P, Verboom J, van Wingerden WKRE, Zobel M, Edwards PJ (2007) Indicators for biodiversity in agricultural landscapes: a pan-European study. J Appl Ecol 45:141–150
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS (2008) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 2:127–135
Bremer LL, Farley KA (2010) Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers Conserv 19:3893–3915
Cook E (2002) Landscape structure indices for assessing urban ecological networks. Landscape Urban Plan. 58:269–280
Crous CJ, Samways MJ, Pryke JS (2013) Exploring the mesofilter as a novel operational scale in conservation planning. J Appl Ecol 50:205–214
Davis ALV (1994) Associations of Afrotropical Coleoptera (Scarabaeidae, Aphodiidae, Staphylinidae, Hydrophilidae, Histeridae) with dung and decaying matter: implications for selection of fly- control agents for Australia. J Nat Hist 28:383–399
Davis ALV, Scholtz CH (2004) Local and regional species ranges of a dung beetle assemblage from the semi-arid Karoo/Kalahari margins, South Africa. J Arid Environ 57:61–85
Davis ALV, Scholtz CH, Chown SL (1999) Species turnover, community boundaries and biogeographical composition of dung beetle assemblages across an altitudinal gradient in South Africa. J Biogeogr 26:1039–1055
Davis AJ, Holloway JD, Huijbregts H, Krikken J, Kirk-Spriggs AH, Sutton SL (2001) Dung beetles as indicators of change in the forests of northern Borneo. J Appl Ecol 38:593–616
Davis ALV, Frolov AV, Scholtz CH (2008a) The African Dung Beetle Genera. Protea Book House, Pretoria
Davis ALV, Scholtz CH, Deschodt C (2008b) Multi-scale determinants of dung beetle assemblage structure across abiotic gradients of the Kalahari-Nama Karoo ecotone, South Africa. J Biogeogr 35:1465–1480
de Andrade RBD, Barlow J, Louzada J, Vaz-de-Mello FZ, Souza M, Silveira JM, Cochrane MA (2011) Quantifying responses of dung beetles to fire disturbance in tropical forests: the importance of trapping method and seasonality. PLoS One 6:e26208
Edwards PB (1991) Seasonal variation in the dung of African grazing mammals, and its consequences for coprophagous insects. Funct Ecol 5:617–628
Escobar F, Halffter G, Solís Á, Halffter V, Navarrete D (2008) Temporal shifts in dung beetle community structure within a protected area of tropical wet forest: a 35-year study and its implications for long-term conservation. J Appl Ecol 45:1584–1592
Filgueiras BKC, Iannuzzi L, Leal IR (2011) Habitat fragmentation alters the structure of dung beetle communities in the Atlantic Forest. Biol Conserv 144:362–369
Fischer J, Lindenmayer DB, Fazey I (2004) Appreciating ecological complexity: habitat contours as a conceptual landscape model. Conserv Biol 18:1245–1253
Forman RTT (1995) Land mosaics: the ecology of landscapes and regions. Cambridge Univ Press, Cambridge
Franklin E, Magnusson WE, Luizao FJ (2005) Relative effects of biotic and abiotic factors on the composition of soil invertebrate communities in an Amazonian savanna. Appl Soil Ecol 29:259–273
Gardner TA, Hernández MIM, Barlow J, Peres CA (2007) Understanding the biodiversity consequences of habitat change: the value of secondary and plantation forests for neotropical dung beetles. J Appl Ecol 45:883–893
Hortal J, Borges PAV, Gaspar C (2006) Evaluating the performance of species richness estimators: sensitivity to sample grain size. J Anim Ecol 75:274–287
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometrical J 50:346–363
Jongman RHG, Bouwma IM, Griffioen A, Jones-Walters L, Doorn AM (2011) The Pan European Ecological Network: PEEN. Landscape Ecol 26:311–326
Jurasinski G, Koch M (2011) Commentary: do we have a consistent terminology for species diversity? we are on the way. Oecologia 167:893–902
Kessler M, Abrahamczyk S, Bos M, Buchori D, Putra DD, Gradstein SR, Höhn P, Kluge J, Orend F, Pitopang R, Saleh S, Schulze CH, Sporn SG, Steffan-Dewenter I, Tjitrosoedirdjo SS, Tscharntke T (2009) Alpha and beta diversity of plants and animals along a tropical land-use gradient. Ecol Appl 19:2142–2156
Kirkman KE, Pott RM (2002) Biodiversity conservation in plantation forestry. In: Pierce SM, Cowling RM, Sandwith T, MacKinnon K (eds) Mainstreaming biodiversity development - case studies from South Africa. The World Bank Environmental Department, Washington DC, pp 33–42
Klein B (1989) Effects of forest fragmentation on dung and carrion beetle communities in central Amazonia. Ecology 70:1715–1725
Kotze DJ, Lawes MJ (2007) Viability of ecological processes in small Afromontane forest patches in South Africa. Austral Ecol 32:294–304
Kotze DJ, Samways MJ (1999) Support for the multi-taxa approach in biodiversity assessment, as shown by epigaeic invertebrates in an Afromontane forest archipelago. J Insect Conserv 3:125–143
Kotze DJ, Samways MJ (2001) No general edge effects for invertebrates at Afromontane forest/grassland ecotones. Biodivers Conserv 10:443–466
Li L, He X, Li X, Wen Q, He HS (2007) Depth of edge influence of the agricultural-forest landscape boundary, southwestern China. Ecol Res 22:774–783
Louzada J, Lima AP, Matavelli R, Zambaldi L, Barlow J (2010) Community structure of dung beetles in Amazonian savannas: role of fire disturbance, vegetation and landscape structure. Landscape Ecol 25:631–641
Mucina L, Rutherford MC (2006) The vegetation of South Africa, Lesotho and Swaziland, Strelitzia 19. South African National Biodiversity Institute, Pretoria
Muriel SB, Kattan GH (2009) Effects of patch size and type of coffee matrix on Ithomiine butterfly diversity and dispersal in cloud-forest fragments. Conserv Biol 23:948–956
Nichols E, Larsen T, Spector S, Davis AL, Escobar F, Favila M, Vulinec K (2007) Global dung beetle response to tropical forest modification and fragmentation: a quantitative literature review and meta-analysis. Biol Conserv 137:1–19
Nichols E, Spector S, Louzada J, Larsen T, Amezquita S, Favila M (2008) Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol Conserv 141:1461–1474
Nielsen ST (2007) Deforestation and biodiversity: effects of bushland cultivation on dung beetles in semi-arid Tanzania. Biodivers Conserv 16:2753–2769
Numa C, Verdú JR, Sánchez A, Galante E (2009) Effect of landscape structure on the spatial distribution of Mediterranean dung beetle diversity. Divers Distrib 15:489–501
Numa C, Lobo JM, Verdú JR (2012) Scaling local abundance determinants in mediterranean dung beetles. Insect Conserv Divers 5:106–117
Pineda E, Moreno C, Escobar F, Halffter G (2005) Frog, bat, and dung beetle diversity in the cloud forest and coffee agroecosystems of Veracruz, Mexico. Conserv Biol 19:400–410
PRIMER-E (2008) PERMANOVA and PRIMER 6. PRIMER-E, Lutton
Pryke JS, Samways MJ (2009) Recovery of invertebrate diversity in a rehabilitated city landscape mosaic in the heart of a biodiversity hotspot. Landscape Urban Plann 93:54–62
Pryke JS, Samways MJ (2010) Significant variables for the conservation of mountain invertebrates. J Insect Conserv 14:247–256
Pryke JS, Samways MJ (2012a) Ecological networks act as extensions of protected areas for arthropod biodiversity conservation. J Appl Ecol 49:591–600
Pryke JS, Samways MJ (2012b) Conservation management of complex natural forest and plantation edge effects. Landscape Ecol 27:73–85
Rodewald AD (2012) Spreading messages about invasives. Divers Distrib 18:97–99
Romero-Alcaraz E, Ávila JM (2000) Effect of elevation and type of habitat on the abundance and diversity of Scarabaeoid dung beetle (Scarabaeoidea) assemblages in a Mediterranean area from Southern Iberian Peninsula. Zool Stud 39:351–359
Samways MJ (2007) Implementing ecological networks for conserving insect and other biodiversity. In: Stewart AJA, New TR, Lewis OT (eds) Insect conservation biology. CABI, Wallingford
Samways MJ, Bazelet CS, Pryke JS (2010) Provision of ecosystem services by large scale corridors and ecological networks. Biodivers Conserv 19:2949–2962
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32
Schlaepfer MA, Sax DF, Olden JD (2011) The potential conservation value of non-native species. Conserv Biol 25:428–437
Spector S, Ayzama S (2003) Rapid turnover and edge effects in dung beetle assemblages (Scarabaeidae) at a Bolivian neotropical forest-savanna ecotone. Biotropica 35:394–404
Svoray T, Bar P, Bannet T (2005) Urban land-use allocation in a Mediterranean ecotone: habitat Heterogeneity Model incorporated in a GIS using a multi-criteria mechanism. Landscape Urban Plann 72:337–351
Tropek R, Spitzer L, Konvicka M (2008) Two groups of epigeic arthropods differ in colonising of piedmont quarries: the necessity of multi-taxa and life-history traits approaches in the monitoring studies. Community Ecol 9:177–184
Tscharntke T, Steffan-Dewenter I, Kruess A, Thies C (2002) Characteristics of insect populations on habitat fragments: a mini review. Ecol Res 17:229–239
Tuomisto H (2010) A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography 33:2–22
Wethered R, Lawes MJ (2003) Matrix effects on bird assemblages in fragmented Afromontane forests in South Africa. Biol Conserv 14:327–340
Yu K, Li D, Li N (2006) The evolution of greenways in China. Landscape Urban Plann 76:223–239
Acknowledgments
We thank Ezemvelo KZN Wildlife, the Merensky Trust and Mondi South Africa for permitting sampling on their holdings. Funding was from the Mondi Ecological Network Programme (MENP) and co-funding from the National Research Foundation of South Africa (NRF).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pryke, J.S., Roets, F. & Samways, M.J. Importance of habitat heterogeneity in remnant patches for conserving dung beetles. Biodivers Conserv 22, 2857–2873 (2013). https://doi.org/10.1007/s10531-013-0559-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-013-0559-4