Abstract
The decline of Aculeata has raised concerns regarding their conservation and the maintenance of their function as important pollinators. Pan trapping is among the most frequently used methods for monitoring these organisms. Numerous researchers have suggested using pan traps of multiple colors in addition to the yellow traps. Here we provide the first data for the color preferences of European Aculeata by analyzing 5,421 individuals from 356 species of bees and wasps obtained using white, yellow, turquoise and pink pan traps. The total capture rates and diversity were higher in the white and yellow traps over the turquoise and pink ones. The species-specific abundance did not follow changes in the total capture rates. Nevertheless, only seven (of the 96 most commonly captured species) were captured at low rates in the yellow traps, but were obtained at high rates in the white traps. Only two species (Apis mellifera and Priocnemis minuta) were captured preferentially to other than white or yellow traps. Importantly, caution is needed when assessing the sex ratios of Aculeata obtained by pan trapping because many Aculeata display sex-specific color preferences. For example, in Dasypoda hirtipes, we captured 14 of 16 males in the yellow traps, but obtained 34 of 38 females in the turquoise traps. We provide experimental evidence that the females of numerous oligolectic bees (collecting pollen for their brood on a few specific plant species) display more stringent trap color preferences than their males, which correlates with their observed flower color preferences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The decline of Aculeata, particularly those considered to be important pollinator species, has raised concerns regarding their conservation and the maintenance of ecosystem services that they provide to wild flowers and crops (Biesmeijer et al. 2006; Nielsen et al. 2011; Burkle et al. 2013). Therefore, there is an urgent need for cost-effective and reproducible methods that allow for unbiased monitoring of the broad spectrum of Aculeata across multiple macro- and micro-habitats. Thus far, pan traps are postulated to collect the highest proportion of total Aculeata species of all of the methods currently available (Nielsen et al. 2011).
Because pan trapping requires low financial and time costs combined with high yields, it has recently been widely and uncritically employed as the sole means for easy sampling of local bee and wasp communities (Cane et al. 2000). Typically, yellow-colored plastic containers (frequently cereal bowls) are set on the ground and partially filled with water, salt and a surfactant. The insects land on the water surface, sink, and can be recovered the same day or within the next several days (Cane et al. 2000; Spafford and Lortie 2013; Tropek et al. 2013). Already three decades ago, William D. J. Kirk hypothesized that there is a relationship between ecological groups of insects and the color of the pan traps (Kirk 1984). Based on the available evidence, he suggested the following: (1) predators and parasites not associated with foliage prefer white and sometimes blue at least as much as yellow; (2) predators and parasites associated with non-grass foliage feeders prefer yellow; and (3) wood borers prefer black or red over yellow. Many studies have claimed that yellow serves as the superior color attractant for Aculeata (Wilson et al. 2008; Gollan et al. 2011; Dirrigl 2012; Ramírez-Freire et al. 2012; Vrdoljak and Samways 2012). However, others did not find any color-specific differences in the total capture rates or the diversity of pan-trapped Aculeata (Toler et al. 2005; Tuell et al. 2009; Abrahamczyk et al. 2010; Bashir et al. 2013). Campbell and Hanula (2007) reported that blue pan traps display the highest capture rates but are only as efficient as the white pan traps in terms of the species spectrum obtained. They suggested that blue and white pan traps are superior to yellow. Several authors, including Campbell and Hanula (2007) and Wilson et al. (2008), reported very low efficiency of red, orange and pink pan traps, which is consistent with Frisch (1971).
Increasing evidence suggests the presence of family-, genus-, or even species-specific color preferences in Aculeata. In the early 1980s, Disney et al. (1982) suggested that particular insect taxa are not caught by yellow traps and sometimes can be attracted in large numbers to other colors. Later, Toler et al. (2005) compared the abundance and diversity of bees captured in the Utah sand dunes with white, yellow and blue pan traps, and they reported that the Sørensen similarity index reached 0.70 between the three colors, and reached even 0.93 when focused on the abundant species only. However, they found that there were extensive differences in the color-specific abundance of particular species. Each color was preferred by at least one species, which was unrelated to the color of the flowers surrounding the traps (Table 1). Despite this, all nine species tested were captured in all three colors of pan traps tested, except for Ashmeadiella gillettei with the yellow pan traps and Eucera lutziana with the white in some cases, the differences in abundance reached over one order of magnitude. The follow-up study conducted in Utah by Wilson et al. (2008) confirmed the presence of species-specific (or genus- or family-specific) differences. Whilst they found Perdita idonea as the most abundant bee in the white, yellow and blue traps, they observed altered color-specific differences in the captures rates in numerous representatives of the Andrenidae, Apidae and Melittidae (Table 1). Green pan traps attracted large numbers of Halictus tripartitus and Lasioglossum hudsoniellus. The species color preferences did not correspond to the colors of their host plants in the case of the specialist pollinators (Wilson et al. 2008). At Michigan, in blueberry plantations, (Tuell et al. 2009) and in New South Wales, Australia (Gollan et al. 2011), the only common species that was more frequently collected in white over yellow pan traps was Apis mellifera, whereas numerous bee species significantly preferred the yellow color (Table 1). In Pakistan, Bashir et al. (2013) observed variations in species-specific responses, but they only morphotyped the predicted species, but did not determine them. In SE Australia, Saunders and Luck (2013) reported that native bees (and surprisingly Apis mellifera as well) were attracted to the yellow pan traps over the blue and white traps, and they also reported that other native Hymenoptera (i.e., excluding the bees) were insensitive to the three colors used.
Only a limited number of studies have addressed the sex-specific responses of bees and wasps. In particular, Leong and Thorp (1999) found that males and females of the oligolectic bee Andrena limnanthis displayed different color preferences, with males only preferring the blue traps, whereas both sexes were attracted to the white traps. The authors did not observe sex-specific differences in any other species examined, similarly to the results obtained for other insects by Kirk (1984) and Barker et al. (1997) (but cf. Kostal and Finch 1996).
All insects possess at least two types of color receptors, one receptor that is sensitive to the ultraviolet part of the spectrum, with a maximum sensitivity near 350 nm, and another receptor that is sensitive to green, with a maximum sensitivity near 540 nm. In addition, a third receptor with a maximum sensitivity near 440 nm is expressed in most insects (Kelber 2001). The data obtained for Apis spp. and Bombus spp. are inconclusive. Repeated behavioral experiments with foraging bees confirmed Apis spp. and Bombus spp. had innate preferences for yellow, white and/or blue–violet (Menzel 1967; Faegri and van der Pijl 1980; Herman et al. 1994; Giurfa et al. 1995; Lunau and Maier 1995; Clare et al. 2000; Gumbert 2000). The results of trapping experiments are not in agreement with the above data for, as yet, unknown reasons. Stephen and Rao (2005) even concluded that Apis mellifera is not attracted to either blue or yellow traps, whereas Bombus spp. were captured in traps of both these colors. As demonstrated by Leong and Thorp (1999), some bee species see white as blue–green, which may partially explain the nearly similar preferences for blue and white documented, e.g., by Campbell and Hanula (2007). Red is seen as dark or black by most Aculeata (Frisch 1971).
Despite the evidence-based conservation monitoring of Aculeata being increasingly dependent on pan trapping, very limited data are available on the possible errors of omission and commission introduced by ad hoc selection of colors used for pan trapping. Particularly, the responses of European bees and wasps are either missing or insufficient, which is true even for the most common species except Apis mellifera. Whilst the colour vision system of bees is conserved (Peitsch et al. 1992; Hempel de Ibarra et al. 2014), the flower color preferences are highly variable even within a particular genus, and are conserved only among the individuals of the same species. Reflecting the relatively restricted distribution of the majority of bee and wasp species, it is not possible to extrapolate the information on color preferences from studies conducted previously in similar climatic regions (such as utilizing the information gained in the U.S. for the European bees). In this study, we thus address the species- and sex-specific responses of European Aculeata to color attractants. Utilizing a large Aculeata dataset acquired across a broad range of habitats, we aim to lift the fog on the existence of the predicted species- and sex-specific differences, and to determine, whether these differences may affect the outcomes obtained by the evidence-based conservation monitoring of Aculeata conducted by pan trapping.
Materials and methods
Study area
The study was performed at multiple sampling sites throughout the Czech Republic, NW Slovakia, and NW Italy. The sampling sites included xerothermophilous habitats of natural origin (such as steppes at the southern slopes), xerothermophilous habitats of industrial or postindustrial origin (such as the south-exposed road verges, sandpits, quarries and ash deposits), wetlands (particularly reed beds and their surroundings), forests (including burned forest patches, forests in rugged sandstone terrain, and alluvial forests), and subalpine habitats (subalpine meadows, bare sand patches in the vicinity of alpine rivers, and ore mine spoil heaps within the subalpine regions). Only a portion of the captures was performed solely for the purpose of this study, the conservation value of the dataset from the reed beds was analyzed by Heneberg et al. (2014), the datasets from the burned and control forest patches were utilized for suggesting the improvements of early post-fire successional stages management by Bogusch et al. (2014). Analyses of several other datasets are either currently under review or under preparation.
Sampling
At each sampling site, we utilized pan traps of four colors: white (RAL 9010), yellow (RAL 1021 or 1003), turquoise (RAL 5018) and pink (RAL 3014). The pan traps were made from round 570 ml polypropylene containers, with a 120 mm upper diameter and a depth of 80 mm (Obal Centrum, Sezemice, Czech Republic), sprayed with one layer of transparent Body Spray Plastofix (HB Body, Thessaloniki, Greece), and overlayed with two layers of the acrylic topcoat Body Spray Irida of indicated RAL colors (HB Body, Thessaloniki, Greece). The traps were filled up to the upper quarter with soapy water containing salt, which acted as a preservative. As a detergent, we used the lemon-scented Jar (Procter and Gamble, Rakovník, Czech Republic). The traps were exposed between April and August 2013 for one to five periods at each site; the individual sampling periods were spanning 2–7 days each. The duration of trap exposure was recorded. The number of traps of each color exposed and assessed was recorded as well. At each site, the pan traps were placed in the microhabitats with the highest proportion of bare sand patches, but preferably in the vicinity of flowering plants because these habitats were expected to attract the most diverse spectrum of bees and wasps (Roulston and Goodell 2011). The traps were placed either in balanced patches of four (each trap of a different color), or were placed in line along transects (again with all four colors included but occasionally with an increased proportion of yellow traps). The collected specimens were temporarily stored in 75 % ethanol until pinned for identification. Petr Heneberg, Petr Bogusch, Lukáš Nývlt and Pavlína Tauchmanová performed the sampling. Petr Bogusch determined and collected the obtained specimens. Jakub Straka and Pavel Tyrner revised certain specimens of Chrysididae and Pompilidae. Categorization of the species captured was performed according to the Czech red list of invertebrates (Farkač et al. 2005).
Statistical analyses
Any members of the infraorder Aculeata were analyzed, except for the Formicidae. Rarefaction curves were computed in PAST v. 2.14 (Hammer et al. 2001) to analyze the species diversity and species richness of the bees and wasps. The rarefaction algorithm was based on the use of the log Gamma function for computing combinatorial terms as described by Krebs (1989). To estimate the species richness, the Chao-1 estimator was calculated (Colwell and Coddington 1994) using the program available at http://www2.biology.ualberta.ca/jbrzusto/rarefact.php [cited as 27 Feb 2014]. The basic diversity indices were calculated for each of the datasets as described (Heneberg et al. 2014). To compare the diversities, a Shannon t test with a bias correction term was used (Poole 1974). All calculations were performed in PAST v. 2.14 (Hammer et al. 2001).
To calculate the capture rates, the number of trap-days (Ndays) was calculated for each species based on the number of traps of a single color exposed at a single sampling site during the single trapping event (Nte), less the number of traps destroyed or that disappeared (Ntd) and the length of exposure of each individual trap (L = 2–7 days). Because we retrieved our data from a wide range of habitats, we calculated the number of trap-days separately for each species and limited the number of the trap-days counted only to those relevant to the sites of occurrence of each respective species. Thus, instead of counting the total number of trap-days from all sampling sites (i), we subtracted all the trapping events performed at sampling sites, where the respective species was absent (a).
The adjusted data were then expressed as the number of individuals per 100 trap-days in pan traps of the selected color.
Following the initial rarefaction and calculation of the diversity indices involving all of the individuals captured, we restricted the additional analyses to the species captured at n ≥10 individuals only (n = 96 species). The χ2 tests with Bonferroni correction (to control the family wise error rate) were used to analyze the significance of color-specific deviations in the sex ratios obtained by pan trapping. The correction was calculated as per the 96 tests performed, thus dividing the threshold p = 0.05 rate by 96–0.0005. To analyze the contribution of multiple variables, the Principal Components Analysis (PCA) approach was used. The data are shown as the mean ± SD unless stated otherwise.
Results
Species diversity of bees and wasps sampled with pan traps of four different colors
We collected a total of 5,421 individuals from 356 species of bees and wasps (Supplementary Table S1). Of these, 5,215 individuals (337 species) were captured at various sites throughout the Czech Republic (including xerothermophilous habitats, wetlands and forests), 116 individuals (26 species) were obtained in NW Slovakia (alluvial forest), and 90 individuals (41 species) were obtained in NW Italy (alluvial subalpine meadows and bare sand patches). The Chao-1 species richness estimator indicated that the total species spectrum of Aculeata attracted to the pan traps at the sampled sites reached 450.6 ± 20.0 species.
The Chao-1 species richness estimator indicated high species diversity in datasets obtained from the pan traps of any of the four colors used. The white pan traps captured 1,138 individuals of 202 species, with the Chao-1 suggesting the potential to capture 327.5 ± 27.6 species. The yellow pan traps captured 2,711 individuals of 265 species, with the Chao-1 suggesting the potential to capture 325.4 ± 14.6 species. The turquoise pan traps captured 954 individuals of 164 species, with the Chao-1 suggesting the potential to capture 262.7 ± 24.3 species. The pink pan traps captured 618 individuals of 120 species, with the Chao-1 suggesting the potential to capture 201.2 ± 22.6 species (Fig. 1). All four assemblages of the bees and wasps were characterized by low dominance, with the yellow and white pan traps attracting assemblages with lower dominance than the turquoise or pink pan traps. High values of the Brillouin’s diversity index (3.7–4.4) also support this finding. The bee and wasp assemblages obtained with the yellow or white pan traps (or for the diversity among the total dataset) displayed twofold higher values of the Margalef’s index and Fisher’s alpha than those obtained in the pink pan traps (p < 0.001 each when assessed by bootstrapping as well as by permutation against the yellow pan traps). The white and yellow pan traps did not show any highly dominant species as suggested by the low values of the Berger-Parker index at both sites (0.10, both). Contrariwise, the Berger-Parker index reached significantly higher values for the dataset from the turquoise (0.14) and particularly the pink pan traps (0.23) (Table 2).
Dominant species of bees and wasps sampled with pan traps of four different colors
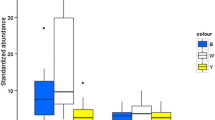
Of the 96 species with ≥10 individuals captured, we found significant species-specific preferences for all four colors tested. The white traps specifically attracted Panurgus calcaratus, which was captured 10-times more efficiently in the white traps than in the other colored traps (Fig. 2a). In sum 8 species (8 %) displayed significantly higher capture rates in the white traps compared to their average capture rate for the four colors tested (χ2 tests at p < 0.05 with Bonferroni correction). In addition to Panurgus calcaratus, a significant preference for white traps over the others was observed for Hedychrum niemelai, H. nobile, Oxybelus trispinosus, Tachysphex pompiliformis, Halictus confusus, Anoplius caviventris and A. infuscatus (χ2 tests at p < 0.05 with Bonferroni correction).
Overview of the dominant species of bees and wasps captured in the four differently colored pan traps but exposed at mutually identical sites and time intervals. The species are sorted according to the frequency of their captures within the white a, yellow b, turquoise c and pink d pan traps. In each case, the figure displays the 20 species with the highest number of individuals relative to the number of trap-days exposed at the sites where the particular species were present. Note that the dominance of the particular species is subject to strong changes across the four datasets, particularly considering the species that is dominant in the yellow pan traps
The yellow traps specifically attracted the most species of Andrena spp. (except A. bicolor, for which the differences were insignificant most likely because of the limited sample size), some of which were captured exclusively in the yellow pan traps (Fig. 2b). In sum, 26 species (27 %) displayed a significantly higher capture rate in yellow traps when compared to their average capture rate for the four colors tested (χ2 tests at p < 0.05 with Bonferroni correction). A significant preference for the yellow traps over the others was observed for Andrena barbilabris, A. flavipes, A. gravida, A. helvola, A. minutula, A. nigroaenea, A. ovatula, A. vaga, Nomada flavoguttata, N. lathburiana, Chrysura dichroa, Colletes cunicularius, Hylaeus confusus, Trypoxylon deceptorium, T. minus, Halictus subauratus, H. tumulorum, Lasioglossum glabriusculum, L. pauxillum, L. politum, L. sabulosum, Osmia aurulenta, O. bicolor, O. rufohirta, Auplopus carbonarius and Priocnemis fennica (χ2 tests at p < 0.05 with Bonferroni correction).
The turquoise traps specifically attracted Dasypoda hirtipes, which was captured exclusively in the turquoise and yellow traps, with turquoise traps 5-times more efficient than the yellow traps (Fig. 2c). In sum, only three species (3 %) displayed significantly higher capture rates in the turquoise traps compared to their average capture rate for the four colors tested (χ2 tests at p < 0.05 with Bonferroni correction). In addition to Dasypoda hirtipes, a significant preference for the turquoise traps over the others was observed for Bombus pascorum and Episyron albonotatum (χ2 tests at p < 0.05 with Bonferroni correction).
The pink traps specifically attracted the most Apis mellifera individuals, which displayed a differential response to the color attractants in the following order: pink > turquoise > white > yellow (Fig. 2d). In sum only 2 species (2 %) displayed significantly higher capture rates in the pink traps compared to their average capture rate for the four colors tested (χ2 tests at p < 0.05 with Bonferroni correction). In addition to Apis mellifera, a marginally significant preference for pink traps over the others was observed for Priocnemis minuta (χ2 tests at p < 0.05 with Bonferroni correction).
The comparison of capture ratios associated with pan traps of the four colors tested is shown in Fig. 3. The results of the χ2 tests with Bonferroni correction utilized in this subchapter are listed in Supplementary Table S2. Numerous species displayed highly differential responses towards the colors used. In addition to those species specifically preferring a single color (which are listed above), there were species that preferred two or three colors over other ones, whereas the other species did not show any preferences. The Venn diagram in Fig. 4 displays all species, which were captured in any of the color pan traps at a ratio ≥5-times higher or lower than those experienced in the yellow pan traps. This analysis included the 96 species with 10 or more individuals captured.
Comparison of the species-specific capture rates between the four colors of pan traps used. a White versus yellow pan traps, b turquoise versus yellow, c pink versus yellow, d white versus turquoise, e white versus pink, f turquoise versus pink pan traps. The abbreviations used indicate the species with the highest capture rates within the pan traps of each respective color. Aba = Andrena barbilabris, Agr = Andrena gravida, Ame = Apis mellifera, Ava = Andrena vaga, Avi = Anoplius viaticus, Ccu = Colletes cunicularius, Cer = Chalicodoma ericetorum, Dhi = Dasypoda hirtipes, Hco = Halictus confusus, Hqu = Halictus quadricinctus, Lmo = Lasioglossum morio, Lpo = Lasioglossum politum, Ltr = Lasioglossum trichopygum, Ndi = Nysson distinguendus, Nla = Nomada lathburiana, Oru = Osmia rufohirta, Pca = Panurgus calcaratus, Pmi = Priocnemis minuta
Species-specific attractiveness of the multiple color attractants in comparison to the most frequently used yellow color. The Venn diagram displays the species, which were captured in any of the color pan traps at a ratio ≥5-times higher or lower than those obtained with the yellow pan traps. The analysis included only species with 10 or more individuals captured. (Color figure online)
Differences in sex-specific capture rates
Of the 96 species with ≥10 individuals captured, we found significant sex-specific color preferences in 11 of these species (Fig. 5) (χ2 tests with Bonferroni correction p < 0.05). In Panurgus calcaratus, both sexes were equally attracted to the yellow pan traps, whereas only the males (n = 62) were attracted to the white pan traps. In Tachysphex pompiliformis, we observed an equal sex ratio in the white pan traps, whereas the three other trap colors attracted females only (n = 10). In Halictus confusus, we observed a male-skewed ratio in the white traps, whereas the females were more attracted to the yellow traps. In Lasioglossum morio, the results obtained for all four trap colors revealed the existence of a female-dominated community. However, the L. morio males were more attracted to the white traps and particularly to the yellow traps over the turquoise and pink traps. In Hoplitis leucomelana, the females were attracted almost exclusively by the turquoise and pink pan traps, whereas the males were captured in traps of all four colors, but predominantly in the yellow traps. In Osmia rufohirta, the yellow traps captured a male-skewed sample, whereas the turquoise traps captured a female-skewed sample, and the yellow traps attracted females only. Similarly, in Dasypoda hirtipes, the yellow traps captured primarily males (14 males vs. 4 females only), whereas the turquoise traps captured primarily females (2 males vs. 34 females). In Arachnospila spissa, the females were found predominantly in the white and yellow traps, whereas the males were distributed across all four colors tested. Finally, in Ammophila sabulosa, the white and yellow traps contained four-times more males than females, whereas the reverse was true for the pink pan traps.
Sex-specific color preferences. The figure displays the data for the 11 species, which displayed statistically significant color-specific deviations in the sex ratios obtained by pan trapping as assessed by χ2 tests at p < 0.05 with Bonferroni correction for the family wise error rate. The relative share of the males (%) is shown. The number of males and females captured into the pan traps of each respective color is indicated just below the graph. (Color figure online)
Effects of habitat, nesting strategy and intra-annual variation
The extensive heterogeneity of our dataset allowed us to address the possible effects of habitat, nesting strategy and intra-annual variation on the pan trap color-specifc capture rates. For this purpose, we performed the PCA analyses of the dataset of 96 species with ≥10 individuals captured listed in Supplementary Table S2. First we sorted the species according to their nesting strategy by assigning them the following categories: burrowers, cavity nesters, parasites, and socially nesting. The PCA analysis revealed that nesting strategies segregate only poorly with the color-specific capture rates. Cavity nesters and parasites were more frequently captured by the yellow traps, whereas the socially nesting Aculeata were more frequently captured by any other color tested and the species burrowing in the soil did not show any color preferences (Fig. 6a). The PCA analysis of the intra-annual variations in Aculeata assemblages revealed that the species with a single spring generation or with two generations preferred the yellow traps, whereas the summer species, long-living species and the species with multiple generations were more frequently captured in any of other colors tested (Fig. 6b). The PCA analysis of habitat preferences revealed that the Aculeata of sandy habitats were most frequently captured in the white-colored traps, whereas those present typically in the other types of open habitats and steppes preferred pink and yellow. The Aculeata associated with wetlands, forest edges and those found across multiple habitats did not show any consistent color preferences (Fig. 6c).
Results of the PCA analysis of the pan trap color preferences of 96 Aculeata species with ≥10 individuals captured, listed in Supplementary Table S2. Scatter plots of the preferences for four pan trap colors among Aculeata assigned with the following categories: a Nesting strategies: burrowers (n = 59 species), cavity nesters (n = 17), parasites (n = 12), and socially nesting (n = 8). b Intra-annual variation: one generation per calendar year (84 species), two generations per year (n = 6), multiple generations per year (n = 6), flying period spanning the entire year (n = 34), spring only (n = 10), spring and summer (n = 7), summer only (n = 45). c Habitat: forest edges (n = 8), wetlands (n = 5), steppes (n = 6), sandy habitats (n = 25), other open habitats (n = 23), multiple habitats (n = 29). (Color figure online)
Discussion
Utilizing the large-scale dataset obtained across multiple habitats in three European countries, we provide the first large-scale evidence for the presence of species- and sex-specific color preferences in Aculeata. Many previous studies addressed the differences in total capture rates or in the total diversity obtained. Consistent with Campbell and Hanula (2007) and Wilson et al. (2008), we found low capture rates and low diversity of Aculeata captured in pink traps. The total capture rates and diversity of Aculeata were only marginally higher in the turquoise traps compared to the pink traps. But the diversity and particularly the capture rates of Aculeata were significantly higher in the white and yellow pan traps. The turquoise colored (RAL 5018) pan traps did not recapitulate the results obtained previously with blue traps (Campbell and Hanula 2007) (Fig. 1). We hypothesize that this is related to the spectrum of turquoise, which is shifted to green and may therefore be perceived differently by the incoming insects.
Importantly, the abundance of particular species did not follow changes in the total capture rates. The most striking example, identified already by the previous authors, is Apis mellifera, which displays the following color preferences: pink > turquoise > white >> yellow (Figs. 2, 4). The similar color preferences of Apis mellifera were previously reported by Tuell et al. (2009) and Gollan et al. (2011). However, the color preferences of other European species were unknown, with data available for only a few American and Australian taxa. Although many of the 96 analyzed species with 10 or more individuals captured displayed significant color preferences, the yellow pan traps captured nearly all the species, although some were at low capture ratios. Because the total capture rates for turquoise and pink pan traps were low, the species-specific benefits gained by the inclusion of turquoise and pink pan traps must be compared with the low overall efficiency of the captures obtained with this color of traps. Only a few species displayed 5-times or higher selectivity over the yellow pan traps, which may be of interest when deciding whether to use the yellow or white–yellow pan traps only or whether to expand the pan trap color spectrum, although this leads to a decrease in the share of yellow traps among the total set of traps exposed. Numerous species that avoided the yellow traps were found in high numbers in the white traps. Therefore, the yellow-white combination may be sufficient to capture these species, which include Agenioideus cinctellus, Hedychrum nobile, Tachysphex psammobius, Anoplius viaticus, Oxybelus trispinosus, Panurgus calcaratus and Rophites quinquespinosus. Only two species, Apis mellifera and Priocnemis minuta, were captured at high capture rates exclusively in the traps that were not white or yellow (Fig. 4). Therefore, the inclusion of multiple colors in the pan trapping experiments (other than white and yellow) was not a reliable approach to increase the number of species found considering that it led (in parallel) to a decreased number of the more efficient yellow and white pan traps exposed when using a constant number of traps per sampling site.
We found sex-specific differences in the color preferences in 11 of the 96 species analyzed. These differences were not previously reported except for Andrena limnanthis (Leong and Thorp 1999). The sex-specific differences in the color preferences may reflect differences in the food collection strategies that exist between the sexes of numerous Aculeata (e.g., Edwards and Telfer 2001; Michener 2007). Importantly, the differences in the observed sex ratios may reach extreme values in some cases. For example in Dasypoda hirtipes, we found 14 of 16 males trapped in the yellow traps, but 34 of the 38 females were obtained from the turquoise traps (Fig. 5). These differences must be accounted for when suggesting a potential skewness of the sex ratio in particular with Aculeata species in datasets obtained by pan trapping.
A large part of the color preferences of bees could be explained by the similarity of these colors to their host flowers. However, the selectivity of each particular species and sex-specific preferences must be considered. We found, e.g., that Panurgus calcaratus is attracted by white traps over the other colors tested (Fig. 2a). Nevertheless, P. calcaratus females are specialists for yellow-colored flowers, whereas only P. calcaratus males feed themselves across a broad range of plants. And indeed, when the P. calcaratus sexes were analyzed separately, we found statistically significant evidence that 62 of 68 males captured were attracted to the white pan traps, whereas six of the seven females captured were found in the yellow pan traps only (Supplementary Table S1). The species that preferred the white traps over the others (Hedychrum niemelai, H. nobile, Oxybelus trispinosus, Tachysphex pompiliformis, Halictus confusus and Anoplius infuscatus) are typical visitors of white-colored flowers, such as those of the family Apiaceae, Matricaria spp. or Achillea spp. The flower preferences of Anoplius caviventris are thus far unknown. Similarly, the species that preferred the yellow traps over the others (most of Andrena spp. except A. bicolor) are typically found on yellow-colored flowers, whereas only A. bicolor collects pollen also from blue flowers. Interestingly, the Andrena spp. males displayed color preferences identical to their females (Supplementary Table S1). The remaining numerous Aculeata species that preferred yellow traps (listed in Supplementary Table S2) consist primarily of the polylectic species and do not include any additional yellow-colored flower specialists. However, the low share of yellow flower specialists may be caused by their low representation in our dataset rather than by the lack of such a relationship. The turquoise color-associated Dasypoda hirtipes (Fig. 2c) is a species, where the females are attracted to blue and violet flowers (typically Cichorium intybus and Knautia arvensis), whereas the males do not appear to be selective. These sex-specific preferences were also consistent with our experiments because the sex ratio of D. hirtipes capture rates in the turquoise traps was one male per each 18.8 females, whereas the yellow traps captured 3.17 males per each single female (Supplementary Table S1). Bombus pascuorum (preferred turquoise over the other colors) is considered a generalist, but is frequently found at blue and violet flowers. Additionally, the last turquoise-associated species identified in this study, Episyron albonotatum, is a frequent visitor of blue-colored flowers, typically Thymus spp. For the species that preferred pink traps, the color preferences of Apis mellifera were repeatedly tested as discussed above, whereas the flower preferences of Priocnemis minuta are unknown. The sex-associated differences observed (Fig. 5) were typically associated with the oligolectic bees. Strict sex-specific flower preferences are known, e.g., for Hoplitis leucomelana and Osmia rufohirta females, which prefer blue, violet and pink flowers, whereas their males visit a much broader range of flowers, with O. rufohirta males frequently visiting yellow-colored flowers, such as Potentilla spp. This behavior corresponds tightly to the species- and sex-specific preferences shown in Fig. 5. In Sphecids, Ammophila sabulosa females are frequent visitors of Thymus spp., whereas the males are less specific and also visit flowers of the family Apiaceae, Tanacetum vulgare, etc., which again corresponds to the sex-specific color preferences observed in this study (Fig. 5). Therefore, the most striking sex-specific differences in color preferences were found for the bee species considered as oligolectic according to Westrich (1989). Females of oligolectic bees collect pollen for their brood from flowers of several plant species (typically from a single family, single color, etc.), whereas the males only feed on nectar and do not display any strict plant species or color preferences. By contrast, both sexes of the adult wasps feed only on nectar but have carnivorous larvae (O’Neill 2001). Thus, the wasp adults usually do not display any flower specialization when searching for their food, and both their sexes are thus attracted to the mutually similar colors of pan traps. In wasps, the prey specialization is more important than the flower specialization. Further research is needed to clarify the species- and sex-specific preferences for those parts of the color spectrum, which are indistinguishable to human eyes and which were not addressed by this study.
Two models of color choice probably apply. The Gumbert and Kunze (2001) model of bee colour vision is followed by the species without any strong innate color preferences, such as Apis mellifera and many Bombus species. In these species, avoidance learning is applied. Thus, they choose the respective color more frequently if they already foraged on more similarly colored flower species in the past. But in the more specialized species, innate color preferences probably apply. In these species, the color of the most effective pan traps is similar to the color of their typical species-specific food source.
Support for the parallel applicability of both, the Gumbert and Kunze model and the innate color preferences model, stems also from the generalized color preferences determined in this study, where the social species with multiple generations occurring throughout the year across multiple habitats (i.e. Apis mellifera and numerous Bombus species) displayed negligible color preferences, whereas the short-living spring cavity nesters of the open landscape were typically found in the yellow traps (Fig. 6). Further specialized species were identified at the species level (Supplementary Table S2). Should the Gumbert and Kunze model be considered as valid, the experiments involving multiple sampling sites and spanning across the whole season should suggest that the particular species display low discrimination between the color attractants, which we observed for the social species. Thus, we have corroborated the validity of the Gumbert and Kunze model in social species, which were already repeatedly shown to behave as proposed by Gumbert and Kunze (Keasar et al. 2013; Dyer et al. 2014; Hegland 2014). In addition, we identified a large number of species, particularly the short-living spring cavity nesters of the open landscape, the color preferences of which were highly uniform across multiple sampling sites and sampling dates. The existence of such species thus suggests the parallel applicability of the innate color preferences model in a subset of Aculeata as well.
Conclusions
Species- and sex-specific responses of Aculeata to color attractants were never addressed in Europe, and only limited data exist worldwide. However, pan trapping is increasingly used as a time-saving method of choice for the monitoring of Aculeata in protected areas and when the evaluation of a conservation value of any habitat of interest is necessary (e.g., those being subject to the environmental impact assessment). However, pan trapping does not lead to the capture of all Aculeata species. Some locally common species can be even severely underrepresented as recently shown, e.g., for Pemphredon fabricii (Heneberg et al. 2014). Although here we identified numerous species-specific color preferences, the number of species preferring colors other than yellow or white was negligible. Further studies are necessary to confirm that some of the rare species underrepresented in our dataset show preferences for the spectral and non-spectral colors underrepresented in the hitherto performed studies. The species-specific color preferences must be considered when performing evidence-based conservation monitoring focusing on any species already known to respond poorly to the colors most often used. Importantly, we show that caution must be used when assessing the sex ratios of Aculeata obtained by pan trapping because numerous Aculeata species display differential sex-specific color preferences.
References
Abrahamczyk S, Steudel B, Kessler M (2010) Sampling Hymenoptera along a precipitation gradient in tropical forests: the effectiveness of different coloured pan traps. Entomol Exp Appl 137:262–268
Barker AM, Sanbrooke KJ, Aebischer NJ (1997) The water trap colour preferences of farmland sawflies. Entomol Exp Appl 85:83–86
Bashir MA, Saeed S, Sajjad A (2013) Monitoring Hymenoptera and Diptera pollinators in a subtropical forest of Southern Punjab, Pakistan. Pak J Agric Sci 50:359–366
Biesmeijer JC, Roberts SPM, Reemer M, Ohlemüller R, Edwards M, Peeters T, Schaffers AP, Potts SG, Kleukers R, Thomas CD, Settele J, Kunin WE (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313:351–354
Bogusch P, Blažej L, Trýzna M, Heneberg P (2014) Forgotten role of fires in Central European forests: critical importance of early post-fire successional stages for bees and wasps (Hymenoptera: Aculeata). Eur J Forest Res. doi:10.1007/s10342-014-0840-4
Burkle LA, Marlin JC, Knight TM (2013) Plant-pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science 339:1611–1615
Campbell JW, Hanula JL (2007) Efficiency of Malaise traps and colored pan traps for collecting flower visiting insects from three forested ecosystems. J Insect Conserv 11:399–408
Cane JH, Minclkey RL, Kervin LJ (2000) Sampling bees (Hymenoptera: Apiformes) for pollinator community studies: pitfalls of pan-trapping. J Kans Entomol Soc 73:225–231
Clare G, Suchling DM, Bradley SJ, Walker JTS, Shaw PW, Daly JM, McLaren GF, Wearing CH (2000) Pheromone trap colour determines catch of non-terget insects. N Zeal Plant Prot 53:216–220
Colwell RK, Coddington JA (1994) Estimating terrestrial biodiversity through extrapolation. Philos Trans R Soc Lond Ser B 345:101–118
Dirrigl FJ (2012) Effectiveness of pan trapping as a rapid bioinventory method of freshwater shoreline insects of subtropical Texas. Southwest Entomol 37:133–139
Disney RHL, Erzinçlioglu YZ, Henshaw DDC, Howse D, Unwin DM, Withers P, Woods A (1982) Collecting methods and the adequacy of attempted fauna surveys with reference to the Diptera. Field Stud 5:607–621
Dyer AG, Dorin A, Reinhardt V, Garcia JE, Rosa MGP (2014) Bee reverse-learning behavior and intra-colony differences: simulations based on behavioral experiments reveal benefits of diversity. Ecol Model 277:119–131
Edwards R, Telfer MG (eds) (2001) Provisional atlas of the aculeate Hymenoptera of Britain and Ireland. Part 3. Biological Records Centre, Huntingdon
Faegri K, van der Pijl L (1980) The principles of pollination ecology, 3rd edn. Pergamon Press, Oxford
Farkač J, Král D, Škorpík M (eds) (2005) [Red list of threatened species in the Czech Republic. Invertebrates]. AOPK ČR, Prague (in Czech)
Giurfa M, Nunez J, Chittka L, Menzel R (1995) Colour preferences of flower-naïve honeybees. J Comp Physiol A 177:247–259
Gollan JR, Ashcroft MB, Batley M (2011) Comparison of yellow and white pan traps in surveys of bee fauna in New South Wales, Australia (Hymenoptera: Apoidea: Anthophila). Aust J Entomol 50:174–178
Gumbert A (2000) Color choices by bumble bees (Bombus terrestris): innate preferences and generalization after learning. Behav Ecol Sociobiol 48:36–43
Gumbert A, Kunze J (2001) Colour similarity to rewarding model plants affects pollination in a food deceptive orchid, Orchis boryi. Biol J Linn Soc 72:419–433
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hegland SJ (2014) Floral neighbourhood effects on pollination success in red clover are scale-dependent. Funct Ecol 28:561–568
Hempel de Ibarra N, Vorobyev M, Menzel R (2014) Mechanisms, functions and ecology of color vision in the honey bee. J Comp Physiol 200:411–433
Heneberg P, Bogusch P, Astapenková A (2014) Reed galls serve as an underestimated but critically important resource for an assemblage of aculeate hymenopterans. Biol Conserv 172:146–154
Herman TJB, Cameron PJ, Walker GP (1994) Effect of pheromone trap position and colour on tomato fruit worm moths and bumblebees. Proc 47th N Zeal Plant Prot Soc Conf, Wellington, pp 154–158
Keasar T, Motro U, Shmida A (2013) Temporal reward variability promotes sampling of a new flower type by bumblebees. Anim Behav 86:747–753
Kelber A (2001) Receptor based models for spontaneous colour choices in flies and butterflies. Entomol Exp Appl 99:231–244
Kirk WDJ (1984) Ecologically selective coloured traps. Ecol Entomol 9:35–41
Kostal V, Finch S (1996) Preference of the cabbage root fly, Delia radicum (L.) for coloured traps: influence of sex and physiological status of the flies, trap background and experimental design. Physiol Entomol 21:123–130
Krebs CJ (1989) Ecological methodology. Harper and Row, New York
Leong JM, Thorp RW (1999) Colour-coded sampling: the pan trap colour preferences of oligolectic and nonoligolectic bees associated with a vernal pool plant. Ecol Entomol 24:329–335
Lunau K, Maier EJ (1995) Innate colour preferences of flower visitors. J Comp Physiol A 177:1–19
Menzel R (1967) Untersuchungen zum Erlernen von Spektralfarben durch die Honigbienen (Apis mellifica). Z Vgl Physiol 56:22–62
Michener CD (2007) The bees of the world, 2nd edn. Johns Hopkins University Press, Baltimore
Nielsen A, Steffan-Dewenter I, Westphal C, Messinger O, Potts SG, Roberts SPM, Settele J, Szentgyörgyi H, Vaissière BE, Vaitis M, Woyciechowski M, Bazos I, Biesmeijer JC, Bommarco R, Kunin WE, Tscheulin T, Lamborn E, Petanidou T (2011) Assessing bee species richness in two Mediterranean communities: importance of habitat type and sampling techniques. Ecol Res 26:969–983
O’Neill KM (2001) Solitary wasps: behavior and natural history. Comstock Publishing Associates, New York
Peitsch D, Fietz A, Hertel H, de Souza J, Ventura DF, Menzel R (1992) The spectral input systems of hymenopteran insects and their receptor-based colour vision. J Comp Physiol A 170:23–40
Poole RW (1974) An introduction to quantitative ecology. McGraw-Hill, New York
Ramírez-Freire L, Alanís-Flores GJ, Ayala-Barajas R, Quiroz-Martýnez H, Velazco-Macías CG (2012) Las abejas del género Agapostemon (Hymenoptera: Halictidae) del estado de Nuevo León, México. Rev Mex Biodivers 83:63–72
Roulston TH, Goodell K (2011) The role of resources and risks in regulating wild bee populations. Annu Rev Entomol 56:293–312
Saunders ME, Luck GW (2013) Pan trap catches of pollinator insects vary with habitat. Aust J Entomol 52:106–113
Spafford RD, Lortie CJ (2013) Sweeping beauty: is grassland arthropod community composition effectively estimated by sweep netting? Ecol Evol 3:3347–3358
Stephen WP, Rao S (2005) Unscented color traps for non-Apis bees (Hymenoptera: Apiformes). J Kans Entomol Soc 78:373–380
Toler TR, Evans EW, Tepedino VJ (2005) Pan-trapping for bees (Hymenoptera: Apiformes) in Utah’s West Desert: the importance of color diversity. Pan-Pac Entomol 81:103–113
Tropek R, Černá I, Straka J, Čížek O, Konvička M (2013) Is coal combustion the last chance for vanishing insects of inland drift sand dunes in Europe? Biol Cons 162:60–64
Tuell JK, Ascher JS, Isaacs R (2009) Wild bees (Hymenoptera: Apoidea: Anthophila) of the Michigan highbush blueberry agroecosystem. Cons Biol Biodivers 102:275–287
von Frisch K (1971) Bees: their vision, chemical senses, and language. Cornell University Press, Ithaca
Vrdoljak SM, Samways MJ (2012) Optimising coloured pan traps to survey flower visiting insects. J Insect Conserv 16:345–354
Westrich P (1989) Die Wildbienen Baden-Württembergs. Eugen Ulmer Verlag, Stuttgart
Wilson JS, Griswold T, Messinger OJ (2008) Sampling bee communities (Hymenoptera: Apiformes) in a desert landscape: are pan traps sufficient? J Kans Entomol Soc 81:288–300
Acknowledgments
The study was supported by the PRVOUK project P31/2012 from the Charles University in Prague, and by the Specifický výzkum projects 2101/2013 and 2102/2013 from the University of Hradec Králové. We thank Lukáš Nývlt and Pavlína Tauchmanová for their help with field sampling. We thank Pavel Tyrner and Jakub Straka for the revision of several specimens from the Chrysididae and Pompilidae families.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10841_2014_9723_MOESM1_ESM.pdf
Supplementary table 1: List of the species found, including the information on their sex, number captured by traps of each of the colors tested, number of trap-days, country where the species were captured, and their Red List status in the Czech Republic (PDF 131 kb)
10841_2014_9723_MOESM2_ESM.pdf
Supplementary table 2: Observed and expected numbers of individuals captured by traps of each of the colors tested. Shown are the species captured at n ≥ 10 individuals only (n = 96 species), the capture rates were tested by the χ2 tests with Bonferroni correction (PDF 130 kb)
Rights and permissions
About this article
Cite this article
Heneberg, P., Bogusch, P. To enrich or not to enrich? Are there any benefits of using multiple colors of pan traps when sampling aculeate Hymenoptera?. J Insect Conserv 18, 1123–1136 (2014). https://doi.org/10.1007/s10841-014-9723-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-014-9723-8