Abstract
Tree plantations may play a role in the conservation of global forest biodiversity. At the landscape scale, plantations with a complex understory may provide surrogate habitats for forest-dwelling organisms. This was tested using a manipulative experiment in which the abundance, movement and survivorship of Ceroglossus chilensis (an endemic and flightless ground beetle) was examined in plantation forest stands where the density of the understory vegetation was manipulated. Between 2009 and 2012, we collected C. chilensis by pitfall trapping in nineteen plots with naturally high, naturally low and experimentally removed understory cover. Beetle movement was evaluated by direct observation, and survival was quantified as the proportion of days that individuals survived in closed and open containers half-buried in the soil. C. chilensis exhibited higher abundance in plots with naturally high than with low or experimentally removed understory cover. Beetles traveled shorter distances and preferred to stay in stands with developed understory. C. chilensis had significantly higher mortality by predators in plantations with scarce understory cover. Therefore, forest plantations with a dense understory can become surrogate habitats for C. chilensis, and may contribute to the conservation of its populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In fragmented landscapes, remnant patches have been considered a main focus in conservation of threatened populations. However, at the landscape scale, the surrounding anthropogenic matrix is increasingly recognized as an important element of biodiversity conservation (Brady et al. 2009; Lindenmayer et al. 2009; Prevedello and Vieira 2010; Simonetti et al. 2012). Forest plantations have increased worldwide in recent decades (Brockerhoff et al. 2008; Felton et al. 2010). According to FAO (2010), forests cover around 4,000 million ha, representing about 31 % of the planet’s terrestrial surface, while forest plantations have grown to 264 million ha by 2010, comprising approximately 7 % of the total forested area. The contribution of forest plantations to the conservation of global forest biodiversity is therefore a question of increasing relevance (Lindenmayer and Hobbs 2004; Brockerhoff et al. 2008; Simonetti et al. 2012).
Although forest plantations have been regarded as “biological deserts” because they harbor impoverished faunal assemblages, there is increasing evidence that some plantations may support several native species (Lindenmayer and Hobbs 2004; Brockerhoff et al. 2008; Felton et al. 2010). A common feature of plantations harboring native species is the presence of a complex understory that provides surrogate habitats or dispersal pathways between remnants of fragmented native forest (Estades et al. 2012). Generally, movement of organisms in the matrix may imply higher dispersal costs because of increased predation risk and the uncertainty of reaching other suitable patches of habitat compared to the dispersal cost of moving through continuous habitat, unless the matrix is structurally complex (Goodwin and Fahrig 2002; Haynes and Cronin 2006). Furthermore, forest plantations with a developed understory may maintain microclimatic and biotic conditions similar to forest remnants and thus promote the survival of many organisms for which the original habitats have been reduced (Lindenmayer and Hobbs 2004). In fact, understory vegetation is considered to be a good predictor of faunal diversity (Bremer and Farley 2010; Estades et al. 2012). Therefore, the conservation of understory vegetation in plantations has been suggested as a way to enhance biological diversity, particularly species richness (Spellerberg and Sawyer 1995).
In Chile, forest plantations were started in the mid-1940s. During the 1970s, forest policy strongly supported them. As a result, between 1995 and 2009 Chile exhibited one of the highest annual rates of afforestation (49,020 ha) and reforestation (53,610 ha) in South America (Nahuelhual et al. 2012). Native forests have been extensively removed and fragmented, originally due to land clearing for farming and subsequently as a result of replacement by exotic plantations (Echeverría et al. 2006; Nahuelhual et al. 2012). This landscape modification has resulted in a mosaic of small forest remnants surrounded by a matrix dominated by Pinus radiata D. Don plantations (Echeverría et al. 2006).
The Maulino forest, a unique and threatened ecosystem, restricted to central Chile (between 35°S and 36°40′S), harbors several distinctive and endangered animal and plant species (Bustamante et al. 2006). Pine plantations surrounding the remnants of Maulino forest might provide habitat for populations of native plants, insects, amphibians, lizards, mammals and birds (Estades et al. 2012 and references therein). These plantations support a developed understory, consisting of Aristotelia chilensis, Cryptocarya alba and Persea lingue trees (Grez et al. 2003; Poch and Simonetti 2013).
Ceroglossus chilensis Eschscholtz (Coleoptera: Carabidae) is an endemic flightless ground beetle and a large (26–29 mm) diurnal predator associated with Nothofagus woods including the Maulino forest (Henríquez et al. 2009). In south-central Chile it is also numerically dominant and persistent in pine plantations, supposedly due to the presence of a developed understory cover in these plantations (Grez et al. 2003). Up to date, there is no experimental evidence regarding the effect of understory in pine plantations on the abundance, dispersal and survival of this species, as an example for other species surviving in plantations worldwide.
In this study we tested the hypothesis that the presence of dense understory in pine plantations is a determining factor for the abundance, movement and survival of C. chilensis. Particularly, we expected that (1) if the abundance of this beetle depends upon understory, it will diminish significantly in pine plantations where understory is experimentally removed, being similar to those observed in pine plantations with naturally low understory cover, (2) this insect will have longer and more linear displacements in pine plantations with low or experimentally removed understory cover. Additionally, they will prefer pine plantations with high understory cover over those with low understory cover, and (3) survival of C. chilensis will be lower in plantations with low or experimentally removed understory than in high understory cover plantations.
Materials and methods
Study site
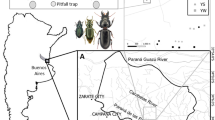
The study was carried out in commercial pine plantations located at Tregualemu in Central Chile (72°43′55.59′′W–35°59′37.12′′S to 72°40′46.34′′W–35°58′5.27′′S). The area covers over 1,370 ha; it is composed of remnants of Maulino forest and pine plantations with different levels of understory development, ranging from 160 to 540 m above sea level. Pine plantations consist of mature 20-year-old stands with 28 m high trees. The understory is dominated by native species such as Aristotelia chilensis and Peumus boldus shrubs, Persea lingue, Cryptocarya alba and Luma apiculata trees, but also includes non-native species such as Rubus ulmifolius shrubs (Poch and Simonetti 2013).
Experimental design
Based on availability and logistics, during November 2009 a total of nineteen 40 × 40 m2 plots located in the mature pine plantations were selected and classified into naturally low cover (L, 7 plots, 0–30 % cover) and naturally high cover (H, 12 plots, >50 %; Fig. 1). The development of the understory was evaluated by vegetation cover measurements (Poch and Simonetti 2013). Three 40 m parallel transects, 15 m apart, were sampled per stand. Within each transect, a 1 m radius-circular plot was established every 10 m, at which vertical plant density measurements were performed by intersection of plant individuals every 0.5 m from the ground up to 3 m. Plantations were classified as low understory cover if woody plant density 1 m above the ground was less than 0.5 individuals/m2, and high understory cover when density was above 0.5 individuals/m2. Plots were interspersed and separated by at least 300 m. This distance is adequate for plots to be statistically independent (Vergara and Simonetti 2004). During the pre-understory removal period (November 2009 and January 2011) the abundance of C. chilensis was assessed in each pine plot (see methods below), establishing a baseline for comparison with the post-understory removal period (February 2011 and July 2012). In February 2011, all woody vegetation was removed in half of the H plots, which were randomly assigned as experimental plots (E; Fig. 1). These plots thus mimic plots with low cover. Debris was removed from the area and disposed of properly. Subsequent regrowth was removed in February 2012. The remaining H and L plots were undisturbed, acting as control (Simonetti et al. 2013).
Abundance of Ceroglossus chilensis
The abundance (i.e. activity-density) of C. chilensis was assessed using dry pitfall traps, consisting of plastic jars of 11 cm diameter and 8 cm depth. In each sampling plot, eighteen pitfall traps were buried at ground level in a grid of 3 × 6 traps. Columns and rows were 10 and 5 m apart, respectively. Traps were opened for four consecutive days during each month from November 2009 to July 2012. Collected beetles were counted, marked with numbered queen bee marks and then released.
The capture probability of pitfall traps may depend on habitat structure, because structural complexity may affect the movement behavior of insects (Melbourne 1999). More captures may be observed in less complex habitats because insects would move more in these habitats with fewer obstacles and thus be more prone to fall in a trap. This possible bias was estimated through mark-release-recapture experiments in six 4 × 4 m2 enclosure plots, delimited by a black plastic barrier. Three of these had less than 10 % understory cover and the other three had more than 70 % understory cover. Nine pitfall traps 1 m apart were placed in a 3 × 3 m2 grid in each of these plots. Individuals were captured in remnant patches adjacent to pine forests with dry pitfall traps. Eight beetles were released at the center of each plot, and then re-captured, counted and released after 4 days. In total 48 different individuals were used for these pitfall bias experiments.
Movement of Ceroglossus chilensis
Movement of C. chilensis was evaluated by direct observation of 76 individual trails (visual tracking), 38 in H, 28 in L and 10 in E plots, during June 2010 and April to June 2011. Beetles were captured in remnant patches adjacent to pine forests with dry pitfall traps. Before beginning the observations, individuals were held in a transparent cage half-filled with soil and pine needles. Beetles were marked with a single white dot at the bottom of one elytron, using an oil paint marker. Each individual was placed on the ground at the center of each pine plot, within an upside-down vial; after several seconds, it was carefully released trying to minimize handling effects on its movement behavior, and then tracked for 30 min or until it was lost, marking its position with a stick every 30 s (i.e. time step; Goodwin and Fahrig 2002). All monitoring sessions were done between 11:00 am and 5:00 pm on warm days. At the end of the visual tracking, the trail was marked with a rope linking the sticks and drawn on paper, estimating total distance (i.e. the sum of linear distances between step points), displacement (i.e. straight-line distance between initial and endpoints of the path), displacement rate (i.e. displacement divided by total time of the monitoring session), the proportion of time that the beetle did not move (i.e. the immobile time steps divided by total time of the monitoring session), step-length (i.e. distance traced in the mobile time steps), and turning angles (i.e. the external angle between the new direction of movement and the previous direction; Goodwin and Fahrig 2002). Low values of the exterior angles (below 45° or above 135°) indicate a nearly linear movement, whereas high values for these angles (between 45° and 135°) show rather tortuous movement.
Probability of edge crossing
Additionally, to assess the preference of C. chilensis for high or low understory cover we studied the probability of edge crossing between areas with High–High, Low–Low and High–Low understory cover. This was assessed in a 6 × 3 m2 enclosure delimited by a black plastic barrier in a pine stand. This enclosure was divided into two similar areas. Initially, both areas of the enclosure had high understory cover (High–High). Then the understory was removed in one area of the enclosure, leaving it with less than 10 % cover, so the enclosure became composed of one high understory cover area adjacent to a low understory cover area (High–Low and Low–High). Finally, the understory was removed from the remaining high understory cover area, so both areas ended with low understory cover (Low–Low). Beetles were captured in remnant patches adjacent to pine forests with dry pitfall traps and individuals were marked with numbered queen bee marks and released at the center of one of the areas of the enclosure, following their movement until they were lost or for 5 min, recording whether they crossed the edge or not. Forty beetles were followed in High–High, 40 in Low–Low, 40 in High–Low, and 40 in Low–High treatments, with half of them released in each area of the enclosure, accounting for all possible treatments of edge crossing.
Survival of Ceroglossus chilensis
Survival of C. chilensis was evaluated in the nineteen pine plots, using 17-L transparent plastic containers, which were cut forming windows in the top, bottom and sides. Windows were covered with 2 mm galvanized metal mesh to allow the movement of prey in and out of the container, and strong enough to prevent the beetles from biting through and escaping (Ewers 2008). Each container was half-buried in the soil; the cage was filled with the same substrate removed from the soil. Consequently, the cages had the typical substrate of the pine stands and were connected to the above- and below-ground environments by the mesh-covered windows (Ewers 2008). Two experimental containers were placed per plot, one was left open to estimate beetle mortality by predators, and the other was closed with the mesh-covered container lid to assess mortality by factors other than predation (e.g., microclimate effect). Beetles were captured in remnant patches adjacent to pine forests with dry pitfall traps (those used initially and those used for the replacement of dead beetles, see below). Three individuals were added to each experimental container, totaling 114 in all containers; their survival was assessed once a month in two periods, from May to December 2010 and from March to December 2011. Dead beetles were removed from the container and replaced with live beetles (Ewers 2008). Individuals that were not found in the open containers were considered as preyed upon, and replaced. Disturbed containers were replaced (Ewers 2008). Parallel to these experiments, microclimatic conditions (i.e. temperature and relative humidity at ground level) were measured at noon inside the experimental containers in all pine plots with a portable thermo-hygrometer.
Data analysis
The effect of understory cover on the cumulative abundance of C. chilensis in pine plantations in the pre-understory removal period was tested using one-way ANOVA. To evaluate the understory manipulation effect on the abundance of C. chilensis in pine plantations, we pooled the abundance observed during equivalent months in the pre- and post-understory removal periods (November, December, January, April, June and July 2009–2010 and the same months in 2011–2012). Changes in abundance were evaluated through the response ratio of the abundance, evaluated as ln(post-understory removal abundance/pre-understory removal abundance). If the abundance of beetles is related to the presence of understory, the response ratio should be negative, with lower abundance after removal compared to the baseline abundance. In contrast, the ratio should be zero if there is no difference in abundance of beetles before and after the removal of the understory (Simonetti et al. 2013). Data was analyzed through a t test of means against the reference value. For bias estimation, the number of recaptured individuals in L and H plots was compared with a Mann–Whitney U test.
The effect of the understory cover on each movement variable was tested using one-way ANOVA and Fisher LSD. As turning angles are circular data, the mean angles, standard error of the mean and the length of the mean vector (r) were estimated for each understory cover type. The r vector is a measure of the concentration of circular data that varies from 0 (when the distribution of the turning angles is completely uniform) to 1 (when all turning angles are in the same direction; Zar 2010). To evaluate the effect of understory cover on this variable, the Watson-Williams F test was used. These circular analyses were performed in Oriana 4.0 (Kovach Computing Services).
The probability of edge crossing (i.e. proportion of beetles that crossed) between areas with High–High and Low–Low, and High–Low and Low–High understory cover in pine stands was compared with a χ2 and a post hoc analysis with a multiple comparison test for proportions (Marascuilo procedure; Marascuilo 1966).
Survival in pine plots was quantified as the proportion of days that beetles survived relative to the total duration of the experiment (Ewers 2008). Data from disturbed cages were not included in the statistical analysis. The effect of understory manipulation on the survival of C. chilensis was assessed with a two-way ANOVA, considering understory cover (H, L and E) and lid (Closed and Open cages) as factors. The Fisher LSD test was used for post hoc comparisons. The same analysis was carried out for microclimate data.
Data transformations [log (x) for abundance, total distance and displacement; log (x + 1) for step-length; \(\sqrt x\) for displacement rate and \(\sin^{ - 1} \left( {\sqrt x } \right)\) for the proportion of time that the individual did not move and for survival] were used to meet the assumptions of ANOVA when necessary. All statistical analyses were run in STATISTICA 7.0.
Results
Abundance of Ceroglossus chilensis
Regarding bias estimation, recaptures in plots with different levels of understory development did not differ significantly (N = 3, U = 4.0, P = 0.83). The rank sum values of recaptures in the enclosures with low and high understory were 11 and 10, respectively. Therefore, the presence of understory does not bias the estimates of abundance.
During the pre-understory removal period, the cumulative abundance was five times higher in H plots (mean ± SE, 70.1 ± 29.1 individuals) than in L plots (14.9 ± 5.0 individuals; F1,17 = 6.50, P = 0.02). The abundance of C. chilensis did not change in H and L plots pre- versus post-understory removal (Fig. 2). In contrast, beetle abundance in the E plots was significantly lower after the understory was removed compared to the same plots before understory removal. Only this response ratio differed significantly from zero, which is the expected outcome if abundances were not affected by understory removal (E: t = −3.06, P = 0.04; H: t = −0.56, P = 0.60; L: t = 0.63, P = 0.55).
Movement of Ceroglossus chilensis
Ceroglossus chilensis traveled significantly shorter distances in H than in L and E plots (total distance, F2,73 = 6.40, P = 0.003; displacement, F2,73 = 5.79, P = 0.005; Fig. 3), and traced significantly shorter steps in H compared to L plots (F2,73 = 8.28, P = 0.0006; Fig. 3). However, no significant differences were observed between plots regarding displacement rate (F2,73 = 2.16, P = 0.12; Fig. 3), proportion of immobile time (F2,73 = 0.08, P = 0.92; Fig. 3), or turning angles [less than 45° in all plots and concentrated in the same direction (r = 0.88–0.97); F2,55 = 1.66, P = 0.20; Fig. 3].
Movement of C. chilensis in High, Experimental and Low understory cover pine plots estimated by visual tracking. a Total distance (mean ± 1 se), b displacement distance (mean ± 1 se), c displacement rate (mean ± 1 se), d step length (mean ± 1 se), e proportion of immobile time (mean ± 1 se) and f turning angle, letters represent the type of plot and colored dots indicate the mean angle of a trail in each treatment. Letters represent Fisher LSD test for post hoc comparisons, with different letters indicating significant differences (P < 0.05)
Probability of edge crossing
The proportion of beetles that crossed edges differed significantly between treatments χ 2 3 = 23.76, P < 0.001. The proportion of individuals crossing from low to high understory cover was significantly greater than any other treatment, which did not differ between them (Table 1).
Ceroglossus chilensis survival
Ceroglossus chilensis survived significantly longer in closed than in open containers (F1,222 = 13.00, P = 0.0004; Closed: 0.63 ± 0.03; Open: 0.40 ± 0.03). No significant differences were detected in beetle survival between plots with different levels of understory cover (F2,222 = 2.89, P = 0.06; H: 0.55 ± 0.03; L: 0.48 ± 0.04; E: 0.52 ± 0.06). However, the beetles survived the lowest proportion of days in the open containers located in the L plots (understory cover × lid interaction, (F2,222 = 3.85, P = 0.02; Fig. 4).
Proportion of days (mean ± 1 SE) that C. chilensis survived in High, Experimental and Low understory cover plots, between May 2010 and December 2011 for High and Low treatments, and between March 2011 and December 2011 for Experimental treatment. Each pine plot had one closed and one opened container. Black bars represent closed containers and white bars represent open containers. Letters represent Fisher LSD test for post hoc comparisons, with different letters indicating significant differences (P < 0.05)
Microclimatic conditions at ground level significantly differed between plots, with temperature values being lower and relative humidity values higher in H plots (temperature: F2,70 = 6.27, P = 0.003; H: 13.9 ± 0.3; L: 15.1 ± 0.2; E: 15.7 ± 0.4; relative humidity: F2,70 = 11.69, P < 0.001; H: 78.4 ± 0.6; L: 74.6 ± 0.8; E: 73.0 ± 1.8). No significant differences were detected in microclimatic conditions between open and closed containers (temperature: F1,70 = 0.001, P = 0.97; relative humidity: F1,70 = 2.38, P = 0.13).
Discussion
This study is the first experimental demonstration that understory is a key factor for supporting carabid beetles in pine plantations. In stands with a naturally dense understory, C. chilensis attains higher abundance, moves shorter distances, and survives predation better than in low or experimentally removed understory plots. Also, they prefer to move toward these stands over those with naturally low or removed cover. These results reaffirm the assertion that plantations with a developed understory can be a surrogate habitat for native fauna including carabid beetles (Lindenmayer and Hobbs 2004; Brockerhoff et al. 2005; 2008; Felton et al. 2010; Simonetti et al. 2012, 2013).
Forest plantations including pine are increasing worldwide, becoming a dominant landscape component, therefore being the matrix in which native remnants and their associated fauna are embedded (Driscoll et al. 2013). Plantations with a developed understory may be structurally similar to the native forests which also have such an understory, enabling these plantations to support high populations of the original fauna (Saura et al. 2014). The complexity offered by the understory can influence the movement and survival of insect populations (Sweaney et al. 2014). The movement of organisms in more complex matrices is generally slower and more tortuous compared to simpler habitats (Goodwin and Fahrig 2002; Fahrig 2007; Hawkes 2009; Kuefler et al. 2010). Indeed, C. chilensis exhibited lower total distance, displacement and step-length in stands with high understory than in those with low or removed understory. Interestingly, as in pine plantations with high understory cover, C. chilensis in native forests displayed shorter steps and lower displacement rates than in pine plantations (Donoso 2011), which suggests that the movement behavior of C. chilensis in plantations with a developed understory is similar to that in native forests. This behavior may imply that insects remain for longer times in these stands, which could account for the observed higher abundance there.
Furthermore, C. chilensis prefers pine plantations with dense understory, as demonstrated by the edge crossing probability experiments. Understory seems to protect C. chilensis from death, particularly from predation, which is suggested by the lower survival observed in open containers, particularly in the low understory cover plantations. This is consistent with what has been observed in other large ground beetles, which select dense vegetation to escape from natural enemies (Brose 2003). Contrary to our expectations, predation risk was similar in plantations with dense and experimentally removed understory. Although carabids have several antipredator defenses, including chemical ones, predation is an important mortality factor for adults (Lövei and Sunderland 1996). The most probable predators feeding on C. chilensis are small mammals, birds and foxes (Silva-Rodríguez et al. 2010; Donoso 2011). All of these have large home-ranges, greater than the 40 × 40 m2 plots where the understory was experimentally removed, which were embedded in a stand with high understory cover. Therefore, quite probably these predators were not able to perceive these stands as low understory plantations. However, microclimatic conditions play no role in survival. First, at the stand level there was no change in temperature and relative humidity in closed versus opened containers, but survival was greater in closed cages. Second, between low and high understory cover stands, despite differences in microclimatic conditions, there were no changes in survival of C. chilensis.
Our results suggest that forest plantations can become surrogate habitats for C. chilensis, as has been proposed for other carabids around the world (Berndt et al. 2008; Pawson et al. 2008; Oxbrough et al. 2010). But for this to occur, forest plantations should maintain some natural structures that mimic natural forests (Niemelä et al. 2007; Lange et al. 2014). In this matter, we demonstrated that pine plantations are suitable habitat for C. chilensis only if the development of the understory is allowed or enhanced. Our results with C. chilensis are not an isolated case, as the role of understory as an enhancer of habitat quality has also been experimentally demonstrated in vertebrate species such as birds in oil palm plantations and carnivores in pine plantations (Nájera and Simonetti 2010; Estades et al. 2012; Simonetti et al. 2013). In a world where the increasing expansion of forestry plantations threatens native forests and their fauna, the challenge for plantations fulfilling an active role in biodiversity conservation has been raised (Hartley 2002; Brockerhoff et al. 2008). Despite being a common element of the landscape, there is a paucity of managerial recommendations to render plantations a more suitable habitat for native fauna (Sweaney et al. 2014). An emerging issue in this matter is to manage stand structure so as to allow for understory growth, which often is removed from plantations in order to enhance productivity (Lindenmayer and Hobbs 2004; van Halder et al. 2008).
According to Aichi Biodiversity Targets (Convention on Biological Diversity 2010), by 2020 areas under forestry regimes should be managed sustainably, ensuring conservation of biodiversity. This goal can be achieved by enhancing the growth of understory in forest plantations. If this management practice is pursued, plantations will reduce the direct pressures on biodiversity, providing surrogate habitats for native fauna and fostering more sustainable forestry practices.
References
Berndt LA, Brockerhoff E, Jactel H (2008) Relevance of exotic pine plantations as a surrogate habitat for ground beetles (Carabidae) where native forest is rare. Biodivers Conserv 17:1171–1185
Brady MJ, Mcalpine CA, Miller CJ, Possingham HP, Baxter GS (2009) Habitat attributes of landscape mosaics along a gradient of matrix development intensity: matrix management matters. Landsc Ecol 24:879–891
Bremer LL, Farley K (2010) Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers Conserv 19:3893–3915
Brockerhoff EG, Berndt LA, Jactel H (2005) Role of exotic pine forests in the conservation of the critically endangered New Zealand ground beetle Holcaspis brevicula (Coleoptera: Carabidae). N Z J Ecol 29:37–43
Brockerhoff EG, Jactel H, Parrota JA, Quine CP, Sayer J (2008) Plantation forests and biodiversity: oxymoron or opportunity? Biodivers Conserv 17:925–951
Brose U (2003) Bottom-up control of carabid beetle communities in early successional wetlands: mediated by vegetation structure or plant diversity? Oecologia 135:407–413
Bustamante RO, Grez AA, Simonetti JA (2006) Efectos de la fragmentación del bosque maulino sobre la abundancia y diversidad de especies nativas. In: Grez AA, Simonetti JA, Bustamante RO (eds) Biodiversidad en ambientes fragmentados de Chile: patrones y procesos a diferentes escalas. Editorial Universitaria, Santiago, pp 83–97
Convention on Biological Diversity (2010) Strategic plan for biodiversity 2011–2020 and the Aichi targets. Secretariat of the Convention on Biological Diversity, Montreal. http://www.cbd.int/doc/strategic-plan/2011-2020/Aichi-Targets-EN.pdf. Accessed Dec 2014
Donoso D (2011) Demografía, movimiento y diversidad genética de Ceroglossus chilensis en el bosque Maulino fragmentado. Dissertation, Universidad de Chile, Santiago, Chile
Driscoll DA, Banks SC, Barton PS, Lindenmayer DB, Smith AL (2013) Conceptual domain of the matrix in fragmented landscapes. Trends Ecol Evol 28(10):605–613
Echeverría C, Coomes D, Salas J, Rey-Benayas JM, Lara A, Newton A (2006) Rapid deforestation and fragmentation of Chilean temperate forests. Biol Conserv 130:481–494
Estades CF, Grez AA, Simonetti JA (2012) Biodiversity in Monterey pine plantations. In: Simonetti JA, Grez AA, Estades CF (eds) Biodiversity conservation in agroforestry landscapes: challenges and opportunities. Editorial Universitaria, Santiago, pp 77–98
Ewers RM (2008) Spatio-temporal variation in mortality rates of Mecodema spp. (Coleoptera: Carabidae) across a forest-grassland edge in New Zealand. Insect Conserv Divers 1:40–47
Fahrig L (2007) Non-optimal animal movement in human-altered landscapes. Funct Ecol 21:1003–1015
FAO (2010) Global Forest Resources Assessment 2010. Main Report. FAO Forestry Paper 163, Rome, pp 346
Felton A, Knight E, Wood J, Zammit C, Lindenmayer D (2010) A meta-analysis of fauna and flora species richness and abundance in plantations and pasture lands. Biol Conserv 143:545–554
Goodwin BJ, Fahrig L (2002) Effect of landscape structure on the movement behaviour of a specialized goldenrod beetle, Trirhabda borealis. Can J Zool 80:24–35
Grez AA, Moreno PO, Elgueta M (2003) Coleópteros (Insecta, Coleoptera) epigeos asociados al bosque maulino y plantaciones de pino aledañas. Rev Chil Entomol 29:9–18
Hartley MJ (2002) Rationale and methods for conserving biodiversity in plantation forests. For Ecol Manag 155:81–95
Hawkes C (2009) Linking movement behaviour, dispersal and population processes: is individual variation a key? J Anim Ecol 78:894–906
Haynes KJ, Cronin JT (2006) Interpatch movement and edge effects: the role of behavioral responses to the landscape matrix. Oikos 113:43–54
Henríquez P, Donoso D, Grez AA (2009) Population density, sex ratio, body size and fluctuating asymmetry of Ceroglossus chilensis (Carabidae) in the fragmented Maulino forest and surrounding pine plantations. Acta Oecol 35:811–818
Kuefler D, Hudgens B, Haddad NM, Morris WF, Thurgate N (2010) The conflicting role of matrix habitats as conduits and barriers for dispersal. Ecology 91:944–950
Lange M, Türke M, Pašalić E, Boch S, Hessenmöller D, Müller J, Prati D, Socher SA, Fischer M, Weisser WW, Gossner MM (2014) Effects of forest management on ground-dwelling beetles (Coleoptera; Carabidae, Staphylinidae) in Central Europe are mainly mediated by changes in forest structure. For Ecol Manag 329:166–176
Lindenmayer DB, Hobbs RJ (2004) Fauna conservation in Australian plantation forests—a review. Biol Conserv 119:151–168
Lindenmayer DB, Wood JT, Cunningham RB, Crane M, Macgregor C, Michael D, Montague-Drake R (2009) Experimental evidence of the effects of a changed matrix on conservating biodiversity within patches of native forest in an industrial plantation landscape. Landsc Ecol 24:1091–1103
Lövei G, Sunderland KD (1996) Ecology and behavior of ground beetles (Coleoptera: Carabidae). Annu Rev Entomol 41:231–256
Marascuilo LA (1966) Large-sample multiple comparisons. Psychol Bull 65:280–290
Melbourne BA (1999) Bias in the effect of habitat structure on pitfall traps: an experimental evaluation. Aust J Ecol 24:228–239
Nahuelhual L, Carmona A, Lara A, Echeverría C, González ME (2012) Land-cover change to forest plantations: proximate causes and implications for the landscape in south-central Chile. Landsc Urban Plan 107:12–20
Nájera A, Simonetti JA (2010) Can oil palm plantations become bird friendly? Agrofor Syst 80:203–209
Niemelä J, Koivula M, Kotze DJ (2007) The effects of forestry on carabid beetles (Coleoptera: Carabidae) in boreal forests. J Insect Conserv 11:5–18
Oxbrough A, Irwin S, Kelly TC, O’Halloran J (2010) Ground-dwelling invertebrates in reforested conifer plantations. For Ecol Manag 259:2111–2121
Pawson S, Brockerhoff E, Meenken E, Didham R (2008) Non-native plantation forests as alternative habitat for native forest beetles in a heavily modified landscape. Biodivers Conserv 17:1127–1148
Poch T, Simonetti JA (2013) Insectivory in Pinus radiata plantations with different degree of structural complexity. For Ecol Manag 304:132–136
Prevedello JA, Vieira MV (2010) Does the type of matrix matter? A quantitative review of the evidence. Biodivers Conserv 19:1205–1223
Saura S, Martín-Queller E, Hunter ML Jr (2014) Forest landscape change and biodiversity conservation. In: Azevedo JC, Perera AH, Pinto MA (eds) Forest landscapes and global change: 167 challenges for research and management. Springer, New York, pp 167–198
Silva-Rodríguez EA, Ortega-Solís GR, Jiménez JE (2010) Conservation and ecological implications of the use of space by chilla foxes and free-ranging dogs in a human-dominated landscape in southern Chile. Aust Ecol 35:765–777
Simonetti JA, Grez AA, Estades CF (2012) Biodiversity conservation in agroforestry landscapes: challenges and opportunities. Editorial Universitaria, Santiago
Simonetti JA, Grez AA, Estades CF (2013) Providind habitat for native mammals through understory enhancement in forestry plantations. Conserv Biol 27:1117–1121
Spellerberg IF, Sawyer JWD (1995) Multiple-use, biological diversity and standards. N Z J For 39:21–25
Sweaney N, Lindenmayer DB, Driscoll DA (2014) Is the matrix important to butterflies in fragmented landscapes? J Insect Conserv 18:283–294
van Halder I, Barbaro L, Corcket E, Jactel H (2008) Importance of semi-natural habitats for the conservation of butterfly communities in landscapes dominated by pine plantations. Biodivers Conserv 17:1149–1169
Vergara PM, Simonetti JA (2004) Does nest site cover reduce nest predation upon rhynocriptids? J Field Ornithol 75:188–191
Zar JH (2010) Biostatistical analysis, 5th edn. Prentice Hall, Upper Saddle River
Acknowledgments
This study was supported by Fondecyt 1095046 and Programa Domeyko-Biodiversidad (Iniciativa Transversal 3), Universidad de Chile. We are grateful to the Corporación Nacional Forestal and Forestal Masisa S.A. for allowing us to work on their property. Thanks are due to A. Rodríguez-San Pedro and R. Zúñiga for their field support, and two anonymous reviewers for their cogent criticisms. We are grateful to Cristián Estades for providing some of the images in Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cerda, Y., Grez, A.A. & Simonetti, J.A. The role of the understory on the abundance, movement and survival of Ceroglossus chilensis in pine plantations: an experimental test. J Insect Conserv 19, 119–127 (2015). https://doi.org/10.1007/s10841-015-9752-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-015-9752-y