Abstract
Background
Radiofrequency ablation (RFA) and cryo-ablation (CRA) have been traditionally performed with fluoroscopy which exposes patients and medical staff to the potential harmful effects of the X-ray. Therefore, we aimed to assess the feasibility, safety, and effectiveness of RFA and CRA of atrioventricular nodal reentry tachycardia (AVNRT) guided by the three-dimensional (3D) electro-anatomical mapping (EAM) system without the use of fluoroscopy.
Methods
We analyzed 168 consecutive patients with AVNRT, 62 of whom were under 19 years of age (128 in RFA (age 34.04 ± 21.0 years) and 40 in CRA (age 39.41 ± 22.8 years)). All procedures were performed completely without the use of the fluoroscopy and with the 3D EAM system.
Results
The acute success rates (ASR) of the two ablation methods were very high and similar (for RFA 126/128 (98.4%) and for CRA 40/40 (100%); p = 0.43). Total procedural time (TPT) was similar in RFA and CRA groups (75.04 ± 42.31 min and 73.12 ± 30.54 min, respectively; p = 0.79). Recurrence rates (1 (2.5%) and 8 (6.25%); p = 0.35) were similar. There were no complications associated with procedures in either group. In pediatric group, ASR (61/62 (98.38%) and 105/106 (99.05%), respectively; p = 0.69) and TPT (75.16 ± 42.2 min and 74.23 ± 38.3 min, respectively; p = 0.88) were similar to the adult group. High ASR was observed with both ablation methods (for RFA 49/50, 98%, and for CRA 12/12, 100%; p = 0.62] with very high arrhythmia-free survival rates (for RFA 98% and for CRA 100%; p = 0.62).
Conclusion
Based on these results, it can be suggested that fluoroless RFA or CRA guided by the 3D EAM system can be routinely performed in all patients with AVNRT without compromising safety, efficacy, or duration of the procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Atrioventricular nodal reentrant tachycardia (AVNRT) is the most common regular supraventricular arrhythmia in adults and children [1]. Catheter radiofrequency ablation (RFA) and cryo-ablation (CRA) procedures are considered to be the first-line treatment option for AVNRT. These procedures have high acute success rates, low recurrence rates, and low incidence of complications [1,2,3,4,5,6,7]. Conventionally, fluoroscopy is used as an effective method to guide catheters and to trail their location during catheter ablation procedures [8]. However, fluoroscopy exposes patients and medical staff to potentially harmful stochastic (non-dose-dependent) and deterministic (dose-dependent) radiation effects with no known safe dose [9,10,11]. Therefore, the ALARA (as low as reasonably achievable) principle should be followed to reduce or eliminate radiation exposure in electrophysiological procedures [12].
Non-fluoroscopic approach with three-dimensional (3D) electro-anatomical mapping (EAM) abolishes radiation exposure in various ablation procedures in adult and pediatric population [13,14,15,16,17,18,19,20,21,22,23,24]. In selected patients with higher risk of atrioventricular (AV) nodal damage, CRA could present a safer option compared with RFA. However, RFA is still the preferred ablation method for supraventricular tachyarrhythmias (SVTs) in children and adults [4, 6]. Moreover, there are only a few studies that focused on the safety and efficacy of CRA without the use of fluoroscopy in both pediatric and adult patients with AVNRT [16, 17].
In the present study, we aimed to compare the feasibility, safety, and effectiveness of fluoroless CRA and RFA procedures in pediatric and adult patients with AVNRT.
2 Materials and methods
2.1 Study population
In this retrospective study, we analyzed 168 consecutive patients with AVNRT that had ablation procedures performed in our institution from December 2014 until December 2019. Only first-time procedures performed with either only CRA or only RFA were included in the analysis. Procedures with crossover between both methods and repeat procedures were excluded from analysis. All patients had a history of paroxysmal palpitations and had ECG documentation of their clinical tachycardia. Clinical examination, laboratory examinations, and echocardiography were performed before the procedure. Anti-arrhythmic drugs were discontinued at least 1 week before the procedure.
2.2 Ablation procedures
Procedures were preferably performed in conscious sedation, except for patients aged 14 years or less in which general anesthesia was used. Femoral vein punctures were performed to access the heart. All procedures were performed completely without the use of the fluoroscopy and with the 3D EAM system (EnSite™ NavX™ Velocity™ and later Precision™, Abbott Park, Illinois, USA). When trans-septal puncture was needed, we used intra-cardiac echocardiography (ICE, Acuson AcuNav™, Biosense Webster, Irvine, California, USA) for puncture guidance.
2.3 Placement of catheters
Once the femoral access was obtained, the 3D EAM system was initialized, and a 10-polar deflectable diagnostic catheter was placed inside the femoral vein and advanced to the right atrium. A 3D anatomical map was created during the advancement of the 10-polar deflectable diagnostic catheter, so the entire path of the catheter from the groin to the right atrium was recorded and used for the advancement of other catheters.
After the 10-polar deflectable diagnostic catheter reached the right atrium, which was recognized by the appearance of endocardial voltage signals on a system for recording and analysis of intra-cardiac electrograms, a partial 3D EAM map of the right atrium was created. In the process, the location of the His bundle was tagged. Finally, the diagnostic catheter was advanced into the coronary sinus, and a shadow of the catheter was used as a location reference in the case of a map shift. Further catheters were advanced from the groin to the right atrium in a similar fashion. Typically, only a 10-polar diagnostic catheter and an ablation catheter were used. Either a non-irrigated 4-mm tip RFA catheter (Celsius®, Biosense Webster, Irvine, California, USA) or CRA catheter (4 mm or 6 mm, Freezor™ or Freezor Xtra™, Medtronic, Minneapolis, USA) was used after the completion of the diagnostic part of the procedure. In our institution, slow pathway ablation in children is routinely performed using cryo energy with a 4-mm tip catheter in order to be more selective in creating the ablation lesion.
2.4 The electrophysiology study
A typical electrophysiology study (EPS) was performed: programmed stimulation from the right ventricle, followed by programmed stimulation from the proximal coronary sinus. We searched for a possible jump of conduction to the slow pathway. The goal of the study was the induction of the tachycardia.
Additional diagnostic maneuvers during ongoing tachycardia were performed when needed.
2.5 Radiofrequency ablation of the slow pathway
Once the diagnosis of AVNRT was confirmed and when radiofrequency (RF) was used as an energy source for ablation, the rest of the procedure was continued in sinus rhythm. The technique used in RFA cases was a slight modification of the established anatomical approach [25, 26]. The RFA catheter tip was positioned in the anatomical location of the slow pathway, and ablation was started at 20 W. If nodal rhythm was observed within 20 s of ablation, the power was increased to 30 W for 10 s and later to 40 W. RF ablation was continued until the disappearance of nodal response or until 60 s of ablation time elapsed.
2.6 Cryo-mapping and cryo-ablation of the slow pathway
In the CRA group, the initial approach in all procedures was cryo-mapping in the region of the slow pathway during ongoing AVNRT, with a switch to cryo-ablation when termination of tachycardia within 20 s of reaching − 30 °C was achieved. When cryo-mapping was not possible due to catheter instability, cryo-ablation was used during ongoing AVNRT for up to 10 s at − 70 °C or lower. When AVNRT was not readily inducible, termination of the slow pathway conduction was targeted with cryo-mapping during programmed stimulation with atrial extra stimuli that induced manifest slow pathway conduction such as atrioventricular prolongation combined with at least one atrial “echo” beat. If slow pathway conduction was blocked, cryo-mapping was converted to cryo-ablation. After the initial successful cryo-ablation, an immediate additional cryo-ablation lesion (i.e., “bonus” lesion) was always added. Cryo-ablation was uniformly applied for 240 s at each location. If any form of AV conduction disturbance occurred during tissue cooling, cryo-mapping or cryo-ablation was at once terminated.
2.6.1 Definition of procedural time, total ablation time, procedural success, complications, and follow-up
Procedural time (PT) was defined as the time interval from the placement of the venous sheaths to their removal at the end of the ablation procedure. Total ablation time (TAT) was defined as time spent delivering radiofrequency energy to the tissue or cooling the tissue with cryo-ablation. TAT was recorded automatically by the RFA generator or the cryo console. After ablation endpoints were achieved, we repeated the EPS, with and without administration of isoproterenol, to test the inducibility of the tachycardia. The procedural endpoint was the non-inducibility of AVNRT. If this was reached, the procedure was deemed acutely successful. If residual slow pathway conduction in the form of a single atrial “echo” beat was recorded, it was not considered a failure.
All patients underwent a post-procedural echocardiogram and clinical examination to exclude pericardial effusion or other acute complications. Any early (during the same hospital stay) or late complications were reported by the physician performing the follow-up. Patients were followed clinically and with the 12-lead ECG recordings 1 month after the procedure and every 6 months thereafter. The 24-h Holter ECG recordings were performed at the discretion of the physician performing the follow-up. Any recorded AVNRT recurrences were used to calculate long-term success rate (LTSR, same as arrhythmia-free survival rate) and recurrence rate (RR).
2.7 Statistical analysis
The statistical analyses were carried out using the IBM SPSS software version 24.0 for Windows (IBM Corporation, Armonk, NY, USA). Continuous variables were presented as mean ± standard deviation (SD), while categorical variables were presented as n or ratio. Since almost all values of baseline demographics and clinical characteristics were normally distributed, unpaired Student’s t test was used to compare groups in terms of these parameters. Multiple group comparisons were performed with one-way ANOVA. Pearson Chi-square test and Fisher exact test were performed to compare categorical variables between groups. p Value was considered significant when it was less than 0.05.
3 Results
There were 40 patients in the CRA group (62.5% females, age 39.41 ± 22.8 years) and 128 patients in the RFA group (63.4% females, age 34.04 ± 21.0 years). There were no patients with congenital heart disease. Conventional cardiovascular risk factors were mostly absent, except for those indicated in Table 1. There were no statistically significant differences in demographic and clinical parameters between both groups.
Detailed demographic and clinical characteristics of the study population are shown in Table 1. The ASR of the ablation procedure was mildly higher in the CRA group compared with the RFA group (40/40 (100%) and 126/128 (98.4%), respectively; p = 0.43). The RFA group was followed for a longer period than the CRA group (503.0 ± 396.9 days and 296.4 ± 181.9, respectively; p < 0.001). LTSR was higher in the CRA group (97.5% (39/40) and 93.8% (120/128), respectively; p = 0.55), resulting in a non-significantly higher RR (CRA and RFA, respectively; 1 patient (2.5%) and 8 patients (6.25%); p = 0.35) in the RFA group. PT was similar in RFA and CRA groups (75.04 ± 42.31 min and 73.12 ± 30.54 min, respectively; p = 0.79); TAT was significantly higher (978.4 ± 505.1 s and 247.7 ± 192.6 s, respectively; p < 0.0001) in the CRA group when compared with the RFA group. At the last follow-up, one patient in the CRA group (1/40, 2.5%) and 14 patients in the RFA group (14/128, 11%) were treated with anti-arrhythmic drugs. Detailed results are shown in Table 2.
Sixty-two pediatric AVNRT patients, under 19 years of age (36/62 females, average age 12.94 ± 3.49 years) were included in our study. When compared with adult patients, ASR (61/62 (98.4%) and 105/106 (99.1%), respectively; p = 0.85), RR (1 patient (1.61%) and 8 patients (7.54%), respectively; p = 0.18), PT (76.6 ± 42.5 min and 74.2 ± 38.3 min, respectively; p = 0.71), and TAT (403.4 ± 589.6 s and 451.8 ± 391.2 s, respectively; p = 0.53) were similar. High ASR (for CRA 12/12, 100%, and for RFA 49/50, 98%) was observed with both ablation methods. This pediatric patient group was followed for a longer period than the adult group (563.03 ± 399.6 days and 393.7 ± 341.2 days, respectively; p = 0.012). Additionally, pediatric group had similar LTSR with both methods (100% with CRA and 98% with RFA; p = 0.62) and no periprocedural complications.
In the CRA group, 28 patients (70%, 28/40) had cryo-mapping performed during ongoing tachycardia (Figs. 1 and 2). In those patients time to termination (TT) of tachycardia during cryo-mapping was 6.94 s. In the rest of patients, AVNRT was not re-inducible after the initial induction, and cryo-mapping was performed during repeated programmed atrial stimulation with clear slow pathway conduction as shown in Figs. 3 and 4.
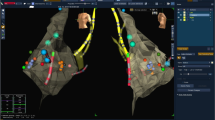
A 3D EAM partial reconstruction of the right atrial anatomy relevant for AVNRT ablation. a Anteroposterior view. b Left lateral view. White dots mark the course of the tricuspid annulus, yellow dots mark the His location, and green dots mark the location of cryo-mapping at the presumed slow pathway location. Blue dots mark cryo-ablation lesions at the initial successful cryo-mapping sites. SVC superior vena cava, IVC inferior vena cava, CS coronary sinus
Surface electrocardiogram (ECG) and intra-cardiac electrogram (iECG) tracings of AVNRT terminated with cryo-mapping at the slow pathway location. The asterisk marks the occurrence of conduction block in the slow pathway and AVNRT termination. I, II, V1, V3, and aVF are surface ECG tracings; CSD-CSP are iECG tracings from the 10-polar catheter placed in the coronary sinus; CRYO-D and CRYO-P are iECG tracings from the distal and proximal bipoles of the cryo-ablation catheter with some signal noise due to cryo-mapping induced cooling of the catheter tip
Surface electrocardiogram (ECG) and intra-cardiac electrogram (iECG) tracings of repeated programmed atrial stimulation. The line with double arrowheads marks the long atrioventricular interval typical for slow pathway conduction; the arrow marks the atrial “echo” beat indicating retrograde fast pathway conduction. I, aVL, and V2 are surface ECG tracings; CSD-CSP are iECG tracings from the 10-polar catheter placed in the coronary sinus; CRYO-D and CRYO-P are iECG tracings from the distal and proximal bipoles of the cryo-ablation catheter
Surface electrocardiogram (ECG) and intra-cardiac electrogram (iECG) tracings of repeated programmed atrial stimulation with the block of slow pathway conduction during cryo-mapping. The double slash marks the occurrence of conduction block in the slow pathway; the arrowhead marks the occurrence of nodal escape beat appearing after the loss of atrioventricular conduction. I, aVL, and V2 are surface ECG tracings; CSD-CSP are iECG tracings from the 10-polar catheter placed in the coronary sinus; CRYO-D and CRYO-P are iECG tracings from the distal and proximal bipoles of the cryo-ablation catheter with prominent signal noise due to cryo-mapping induced catheter tip cooling
There were no complications associated with procedures. No fluoroscopy was used during procedures.
4 Discussion
The results of our study show that CRA and RFA procedures can be successfully and safely performed in AVNRT patients without the use of fluoroscopy. ASR was excellent (100% for CRA and 98.4% for RFA), and RR was low (2.5% in the CRA group and 6.25% in the RFA group) in adult and pediatric patients. Most of CRA procedures were successfully performed with cryo-mapping during ongoing AVNRT with a reasonable TT. In addition, there was a trend toward lower RR in the CRA group.
RFA and CRA with the use of fluoroscopy are well-established techniques for AVNRT treatment [2,3,4,5,6,7]. Santangeli et al. [4] analyzed a total of 2340 patients in 5 prospective randomized and 9 retrospective studies in a systematic review. Although RFA was associated with shorter PT, there was no significant difference in ASR between RFA and CRA (88% versus 83, respectively; p = 0.157). However, permanent AV block was reported in 0.87% of cases in the RFA group and none in the CRA group (p = 0.035). During median follow-up of 10.5 months, arrhythmia-free survival rate was significantly higher in the RFA group (96.5% versus 90.9%; p < 0.001). Similarly, Hanninen et al. [5] analyzed a total of 5617 patients in 14 trials and reported that ASR with RFA was non-significantly higher than with CRA. CRA was associated with the reduced risk of permanent AV block (0.75% vs 0%) and shorter fluoroscopy time (13.5 min vs 15.9 min). However, these advantages were balanced by a lower arrhythmia-free survival rate (93.3% vs 96.2%) and longer PT. Nonetheless, this analysis suggests that CRA technique could represent a reasonable alternative especially when procedural safety is preferred over long-term arrhythmia-free survival.
Despite all these positive aspects of fluoroscopy-guided RFA and CRA, new methods were introduced to minimize the exposure to ionizing radiation. There are several studies that report feasibility of 3D EAM-guided RFA or CRA in SVT patients [16, 18,19,20,21,22,23,24]. A multi-center randomized prospective trial comparing 3D EAM-guided minimal fluoroscopy procedures with conventional fluoroscopy-guided procedures for treatment of SVTs showed similar success and complication rates between both approaches [21]. Based on these results, Casella et al. [21] proposed that the use of the 3D EAM system in SVT ablation could significantly reduce radiation exposure without compromising safety and efficacy. In a population similar to ours, Stec et al. [19] showed that totally fluoroless approach in SVT patients produces comparable results to standard fluoroscopy-based technique. Unlike the results of our study, they determined that pediatric patients had significantly higher RR compared with adults (16.0% vs 5.3%) [19]. Based on our results, we could hypothesize that the use of CRA in our cohort produced lower RR. Moreover, retrospective studies of combined use of the 3D EAM and the RFA in patients with SVTs by Giaccardia et al. [18] and Santoro et al. [24] produced similar findings regarding success rates and safety. In addition, meta-analysis based on 10 clinical articles involving 2.261 patients suggested that zero or near-zero fluoroscopic ablation significantly reduced fluoroscopic time, radiation dose, ablation time, and lifetime risk of malignancy without compromising success rates and causing significant procedural prolongation [27]. However, most of these studies included only adult patients with various types of SVTs, and specific analyses related to AVNRT patients were not provided in detail. Additionally, although different 3D EAM technologies were used, only RFA was used as the ablation method. Recently, Balli et al. [14] reported low RR with fluoroless CRA approach in 109 pediatric patients with AVNRT. In a single-center study similar to ours, Scaglione et al. [15] examined the efficacy of fluoroless CRA with the use of the 3D EAM system in a small-sized pediatric population with AVNRT. In this study, they reported a 92% overall efficacy of CRA with a few patients requiring a second ablation and fluoroscopy-guided RFA.
4.1 Clinical implications
Two clinical implications of our analysis should be highlighted.
First, it seems that fluoroless AVNRT ablation procedures in adult and pediatric patients can be performed without compromising efficacy and procedural duration. The results of our study are in line with recently published data of fluoroless CRA and RFA in patients with AVNRT that show a positive trend regarding higher success rates combined with lower PT [13,14,15,16,17].
Second, fluoroless CRA might not be less effective than fluoroless RFA for treatment of AVNRT.
Favorable results in the CRA group in our study can at least partly be attributed to utilization of two ablation strategies as needed. The first was cryo-mapping during the ongoing AVNRT with a low average TT. A technique used in 65% of CRA procedures in our study and previously published by Eryazici et al. [28] resulting in similar LTSR (98.9%) and RR (3.7%).
The second was cryo-mapping with targeting slow pathway during programmed stimulation with atrial extra stimuli that induced manifest slow pathway conduction.
Additionally, it can be assumed that the 3D EAM system provides more accurate and reliable information about anatomy and catheter position compared with fluoroscopy, which might have an additional contribution to efficacy when combined with cryo-mapping and subsequent cryo-ablation at the same micro-location in the Koch triangle.
The described favorable CRA outcomes should be confirmed in larger, randomized studies.
4.2 Study limitations
There are some study limitations decreasing the value of our conclusions. Firstly, this study is observational without a control group, and it is not randomized to compare the fluoroscopy-based and fluoroless CRA and RFA. Secondly, the protocol used for RF ablation of the slow pathway in this study might differ slightly from RFA protocols in other published studies, which might limit the ability to compare the results. Thirdly, all procedures were performed by a single operator, already experienced in fluoroless AVNRT ablation, thus making any analysis of a possible learning curve difficult. Fourthly, the follow-up period for the detection of possible recurrences of arrhythmias was short in the CRA compared with the RFA group. It is reasonable to assume that with a longer follow-up, more patients would experience recurrences. Fifthly, due to exclusion criteria used in our analysis, it is possible that in the “real life” clinical setting, CRA or RFA of AVNRT might result in less favorable outcome. Finally, sizes of the compared CRA and RFA groups are very different (40 vs 128 patients), which limit the value of our analysis.
5 Conclusions
Both CRA and RFA can be effectively and safely performed without the use of fluoroscopy in AVNRT patients. Moreover, CRA may present a safer and more effective alternative to RFA, especially in pediatric patients with AVNRT.
Availability of data and material
All data generated and analyzed during this study are included in this published article or can be provided upon request.
References
Blomström-Lundqvist C, Sheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines and the European society of cardiology committee for practice guidelines. J Am Coll Cardiol. 2003;42:1493–531.
Calkins H, Yong P, Miller JM, Olshansky B, Carlson M, Saul JP, et al. Catheter ablation of accessory pathways, atrioventricular nodal reentrant tachycardia, and the atrioventricular junction: final resuls of a prospective, multicenter clinical trial. Circulation. 1999;99:262–70.
Jackman WM, Beckman KJ, McClelland JH, Wang X, Friday KJ, Roman CA, et al. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry by radiofrequency catheter ablation of slow-pathway conduction. N Engl J Med. 1992;327:313–8.
Santangeli P, Proietti R, Di Biase L, Bai R, Natale A. Cryoablation versus radiofrequency ablation of atrioventricular nodal reentrant tachycardia. J Interv Card Electrophysiol. 2014;39:111–9.
Hanninen M, Yeung-Lai-Wah N, Massel D, Gula LJ, Skanes AC, Yee R, et al. Cryoablation versus RF ablation for AVNRT: a meta-analysis and systematic review. J Cardiovasc Electrophysiol. 2013;24:1354–60.
Papagiannis J, Papadopoulou K, Rammos S, Katritsis D. Cryoablation versus radiofrequency ablation for atrioventricular nodal reentrant tachycardia in children: longterm results. Hell J Cardiol. 2010;51:122–6.
Spector P, Reynolds MR, Calkins H, Sondhi M, Xu Y, Martin A, et al. Metaanalysis of ablation of atrial flutter and supraventricular tachycardia. Am J Cardiol. 2009;104:671–7.
Cappato R, Kuck KH. Catheter ablation in the year 2000. Curr Opin Cardiol. 2000;15:29–40.
Ait-Ali L, Andreassi MG, Foffa I, Spadoni I, Vano E, Picano E. Cumulative patient effective dose and acute radiation-induced chromosomal DNA damage in children with congenital heart disease. Heart. 2010;96:269–74.
Kovoor P, Ricciardello M, Collins L, Uther JB, Ross DL. Risk to patients from radiation associated with radiofrequency ablation for supraventricular tachycardia. Circulation. 1998;98:1534–40.
Picano E, Vañó E, Rehani MM, Cuocolo A, Mont L, Bodi V, et al. The appropriate and justified use of medical radiation in cardiovascular imaging: a position document of the ESC associations of cardiovascular imaging, percutaneous cardiovascular interventions and electrophysiology. Eur Heart J. 2014;35:665–72.
Gellis LA, Ceresnak SR, Gates GJ, Nappo L, Pass RH. Reducing patient radiation dosage during pediatric SVT ablations using an “ALARA” radiation reduction protocol in the modern fluoroscopic era. Pacing Clin Electrophysiol. 2013;36:688–94.
Jan M, Žižek D, Rupar K, Mazić U, Kuhelj D, Lakič N, et al. Fluoroless catheter ablation of various right and left sided supraventricular tachycardias in children and adolescents. Int J Card Imaging. 2016;32:1609–16.
Balli S, Kucuk M, Orhan Bulut M, Kemal Yucel I, Celebi A. Transcatheter cryoablation procedures without fluoroscopy in pediatric patients with atrioventricular nodal reentrant tachycardia: a single-center experience. Acta Cardiol Sin. 2018;34:337–43.
Scaglione M, Ebrille E, Caponi D, Blandino A, DI Donna P, Siboldi A, et al. Single center experience of fluoroless AVNRT ablation guided by electroanatomic reconstruction in children and adolescents. Pacing Clin Electrophysiol. 2013;36:1460–7.
Kozluk E, Rodkiewicz D, Piątkowska A, Opolski G. Safety and efficacy of cryoablation without the use of fluoroscopy. Cardiol J. 2018;25:327–32.
Seizer P, Bucher V, Frische C, Heinzmann D, Gramlich M, Müller I, et al. Efficacy and safety of zero-fluoroscopy ablation for supraventricular tachycardias. Use of optional contact force measurement for zero-fluoroscopy ablation in a clinical routine setting. Herz. 2016;41:241–5.
Giaccardi M, Del Rosso A, Guarnaccia V, Ballo P, Mascia G, Chiodi L, et al. Nearzero x-ray in arrhythmia ablation using a 3-dimensional electroanatomic mapping system: a multicenter experience. Heart Rhythm. 2016;13:150–6.
Stec S, Śledź J, Mazij M, Raś M, Ludwik B, Chrabąszcz M, et al. Feasibility of implementation of a “simplified, no-X-ray, no-lead apron, two-catheter approach” for ablation of supraventricular arrhythmias in children and adults. J Cardiovasc Electrophysiol. 2014;25:866–74.
Haegeli LM, Stutz L, Mohsen M, Wolber T, Brunckhorst C, On CJ, et al. Feasibility of zero or near zero fluoroscopy during catheter ablation procedures. Cardiol J. 2019;26:226–32.
Casella M, Dello Russo A, Pelargonio G, Del Greco M, Zingarini G, Piacenti M, et al. Near zerO fluoroscopic exPosure during catheter ablAtion of supRavenTricular arrhYthmias: the NO-PARTY multicentre randomized trial. Europace. 2016;18:1565–72.
Earley MJ, Showkathali R, Alzetani M, Kistler PM, Gupta D, Abrams DJ, et al. Radiofrequency ablation of arrhythmias guided by non-fluoroscopic catheter location: a prospective randomized trial. Eur Heart J. 2006;27:1223–9.
Razminia M, Willoughby MC, Demo H, Keshmiri H, Wang T, D'Silva OJ, et al. Fluoroless catheter ablation of cardiac arrhythmias: a 5-year experience. Pacing Clin Electrophysiol. 2017;40:425–33.
Santoro A, Di Clemente F, Baiocchi C, Zacà V, Bianchi C, Bellini C, et al. From near-zero to zero fluoroscopy catheter ablation procedures. J Cardiovasc Electrophysiol. 2019;30:2397–404.
Kalbfleisch SJ, Strickberger SA, Williamson B, Vorperian VR, Man C, Hummel JD, et al. Randomized comparison of anatomic and electrogram mapping approaches to ablation of the slow pathway of atrioventricular node reentrant tachycardia. J Am Coll Cardiol. 1994;23:716–23.
Katritsis DG, Zografos T, Siontis KC, Giannopoulos G, Muthalaly RG, Liu Q, et al. Endpoints for successful slow pathway catheter ablation in typical and atypical atrioventricular nodal re-entrant tachycardia: a contemporary, Multicenter Study. JACC Clin Electrophysiol. 2019;5:113–9.
Yang L, Sun G, Chen X, Chen G, Yang S, Guo P, et al. Meta-analysis of zero or near-zero fluoroscopy use during ablation of cardiac arrhythmias. Am J Cardiol. 2016;118:1511–8.
Eryazici PL, Razminia M, D'Silva O, Chavez JR, Ciftci FD, Turner M, et al. Time-limited cryomapping during tachycardia: improved long-term outcomes for cryoablation of AVNRT. J Interv Card Electrophysiol. 2016;47:125–31.
Author information
Authors and Affiliations
Contributions
All authors (MJ, MY, TPK, DŽ, DK, AP, NL) made substantial contribution to the design of the study, acquisition, analysis, and interpretation of data. All authors (MJ, MY, TPK, DŽ, DK, AP, NL) also actively participated in drafting the manuscript, its final revisions, and corrections. All authors (MJ, MY, TPK, DŽ, DK, AP, NL) read and approved the final version of the manuscript. All authors (MJ, MY, TPK, DŽ, DK, AP, NL) agree to be personally accountable for their personal contributions to the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The study complies with the Declaration of Helsinki. The fluoroless approach to catheter ablation was approved by the National Medical Ethics Committee (Komisija za medicinsko etiko Republike Slovenije).
Informed consent
All patients (or their parents in case of pediatric patients) gave written informed consent before ablation procedures were performed.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jan, M., Yazici, M., Kalinšek, T.P. et al. Fluoroless radiofrequency and cryo-ablation of atrioventricular nodal reentry tachycardia in adults and children: a single-center experience. J Interv Card Electrophysiol 61, 155–163 (2021). https://doi.org/10.1007/s10840-020-00791-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-020-00791-1