Abstract
Objectives
Atrial fibrillation (AF) is thought to be a progressive arrhythmia, starting with short paroxysmal episodes, until eventually, it becomes permanent. Evidence for this is limited to studies with short follow-up or with minimal cardiac rhythm monitoring. We utilised the continuous rhythm monitoring capabilities of implanted pacemakers to define better the natural history of AF.
Methods
The study included 356 patients with pacemaker devices capable of continuous atrial rhythm monitoring (186 male, mean age (±SD) 79.5 ± 8.9 years). All clinical records, including history/physical examination reports, laboratory results, ECGs and Holter monitoring data were reviewed. Patients were included if AF episodes >30 s were documented. Permanent pacemaker diagnostic data were reviewed at least every 12 months. ACC/AHA/ESC guidelines were used to define AF episodes as paroxysmal, persistent or long-standing persistent/permanent.
Results
Study follow-up period (±SD) was 7.2 ± 3.1 years. Over the study period, 179 of 356 patients (50.3 %) had at least one episode of persistent AF. Of the 356 patients, 314 (88.2 %) had paroxysmal AF and 42 (11.8 %) had persistent AF at the time of diagnosis. The predominant AF subtype, at latest follow-up, was paroxysmal for 192 patients (53.9 %), persistent for 77 (21.6 %) and long-standing persistent/permanent for 87 (24.4 %). Univariable predictors of progression to persistent AF were (1) male gender, (2) increasing left atrial diameter (LAD), (3) reduced atrial pacing (AP) and (4) increasing ventricular pacing.
Conclusions
Although many patients with AF will have persistent episodes, long-term continuous pacemaker follow-up demonstrates that the majority will have a paroxysmal, as opposed to persistent, form of the arrhythmia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Atrial fibrillation (AF) is the most commonly encountered and most commonly treated cardiac arrhythmia worldwide [1]. It is a clinically varied disorder with a poorly defined natural history. Episodes of AF may be (i) paroxysmal—short lived (<7 days) and self-terminating; (ii) persistent—more long lasting (>7 days) or requiring chemical or electrical cardioversion to terminate or (iii) long-standing persistent/permanent—very long standing, usually more than 1 year, or attempts to restore sinus rhythm have proven unsuccessful [2].
In experimental animal models, ‘atrial fibrillation begets atrial fibrillation’, thus generating hypotheses that in humans, the condition progresses inexorably from paroxysmal through persistent to a permanent form of the arrhythmia [3].
Defining the natural history of atrial fibrillation is difficult. Although in some patients, arrhythmia episodes are frequently highly symptomatic, some patients have entirely asymptomatic episodes or have both symptomatic and asymptomatic episodes [4–6]. Patients and their physicians are thus often unaware of the true arrhythmia burden and if there is any disease progression.
Modern dual chamber pacemaker devices are capable of continuous beat-to-beat monitoring of the cardiac rhythm and are highly sensitive and specific for the detection of AF episodes and overall AF burden [7–10].
The primary objectives of this study were to define the long-term natural history of atrial fibrillation using the continuous monitoring capabilities of permanent pacemaker devices and to assess for independent risk factors of arrhythmia progression.
2 Methods
2.1 Study design and population
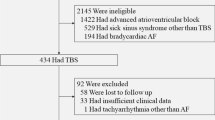
The study protocol was approved by a local institutional review committee. We performed a retrospective cohort study. Patients with pacemaker devices capable of continuous atrial rhythm monitoring at our institution were eligible for study inclusion. All clinical records, including history and physical examination reports, laboratory results, ECGs and Holter monitoring data for every outpatient encounter and hospital admission, were reviewed. Patients were included if AF episodes >30 s were documented by means of 12 lead ECG, ambulatory cardiac monitoring or on pacemaker device Holters.
Demographic data were gained by case note review. Information gathered included first onset of arrhythmia symptoms, method of arrhythmia diagnosis, presence of co-morbidities, medication usage (anti-arrhythmic/anticoagulant/angiotensin-converting enzyme (ACE) and angiotensin-II (AT2) inhibitors/statins), echocardiographic data and requirement for AF interventions: DC cardioversion, atrioventricular (AV) node ablation and AF ablation. Permanent pacemaker diagnostic data were reviewed at least every 12 months in all patients. Data recorded included the following: AF burden, AF episode number and atrial and ventricular pacing percentage; stored electrograms were reviewed when available. Modern pacemaker devices have sophisticated AF/AT diagnostic algorithms, operational details have previously been described, a rate of 200 bpm was used to detect atrial arrhythmias [8, 11, 12].
2.2 Definitions
AF was defined as any episode of sustained atrial fibrillation >30 s. AF burden was defined as the total duration of recorded AF divided by the duration of cardiac monitoring expressed as a percentage. AF episode duration was classified as per ACC/AHA/ESC guidelines: (i) AF episodes terminating spontaneously within 7 days are referred to as paroxysmal atrial fibrillation; (ii) AF episodes lasting longer than 7 days or requiring chemical or electrical cardioversion are referred to as persistent atrial fibrillation; (iii) long-standing persistent AF is defined as a continuous arrhythmia >1 year in duration and (iv) permanent AF refers to AF accepted by patient and physicians or where attempts to restore sinus rhythm have proven unsuccessful [2, 13].
At study census, patients were categorised into paroxysmal or persistent AF subtypes if AF episodes were predominantly less than or greater than 7 days, respectively. If AF episodes were not interrupted by sinus rhythm in the prior 12 months, their AF subtype was categorised as long-standing persistent/permanent.
2.3 Statistical analysis
Data was analysed using SPSS Version 19.0 software (IBM Corporation, Armonk, New York), and SAS 9.3 (SAS Institute, Cary, North Carolina). Descriptive data were described using standard methods. Continuous variables are expressed as mean ± standard deviation. Means were compared using independent or related sample t tests for parametric data and the Wilcoxon test or Mann Whitney U test for non-parametric data as appropriate. Categorical variables were compared using the chi squared test or Fisher’s exact test as appropriate. Rates of progression of AF, ischaemic cerebrovascular events or death were estimated using Kaplan-Meier survival analysis. Comparison of survival curves were made with the log rank test. The ability of multiple variables to predict progression to persistent AF and risk of death or stroke were assessed using Cox proportional hazards modelling including time-dependent covariates of explanatory variables listed as time dependencies. Univariable analysis was performed followed by multivariable analysis with backward elimination. A P value of <0.05 was considered statistically significant.
3 Results
Four hundred fourteen patients were screened for study inclusion, of which 58 were excluded with no documented atrial fibrillation. Three hundred fifty-six patients (186 male, mean age (±SD) 79.5 ± 8.9 years) were included in the study analysis. The study follow-up period (±SD) was 7.2 ± 3.1 years (min. 1 year, max. 18 years) with continuous cardiac rhythm monitoring for the duration of follow-up. The average number of pacemaker device checks (±SD) for each patient was 9.4 ± 4.6. Age at diagnosis of atrial fibrillation was 70.5 ± 10.4 years. Other patient demographics and initial method of AF diagnosis are presented in Table 1. Pacing indication was sinus node disease, atrioventricular node disease and AF in 65.1, 15.9 and 18.4 %, respectively; pacing was indicated for other reasons in 0.6 % of the study population. AF represented the pacing indication as a prelude to AV node ablation or to receive AF prevention pacing algorithms. The devices utilised in the study are listed in Table 2; there are more devices than patients due to requirement for elective generator change in some cases. In patients with AF documented prior to device implantation, diagnosis was made 2.8 ± 3.3 years (mean ± SD) prior. In patients with no previous history of AF, AF was detected via the device 3.5 ± 3.1 years (±SD) following device implant. The number of anti-arrhythmic drugs used (±SD), per patient, was 1.7 ± 1.1.
3.1 Progression of atrial fibrillation
One hundred seventy-nine of the 356 patients (50.3 %) developed at least one episode of persistent AF over the study period, mean time to persistent AF was 4.9 ± 4.3 years and the annual attrition rate was 7.0 %. Kaplan-Meier estimates of progression to persistent AF are demonstrated in Fig. 1. Significant univariable predictors of progression to persistent AF were (1) male gender, (2) increasing left atrial diameter, (3) reduced atrial pacing and (4) increasing ventricular pacing (hazard ratio (HR) 1.67, 1.38, 0.99 and 1.01, respectively; Table 3a). Multivariable analysis demonstrated independent predictors of progression to persistent AF to be (1) presence of co-morbidity, (2) increasing left atrial diameter and (3) reducing atrial pacing (HR 1.89, 1.50 and 0.99, respectively; Table 3b).
3.2 Predominant AF subtype at diagnosis and study census
Prevalence of AF subtype (paroxysmal, persistent, long-standing persistent) at initial diagnosis and at latest follow-up are demonstrated in Fig. 2. Of the 356 patients, 314 had paroxysmal AF and 42 had persistent AF at the time of diagnosis. The predominant AF subtype, at latest follow-up, was paroxysmal for 192 patients (53.9 %), persistent for 77 (21.6 %) and long-standing persistent/permanent for 87 (24.4 %). Table 4 demonstrates demographic data for patients who continued to have predominantly paroxysmal AF compared with those who had predominantly persistent or long-standing persistent AF. Patients who remained predominantly in paroxysmal AF at study census had smaller left atrial diameters, higher LV ejection fractions, higher levels of atrial pacing, lower levels of ventricular pacing and took fewer anti-arrhythmic drugs.
3.3 Ventricular pacing and progression of AF
Average ventricular pacing percentages were shown to be associated with increased risk of development of a persistent AF episode. Kaplan-Meier estimates of progression to episodes of persistent AF are demonstrated in Fig. 3 with average ventricular pacing divided in to quartiles.
3.4 Mortality and ischaemic stroke over study period
Over the period of study follow-up, 62 patients died from all causes at a mean age of 82.6 ± 5.2 years. Over the period of study follow-up, 40 patients experienced an ischaemic cerebrovascular incident. At the time of AF diagnosis, CHA2DS2-VASc scores were higher in patients who had a stroke versus those who did not (mean ± SD), 2.7 ± 1.3 versus 2.2 ± 1.6, respectively, although this difference was not statistically significant, P = 0.067. Thirty-four of the 40 patients were not on warfarin at the time of their cerebrovascular event. Figure 4 demonstrates Kaplan-Meier estimates of time to cerebrovascular event according to treatment with warfarin. Univariate analysis demonstrated predictors of mortality or stroke to be (1) increasing age, (2) co-morbidity and (3) increasing ventricular pacing (odds ratio (OR) 1.07, 2.66 and 1.01, respectively; Table 5a). AF burden was not demonstrated to be a predictor of stroke (Table 5a). Multivariable analysis demonstrated independent predictors of mortality or stroke to be (1) increasing age and (2) co-morbidity (HR (95 % confidence interval, CI)) 1.06 (1.03–1.09) and 2.16 (1.35–3.47), respectively; Table 5b).
4 Discussion
This study demonstrates that over nearly 8 years of follow-up from devices, although many patients with atrial fibrillation do ‘progress’ and develop persistent episodes, the majority continue to have predominantly paroxysmal episodes of AF.
International guidelines and expert consensus for the management of atrial fibrillation state that the natural history of the disease consists of ‘short, rare episodes’ progressing to ‘longer, more frequent attacks’ and that only a small minority of patients remain in paroxysmal AF [2]. This progression of atrial fibrillation from paroxysmal to persistent and permanent forms of the arrhythmia is concluded from the results of a number of studies [14–18]. Previous studies are significantly limited by either short study follow-up times or very intermittent and infrequent cardiac rhythm monitoring that reveals a less precise natural history of AF than our own study utilising the continuous monitoring capabilities of implanted pacemakers.
4.1 Comparison with previous studies
The Euro Heart Survey on AF followed 5333 patients and reported follow-up date at 1 year [17]. Of patients with paroxysmal AF, 80 % continued to have predominantly paroxysmal AF whilst 30 % of patients with persistent AF progressed to permanent AF. The follow-up of this study was short and detected AF episodes if patients reported symptoms or sporadic ECG recording.
Jahangir et al. reported a natural history of lone atrial fibrillation over a 30-year period [15]. Seventy-six patients, mean age of 44 years at diagnosis, were followed. Patient symptoms, intermittent 12 lead ECGs and Holter monitors were used to diagnose AF episodes. Thirty-one percent of the cohort reportedly progressed to permanent AF. At the end of the study, 57, 8 and 35 % were in paroxysmal, persistent or permanent AF, respectively.
The Canadian Registry of Atrial Fibrillation followed 757 patients with an initial diagnosis of paroxysmal AF for a median of 8.0 years [16]. The study reported progression to ‘chronic’ AF, defined as the presence of AF on two ECGs at least 1 week apart with no evidence of intervening sinus rhythm; 8.6 and 24.7 % of patients developed chronic AF at 1 and 5 years, respectively.
Saksena et al. reported on AF progression in patients with paroxysmal AF following pacemaker implantation [19]. Three hundred thirty patients were included and over the period of follow-up, 24 % developed an episode of persistent AF. Whilst continuous rhythm monitoring was utilised, the duration of follow-up, a mean of 401 days, was much shorter than our own study. Nagarakanti et al. reported further analysis of this cohort and elegantly demonstrated recurrent episodes of persistent AF interspersed by decrementing time in sinus rhythm [20]. After the third episode of persistent AF, spontaneous return to SR was not seen but had the follow-up period been longer, this may have been observed.
Common to all these studies, and our own study, is the high incidence of time-dependent progression to episodes of persistent AF. The conclusion that these results represent inevitable disease progression to permanent AF is, however, questionable. All of these studies, except the study by Saksena et al., lacked continuous cardiac rhythm monitoring that enable insight in to the true natural history of AF. Whilst AF may become persistent or be deemed permanent (46 % of study cohort by censorship), the majority of patients (54 %) remain in a paroxysmal form and persistent episodes are frequently interspersed by periods of sinus rhythm.
4.2 Ventricular pacing and persistent AF
Recently, several studies have reported the deleterious effects of right ventricular pacing with regards to development of persistent atrial fibrillation [21, 22]. The current study supports these data and illustrates that ventricular pacing may alter a natural history of the AF in patients with implanted devices. Our analyses have considered this and our results assess for changes in AF subtypes independent of ventricular pacing percentage.
4.3 Predictors of persistent AF, mortality and cerebrovascular events
Several studies have suggested that co-morbidities and gender are important factors in the development of persistent and permanent AF [23–26]. Our study supports these findings and also demonstrates that structural factors such as left atrial diameter are important.
This study supports the high mortality rates associated with AF that have been found in numerous patient populations [15, 27–30]. This has also been demonstrated in patients with permanent pacemakers with episodes lasting longer than 5 min being associated with higher mortality rates [31].
This study demonstrates the high rates of cerebrovascular events in patients with atrial fibrillation. The strongest predictor of these events was a lack of anticoagulation treatment. There are increasing data highlighting the increased risk of thromboembolic events associated with both symptomatic and subclinical AF detected via devices [32, 33]. There are still high rates of under anticoagulation in these patient populations; the increased use of novel oral anticoagulants, with easier administration and lower bleeding risks, may help to address this concern. The amount of atrial fibrillation experienced (AF burden) was not a predictor of mortality or stroke in our study (OR 1.005, 95 % CI 0.997–1.013). This highlights the importance of anticoagulation in patients with episodes of atrial fibrillation regardless of overall AF burden.
4.4 Study limitations and suggested further research
Our study has a number of limitations. It is a retrospective analysis of patients with an indication for permanent pacemaker implantation and results may not be applicable to a more general population. Ventricular pacing may impact on a natural history of AF but our analyses have accounted for this.
Future studies to further describe the natural history of AF require continuous cardiac monitoring to achieve this. Implantable loop recorders are likely to be the best way to achieve this but, at present, follow-up is limited by the battery life of these devices, typically 2–3 years.
5 Conclusions
Although many patients with atrial fibrillation will have persistent episodes, long-term follow-up with the aid of extensive continuous rhythm monitoring from implanted cardiac pacemakers demonstrates, contrary to dogma, that the majority will continue to have a paroxysmal form of the arrhythmia. Evaluation and treatment of patients with atrial fibrillation should not assume a natural and inevitable progression to persistent or permanent forms of atrial fibrillation.
References
Bagai, J., & Avitall, B. (2001). Atrial fibrillation. Current Treatment Options in Cardiovascular Medicine, 3(4), 261–276.
Camm, A. J., Kirchhof, P., Lip, G. Y., et al. (2010). Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). European Heart Journal, 29.
Wijffels, M. C., Kirchhof, C. J., Dorland, R., et al. (1995). Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation, 92(7), 1954–1968.
Page, R. L., Wilkinson, W. E., Clair, W. K., et al. (1994). Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation, 89(1), 224–227.
Silberbauer, J., Veasey, R. A., Cheek, E., et al. (2009). Electrophysiological characteristics associated with symptoms in pacemaker patients with paroxysmal atrial fibrillation. Journal of Interventional Cardiac Electrophysiology, 26(1), 31–40.
Chugh, S. S., Blackshear, J. L., Shen, W. K., et al. (2001). Epidemiology and natural history of atrial fibrillation: clinical implications. Journal of the American College of Cardiology, 37(2), 371–378.
Carlioz, O., Perrier, E., Thomas, O., et al. (2000). Accuracy of atrial tachyarrhythmia monitoring in a Selection device: correlation with an external Holter recording. Europace, 1(Suppl D), 9–10.
Israel, C. W. (2002). Analysis of mode switching algorithms in dual chamber pacemakers. Pacing and Clinical Electrophysiology, 25(3), 380–393.
Jarwe, M., Klug, D., Marquie, C., et al. (2001). Description of an atrial tachyarrhythmia patient population with a dual chamber pacemaker. Europace, 2(Abstract Suppl), A20.
Purerfellner, H., Gillis, A. M., Holbrook, R., et al. (2004). Accuracy of atrial tachyarrhythmia detection in implantable devices with arrhythmia therapies. Pacing and Clinical Electrophysiology, 27(7), 983–992.
Silberbauer, J., Arya, A., Veasey, R. A., et al. (2010). The effect of bipole tip-to-ring distance in atrial electrodes upon atrial tachyarrhythmia sensing capability in modern dual-chamber pacemakers. Pacing and Clinical Electrophysiology, 33(1), 85–93.
Veasey, R. A., Arya, A., Freemantle, N., et al. (2010). The usefulness of minimal ventricular pacing and preventive AF algorithms in the treatment of PAF: the 'MinVPace' study. J Intervent Cardiac Electrophysiol, 28(1), 51–57.
Fuster, V., Ryden, L. E., Cannom, D. S., et al. (2006). ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: full text: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace, 8(9), 651–745.
Al-Khatib, S. M., Wilkinson, W. E., Sanders, L. L., et al. (2000). Observations on the transition from intermittent to permanent atrial fibrillation. American Heart Journal, 140(1), 142–145.
Jahangir, A., Lee, V., Friedman, P. A., et al. (2007). Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation, 115(24), 3050–3056.
Kerr, C. R., Humphries, K. H., Talajic, M., et al. (2005). Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. American Heart Journal, 149(3), 489–496.
Nieuwlaat, R., Prins, M. H., Le Heuzey, J. Y., et al. (2008). Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow-up of the Euro Heart Survey on atrial fibrillation. European Heart Journal, 29(9), 1181–1189.
Ruigomez, A., Johansson, S., Wallander, M. A., et al. (2005). Predictors and prognosis of paroxysmal atrial fibrillation in general practice in the UK. BMC Cardiovascular Disorders, 5, 20.
Saksena, S., Hettrick, D. A., Koehler, J. L., et al. (2007). Progression of paroxysmal atrial fibrillation to persistent atrial fibrillation in patients with bradyarrhythmias. American Heart Journal, 154(5), 884–892.
Nagarakanti, R., Saksena, S., Hettrick, D., et al. (2011). Progression of new onset to established persistent atrial fibrillation: an implantable device-based analysis with implications for clinical classification of persistent atrial fibrillation. Journal of Interventional Cardiac Electrophysiology, 32(1), 7–15.
Sweeney, M. O., Bank, A. J., Nsah, E., et al. (2007). Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. New England Journal of Medicine, 357(10), 1000–1008.
Veasey, R. A., Arya, A., Silberbauer, J., et al. (2011). The relationship between right ventricular pacing and atrial fibrillation burden and disease progression in patients with paroxysmal atrial fibrillation: the long-MinVPACE study. Europace, 13(6), 815–820.
Benjamin, E. J., Levy, D., Vaziri, S. M., et al. (1994). Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA, 271(11), 840–844.
Buch, P., Friberg, J., Scharling, H., et al. (2003). Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. European Respiratory Journal, 21(6), 1012–1016.
Healey, J. S., & Connolly, S. J. (2003). Atrial fibrillation: hypertension as a causative agent, risk factor for complications, and potential therapeutic target. American Journal of Cardiology, 91(10A), 9G–14G.
Humphries, K. H., Kerr, C. R., Connolly, S. J., et al. (2001). New-onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation, 103(19), 2365–2370.
Benjamin, E. J., Wolf, P. A., D'Agostino, R. B., et al. (1998). Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation, 98(10), 946–952.
Stewart, S., Hart, C. L., Hole, D. J., et al. (2002). A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. American Journal of Medicine, 113(5), 359–364.
Kopecky, S. L., Gersh, B. J., McGoon, M. D., et al. (1987). The natural history of lone atrial fibrillation. A population-based study over three decades. New England Journal of Medicine, 317(11), 669–674.
Kopecky, S. L., Gersh, B. J., McGoon, M. D., et al. (1999). Lone atrial fibrillation in elderly persons: a marker for cardiovascular risk. Archives of Internal Medicine, 159(10), 1118–1122.
Glotzer, T. V., Hellkamp, A. S., Zimmerman, J., et al. (2003). Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation, 107(12), 1614–1619.
Glotzer, T. V., Daoud, E. G., Wyse, D. G., et al. (2009). The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circulation. Arrhythmia and Electrophysiology, 2(5), 474–480.
Healey, J. S., Connolly, S. J., Gold, M. R., et al. (2012). Subclinical atrial fibrillation and the risk of stroke. New England Journal of Medicine, 366(2), 120–129.
Author contributions
RA Veasey did the conception and design, analysis and interpretation of data and drafted of the manuscript. C Sugihara revised the manuscript critically for important intellectual content. K Sandhu revised the manuscript critically for important intellectual content. G Dhillon revised the manuscript critically for important intellectual content. N Freemantle did the analysis and interpretation of data and revised the manuscript critically for important intellectual content. SS Furniss revised the manuscript critically for important intellectual content. N Sulke revised the manuscript critically for important intellectual content and final approval of the manuscript submitted.
Funding
None
Competing interests
The authors declare that they have no competing interests.
Conflict of interest
The authors state no interests which might be perceived as posing a conflict or bias.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Veasey, R.A., Sugihara, C., Sandhu, K. et al. The natural history of atrial fibrillation in patients with permanent pacemakers: is atrial fibrillation a progressive disease?. J Interv Card Electrophysiol 44, 23–30 (2015). https://doi.org/10.1007/s10840-015-0029-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-015-0029-x