Abstract
In the clinical setting, atrial fibrillation (AF) is the most commonly presented arrhythmia of clinical significance. It is estimated that it affects 46.1 million people globally. Up to a half-million hospitalizations annually in the USA have AF as their primary diagnosis, and AF is estimated to contribute to >100,000 deaths per year in the USA. AF has a significant impact on health-care costs, with the major cost drivers being hospitalizations, stroke, and loss of productivity.
The real prevalence of AF could be underrepresented due to the fact that in up to 25 % of cases, AF occurs in the absence of symptoms, potentially underestimating the real prevalence of the disease. Monitoring techniques to detect asymptomatic, or subclinical AF, also have an impact on prevalence. AF is associated with significant morbidity. As the population age increases, so does the prevalence. Gender also had an impact on prevalence, with AF occurring more frequently in males than females, but despite a greater prevalence in men, women represent the bulk of patients with AF due to their longer survival. The impact of race is less clear.
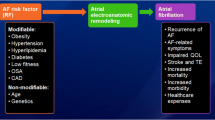
The Pathogenesis of AF: Notwithstanding the underlying risk factors, electrophysiological changes of atrial myocardium are likely to play a role. Sustained AF seems to require the development of multiple wavelets, rather than the single wavefront seen in atrial flutter. As AF becomes established, the refractory period of the atrial muscle shortens; this electrophysiological change predisposes to further AF. In some cases, AF results from another supraventricular tachycardia.
Risk Factors: AF has been associated with cardiovascular disease, in particular with hypertension, coronary artery disease, cardiomyopathy, and valvular disease; it can also occur after cardiac surgery and in the presence of myocarditis or pericarditis. Venous thromboembolic disease, chronic obstructive pulmonary disease, obesity, diabetes, and renal disease have also been described as risk factors. Certain medications can cause or contribute to AF development. However, the absence of an accepted biologic mechanism and the susceptibility of case-control studies to unmeasured confounders make us cautious about the strength of this association.
Classification and Progression: AF has been classified as paroxysmal, persistent, permanent, or lone. Lone atrial fibrillation refers to the presence of AF with no underlying structural heart disease; it can be present in as much as 45 % of patients with paroxysmal AF.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
First identified on electrocardiograms over 100 years ago [1, 2], atrial fibrillation (AF) is the most commonly presented arrhythmia of clinical significance [3, 4]. It is estimated that it affects 46.1 million people globally [5]. Up to a half-million hospitalizations annually in the USA have AF as their primary diagnosis, and AF is estimated to contribute to >100,000 deaths per year in the USA [6]. And hospitalizations have increased by 23 % between 2000 and 2010 [7].
AF has a significant impact on health-care costs, with the major cost drivers being hospitalizations, stroke, and loss of productivity [8–11]. In 2001, it was estimated that $6.65 billion US dollars was spent on AF treatment [12], with more recent findings estimating the costs to have ballooned from $16 to $26 billion US dollars a year [13], and according to findings by the Cost of Care in Atrial Fibrillation survey, hospitalizations represented a large portion of the cost of treating AF: 52 % vs. 23 % for drug therapy [14].
In the USA, the prevalence of AF increased by 4.5 % (from 4.1 to 8.6 %) between 1993 and 2007, with a 0.3 % increase per year, in Medicare beneficiaries older than 65 years of age [15–20]. Over 2.3 million adults in the USA had a diagnosis of AF in the years 1996 and 1997, with estimations that the number would go up to 5.6 million by 2050 [3]; this numbers climbed to 3.03 million in 2005 in the USA alone, and the prediction now is that 7.56 million will have AF by 2050 [21]. Worldwide, a total of 33.5 million patients had a diagnosis of AF in the year 2010, and it’s estimated that there will be 5.5 million new cases diagnosed each year [5]; with such a dynamic prevalence, the impact and burden in our health-care system cannot be overlooked. Specially, when the real prevalence of AF could be underrepresented due to the fact that in up to 25 % of cases, AF occurs in the absence of symptoms, potentially underestimating the real prevalence of the disease [22, 23]. Monitoring techniques to detect asymptomatic, or subclinical AF, also have an impact on prevalence. Using a 24-h Holter monitor or an event-loop recording device for 7 days, AF was detected in 11 % of the patients sampled [24]. When using a dual-chamber pacemaker or implantable cardioverter defibrillator for 3 months, subclinical AF was detected in 10 % of patients, and at 2.5 years, subclinical AF was detected in 35 % of patients, of whom 16 % had developed clinical AF [25].
AF is associated with significant morbidity, including a 3–5 times increase in the risk of stroke, and a three times increase in the risk of congestive heart failure (CHF) [26, 27], and it’s also associated with a twofold increase in comorbidity-adjusted mortality [9]. AF has also been described as a risk factor for dementia, even in patients without a history of stroke. Studies have reported that decreased cognitive scores (OR 1.5–3.5) are associated with the presence of AF in different populations [30–34]. In the ONTARGET and TRANSCEND studies [35], patients with AF showed faster worsening of Modified Mini-Mental State Examination and Digit Symbol Substitution Test scores over the subsequent 5 years than did patients without AF [36]. It has also been described that individuals with AF had an odds ratio of 2.8 for dementia compared with individuals without AF [31]. The increased risk of dementia in patients with AF is also associated with increased mortality (HR 2.9) [37].
The increase in prevalence has been attributed to a greater ability to treat chronic cardiac and noncardiac disease, the aging population, and the improved ability to suspect and diagnose AF [22]; therefore, when talking about prevalence, we must keep in mind it depends on the population we study. AF is not common in infants and children; if present, it will almost always be associated with a structural heart defect: congenital heart disease, cardiomyopathy, or Wolff-Parkinson-White (WPW) syndrome [28].
As the population age increases, so does the prevalence of AF. It is present in 0.12–0.16 % of subjects younger than 49 years of age, 3.7–4.2 % in those aged 60–70 years, and 10–17 % in patients 80 years or older [5, 15–20, 29]. In subjects over 50 years of age, AF was more frequent in Whites than Blacks (2.2 % vs. 1.5 %). Gender also had an impact on prevalence, with AF occurring more frequently in males (1.1 % vs. 0.8 %) than females [3, 4], but despite a greater prevalence in men, women represent the bulk of patients with AF due to their longer survival [5, 15–20, 29].
The impact of race is less clear. Multiple studies have found the risk of developing AF to be lower in Blacks compared to Whites, but it has not been determined whether Blacks are at lower risk or if Whites are at higher risk [38]. Data on Native American patients is scarce, but in a study of 664,754 subjects, of which 27,697 were Native Americans, an analysis looking at AF trends was conducted in a subpopulation of 67 % White and 55 % Native Americans and found that AF was less prevalent in Native Americans than in White adult males after adjusting for age, BMI, and predisposing comorbidities (adjusted odds ratio 1.15; CL 1.04–1.27) [39].
The lifetime risk for developing AF was analyzed in a report from the Framingham Heart Study. They found the risk for developing AF from age 40 to 95 was 26 % for men and 23 % for women. The risk of developing AF from age 80 to 95 was 23 % for men and 22 % for women. Lifetime risk didn’t change significantly with increased age due to the fact that AF incidence increases with age [40].
The exact reason for the current trends in prevalence are unknown, but could be partially explained by aging trends in the global population. Temporal trends in prevalence may result from a lead time bias, increased survival from coexistent cardiovascular conditions such as ischemic heart disease and heart failure due to improved management of cardiovascular comorbidities, resulting in a larger high-risk group [41].
Risk Factors
Cardiovascular Disease
AF has been associated with cardiovascular disease, in particular with hypertension, coronary artery disease, cardiomyopathy, and valvular disease; it can also occur after cardiac surgery and in the presence of myocarditis or pericarditis [42]. Hypertension can increase the risk of developing AF by 1.42-fold [43], which represents a relatively small increase in risk; however, given the high frequency of hypertension in the general population, hypertensive heart disease is the most common underlying disorder in patients with AF [44].
Coronary Heart Disease
AF is not commonly associated with coronary heart disease unless it’s complicated by an acute myocardial infarction (MI) or heart failure (HF). AF can occur in 6–10 % of patients with an acute MI, presumably due to atrial ischemia or atrial stretching secondary to HF [41–48]. In patients with chronic stable coronary heart disease, AF can be found in less than 1 % of patients, with a relative risk of 1.98 at 7 years [49].
Valvular Lesions
Lesions that lead to significant stenosis or regurgitation are associated with the development of AF. The rate of development of AF can be up to 5 % per year in patients with valvular disease, and the major independent risk factors were age (65 years or older) and baseline left atrial dimension >50 mm [50]. When AF complicates severe mitral regurgitation, valve repair or replacement is indicated [42].
Rheumatic Heart Disease (RHD)
RHD and its associated mitral valve disease were a major cause of AF in the Western world in the past, but the availability of early treatments has made the disease rare in developed countries. However, several studies from Africa, Asia, and the Middle East report a substantial prevalence of RHD in their population with AF [51–55].
Cardiomyopathy
AF has been reported in 10–28 % of patients with hypertrophic cardiomyopathy [49, 56, 57], although the prognostic importance remains unclear, with some literature showing a negative impact on prognosis [57], while others show no increase in mortality [56].
Venous Thromboembolic Disease
Deep vein thrombosis and pulmonary embolism are associated with an increased risk of AF. The exact mechanism for this is not known, but it has been speculated to be related to an increase in pulmonary vascular resistance and cardiac afterload, which may lead to atrial strain [58, 59]. It has been reported that up to 14 % of patients with a documented pulmonary embolism develop AF [60, 61].
AF can also occur in chronic obstructive pulmonary disease [61–63], peripartum cardiomyopathy [64], lupus myocarditis [65], and both idiopathic and uremic pericarditis [66, 67]. Obstructive sleep apnea may also be related, in which case the provision of continuous positive airway pressure reduces the risk of the recurrence of AF [68].
Obesity
This has also been described as a risk factor. Increased left atrial pressure and volume as well as a shortened effective refractory period in the left atrium and proximal and distal pulmonary veins have been identified as potential factors that facilitate and perpetuate AF in this population [69]. The risk of AF among individuals who are obese was >1.6 times that of counterparts with a normal BMI [70]. In the Atherosclerosis Risk in Communities Study (1989) from the USA, the population-attributable fraction of the risk of AF contributed by being overweight or obese was estimated at 17.9 %, making it the second most important risk factor after hypertension [71].
Diabetes
This has also been associated with an increased risk of developing AF (OR 1.1 for men and 1.5 for women); increased left ventricular mass and increased arterial stiffness have been put forth as possible mechanisms [72, 73].
Secondhand Smoke
This is an emerging risk factor linked to the development of AF in both current and former smokers [74, 75]. Current hypotheses regarding the mechanism by which secondhand smoke leads to cardiac disease include induction of an inflammatory state [76] and direct effects of nicotine on atrial structural remodeling [77, 78] and effects on autonomic function [79, 80]. Each of these mechanisms has been implicated in the pathogenesis of AF [70, 81]. Dixit et al. looked into a subpopulation if 4976 subjects enrolled in the Health eHeart Study and found that patients with AF were more likely to have been exposed to SHS in utero, as a child, as an adult, at home, and at work. However, those without AF were more likely to have visited social environments with significant SHS. When in utero exposure was separated by parent, maternal or paternal smoking during that period was associated with AF: 50 % of those with AF exposed to maternal smoke compared to 32 % of those without AF, p < 0.001, and 66 % of those with AF exposed to paternal smoke compared to 48 % of those without AF, p < 0.001 [82].
Renal Disease
This has also been linked as a risk factor for the development of AF. Compared to individuals with eGFRcys >90 mL/min/m2, a multivariable hazard ratios for the development of AF were significantly increased at 1.3, 1.6, and 3.2 in those with eGFRcys of 60–89, 30–59, and 15–29 mL/min/m2, respectively, during a median follow-up of 10.1 years [83].
In the Framingham study, 1409 patients with new-onset AF were evaluated for the risk of subsequent occurrence based on whether they develop a secondary precipitant or not. A precipitant was found in 31 % of patients. They were cardiothoracic surgery (30 %), infection (23 %), noncardiothoracic surgery (20 %), and acute myocardial infarction (18 %). Other precipitants found were acute alcohol consumption, thyrotoxicosis, acute pericardial disease, acute pulmonary embolism, and other acute pulmonary pathologies. While the 15-year cumulative incidence of recurrent AF was lower among those with secondary causes (62 % vs. 71 %), finding a recurrence of AF among the population with secondary causes was still unexpected [84].
Postoperative Period
AF has been reported in up to 30–40 % of patients in the postoperative period for those who underwent coronary artery bypass grafting (CABG) surgery [85–88], in 37–50 % after valve surgery [85, 88, 89], and in up to 60 % of those undergoing a valve replacement plus CABG [65, 68, 85, 88]. AF has also been described in up to 24 % of patients with a denervated transplanted heart and often in the absence of significant rejection [88, 89]. Most occur within the first 2 weeks, while developing AF after the 2-week mark has been associated with an increased risk of subsequent death [89, 90]. AF is less common after noncardiac surgeries with incidence rates that vary, from 1 to 40 %. This wide range could likely be due to the variability in patients and surgical characteristics [91, 92].
Hyperthyroidism
Patients with hyperthyroidism are at an increased risk of developing AF [93]. This is believed to occur due to an increased beta-adrenergic tone that may contribute to the rapid ventricular response [94]. It has also been suggested that it may be related to an increased automaticity and enhanced triggered activity of pulmonary vein cardiomyocytes, which can be a source of ectopic beats that initiate AF [95]. AF can be seen in up to 20 % of patients over the age of 60 but less than 1 % of patients under 40; men are also more likely to have AF than women (12.1 % vs. 7.6 %) [96].
Other factors associated with an increased risk have also been described:
Family History
The presence of AF in a first-degree relative, particularly a parent, has been associated with an increase in risk, independent of standard risk factors such as age, sex, hypertension, diabetes, or clinically overt heart disease [97]. The strength of this connection seems to be greater with first-degree relatives with premature onset (less than 65 years of age) [98].
Polygenic Inheritance
This seems to be more common and could explain the modest elevation in the relative risk of AF in first- and second-degree relatives of affected individuals [97, 99].
Monogenic Inheritance
Both autosomal dominant and autosomal recessive forms have been identified. Genetic linkage analysis has identified loci at 10q22-q24, 11p15.5, 6q14-16, 3p22-p25, and 4q25 [100–104]. At the 4q25 locus, several single-nucleotide polymorphisms have been identified [105].
Birth Weight
A possible relationship between birth weight and AF development has been described. The age-adjusted hazard ratios (with <2.5 kg [5.5 lb] being the referent group) for incident AF increased significantly from the lowest to the highest birth weight category, during a median follow-up of 14.5 years [106].
Inflammation and Infection
A relationship has been described after studies looking at levels of C-reactive protein (CRP) and finding elevated levels in people with later development of AF [107], history of atrial arrhythmias [108], failed cardioversion [109], recurrence of AF after cardioversion [110], and development of AF after cardiac surgery. However, inflammation is more likely a marker of conditions associated with AD, as opposed to being a direct or perpetuating cause [111].
Pericardial Fat
Pericardial fat is a visceral adipose tissue with inflammatory properties, and patients with AF had a significantly higher pericardial fat volume (102 mL vs. 76 mL in controls with no AF) [112]. Using data from over 3000 individuals in the Framingham Offspring Cohort, a 40 % higher odds ratio of AF per standard deviation increase in pericardial far volume was observed. This association remained significant after adjustments for age, gender, heart disease (myocardial infarction, heart failure), BMI, and other regional fat deposits [113].
Autonomic Dysfunction
The autonomic nervous system may be involved in the initiation and maintenance of AF. It may be particularly important in patients with paroxysmal AF, as both heightened vagal and sympathetic tone can promote AF. Heightened vagal tone in predominantly normal hearts, which may explain why vagally mediated AF is often seen in athletic young men without apparent heart disease who have slow heart rates during rest or sleep; such patients may also have an electrocardiogram (ECG) pattern of typical atrial flutter alternating with AF [114, 115]. In comparison, AF induced by increased sympathetic tone may be observed in patients with underlying heart disease or during exercise or other activity [115].
Corrected QT Interval
Individuals with either congenital long QT syndrome or short QT syndrome have an increased risk of AF [116, 117]. Individuals with a QTc <372 ms (1st percentile) or >419 ms (60th percentile) had an increased risk (adjusted hazard ratios 1.45 up to 1.44, respectively) compared to the reference group (411–419 ms) [118].
Premature Atrial Contractions
Premature atrial contractions (PAC) are known triggers of PAC. PAC count (by quartile) on Holter monitoring was associated with incident AF [119].
Other Supraventricular Tachyarrhythmias
Spontaneous transition between typical atrial flutter and AF has been observed, although little is known about the mechanism of this [120, 121]. AF is, in some patients, associated with paroxysmal supraventricular tachycardia (PSVT) [122–124]. The most common causes of PSVTs are atrioventricular nodal reentrant tachycardia and atrioventricular reentrant tachycardia, which occurs in patients with WPW syndrome or concealed accessory pathways. Among patients with WPW syndrome, the mechanism may be retrograde conduction via the accessory pathway of a premature beat, stimulating the atrial myocardium during its vulnerable period [125]. Ablation of the accessory pathway reduces the incidence of subsequent AF [125, 126].
Low Serum Magnesium
Low serum magnesium in patients undergoing cardiac surgery has been identified as a risk factor for the development of postoperative AF. Patients in the lower quartile of serum magnesium (≤1.77 mg/dL) were approximately 50 % more likely to develop AF compared to patients in the upper quartiles (≥1.99 mg/dL) after multivariable adjustments [127].
Alcohol
AF occurs in up to 60 % of binge drinkers with or without an underlying alcoholic cardiomyopathy [128]. Most cases occur during and following weekends or holidays when alcohol intake increases, a phenomenon which has been termed “the holiday heart syndrome.” Moderate, long-term alcohol consumption does not appear to be a risk factor for AF and has no significant association in either men or women [72, 129, 130]. In contrast, heavy alcohol consumption is associated with an increased incidence of AF. Two large cohort studies found an increased incidence among men with heavy alcohol consumption (hazard ratio 1.45 in both) [131, 132]. Neither study found a correlation in female patients, but the ability to detect such a correlation was limited by the small sample size of women with alcohol consumption in this range. Another study found an increased risk (relative risk 1.34, 95 % CI) with consumption of more than 36 g per day (>3 drinks/day) [130].
Fish and Fish Oil Supplements
It has been suggested the intake of fish and fish oils rich in long-chain n-3 fatty acids may reduce the incidence of arrhythmias [133], but the data is mixed. Three cohort studies, with sample sizes of 45,000, 48,000, and 5000 patients, found no relationship [134–136], while one study with a cohort of approximately 5000 patients suggested a reduction in AF burden [136].
Medications
Certain medications can cause or contribute to AF development [137]. These include theophylline [138], adenosine [114], and drugs that enhance vagal tone, such as digitalis [139]. Case-control studies have suggested an increased risk for developing AF in patients taking nonsteroidal anti-inflammatory drugs (NSAIDs) [140–142]. However, the absence of an accepted biologic mechanism and the susceptibility of case-control studies to unmeasured confounders make us cautious about the strength of this association [143].
Classification and Progression
AF has been classified as paroxysmal, persistent, permanent, or lone [144]. Lone atrial fibrillation refers to the presence of AF with no underlying structural heart disease; it can be present in as much as 45 % of patients with paroxysmal AF [145].
In patients recently diagnosed, it’s been found that up to 15 % of patients progress to persistent or permanent AF within 1 year. Heart failure, hypertension, and rate-control therapy, rather than rhythm-control (26 % vs. 11 %), were independently associated with AF progression. A risk stratification rule to assess the probability of AF progression found that age, previous TIA or stroke, and COPD were also associated with AF progression. The clinical outcome of patients demonstrating AF progression is worse compared with patients demonstrating no AF progression [146]. Up to 52 % of newly diagnosed had paroxysmal AF [147]. Patients with a higher CHADs2 score showed more AF progression: 19 % progression when CHADs2 > 1 vs. 14 % when CHADs2 is 1 and 9 % when CHADs2 is 0 (p < 0.0001). The use of class 1c antiarrhythmic drugs was also associated with less AF progression, whereas the use of cardiac glycosides was associated with more AF progression at 1 year follow-up [146].
The World Health Organization (WHO) quantifies the burden of disease from mortality and morbidity utilizing disability-adjusted life year (DALY) measurements; one DALY can be thought of a one lost year of “healthy” life. The sum of these DALYs across a population, or the burden of disease, is considered a measurement of the gap between current health status and an ideal health situation where the entire population lives to be of advanced age, free of disease and disability [147].
In the 2010 Global Burden of Disease Study, DALYs were utilized to indicate the overall morbidity contributed by a given disease in the population. For AF, the age-standardized DALYs in Central Asia, China, Russia, South Asia, Southeast Asia, and sub-Saharan Africa were 35–50 per 100,000 people. In comparison, age-standardized DALYs for patients with AF in Australia, Canada, the USA, and Western Europe were >60 per 100,000 people [148, 149]. A comparison of these values with data from the 1990 Global Burden of Disease Study shows that the burden of AF is steadily rising and now comprises an increased percentage of total DALYs for each nation [148].
References
Einthoven W. Le telecardiogramme. Arch Int Physiol. 1906;4:132–64.
Lewis T, Report CXIX. Auricular fibrillation: a common clinical condition. Br Med J. 1909;2(2552):1528.
Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–5.
Fuster V, Rydén LE, Cannom DS, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. Circulation. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006;114(7):e257–354.
Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–47.
Go AS, Mozaffarian D, Roger VL, American Heart Association Statistics Committee and Stroke Statistics Subcommittee, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–245.
Patel NJ, Deshmukh A, Pant S, et al. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129:2371.
Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82(8A):2N–9.
Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death. Circulation. 1998;98:946–52.
Thrall G, Lane D, Carroll D, Lip GY. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119(5):448.e1–19.
Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–5.
Coyne KS, Paramore C, Grandy S, et al. Assessing the direct costs of treating non valvular atrial fibrillation in the US. Value Health. 2006;9(5):348–56.
Lee WC, Lamas GA, Balu S, et al. Direct treatment cost of atrial fibrillation in the elderly American population: a Medicare perspective. J Med Econ. 2008;11(2):281–98.
Le Heuzey JY, Paziaud O, et al. Cost of care distribution in atrial fibrillation patients: the COCAF study. Am Heart J. 2004;147(1):121–6.
Friberg L, Bergfeldt L. Atrial fibrillation prevalence revisited. J Intern Med. 2013;274:461–8.
Wilke T, Groth A, Mueller S, et al. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace. 2013;15:486–93.
Zoni-Berisso M, Filippi A, Landolina M, et al. Frequency, patient characteristics, treatment strategies, and resource usage of atrial fibrillation [From the Italian Survey of Atrial Fibrillation Management (ISAF) Study]. Am J Cardiol. 2013;111:705–11.
Piccini JP, Hammil BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries: 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93.
Stefansdottir H, Appelund T, Gudnason V, Adnar DO. Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projection. Europace. 2011;13:1110–7.
Cowan C, Healicon R, Robson I, et al. The use of anticoagulants in the management of atrial fibrillation among general practices in England. Heart. 2013;99:1166–72.
Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104(11):1534–9.
Fitzmaurice DA, Hobbs DR, Jowet S, et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomized controlled trial. BMJ. 2007;335:386–91.
Rho RW, Page RL. Asymptomatic atrial fibrillation. Progr Cardiovasc Dis. 2005;48:78–87.
Jabaudon D, Sztajzel J, Sievert K, et al. Usefulness of ambulatory 7-day ECG monitoring for the detection of atrial fibrillation and flutter after acute stroke and transient ischemic attack. Stroke. 2004;35:1647.
Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120.
Camm AJ, Lip GY, De Caterina R, ESC Committee for Practice Guidelines(CPG), et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–47.
Patel PJ, Keating RJ, Gersh BJ, et al. Outcome of patients with newly diagnosed atrial fibrillation. Am J Cardiol. 2004;94(11):1379–82.
Radford DJ, Izukawa T. Atrial fibrillation in children. Pediatrics. 1977;59(2):250–6.
Gomez-Doblas JJ, Muniz J, Alonso Martin JJ, et al. Prevalence of atrial fibrillation in Spain. OFRECE study results. Rev Esp Cardiol. 2014;67(4):259–69.
Udompanich S, Lip GY, Apostolakis S, Lane DA. Atrial fibrillation as a risk factor for cognitive impairment: a semi-systematic review. QJM. 2013;106:795–802.
Kawabata-Yoshihara LA, Scazufca M, Santos IS, et al. Atrial fibrillation and dementia: results from the Sao Paulo Ageing & Health Study. Arq Bras Cardiol. 2012;99(6):1108–14.
Stefansdottir H, Arnar DO, Aspelund T, et al. Atrial fibrillation is associated with reduced brain volume and cognitive function independent of cerebral infarcts. Stroke. 2013;44(4):1020–5.
Thacker EL, McKnight B, Psaty BM, et al. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology. 2013;81(2):119–25.
Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158(5 Pt 1):338–46.
Marzona I, O’Donnell M, Teo K, et al. Increased risk of cognitive and functional decline in patients with atrial fibrillation: results of the ONTARGET and TRANSCEND studies. CMAJ. 2012;184(6):E329–36.
Thacker EL, McKnight B, Psaty BM, et al. Association of body mass index, diabetes, hypertension, and blood pressure levels with risk of permanent atrial fibrillation. J Gen Intern Med. 2013;28(2):247–53.
Miyasaka Y, Barnes ME, Petersen RC, et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a community-based cohort. Eur Heart J. 2007;28(16):1962–7.
Marcus GM, Alonso A, Peralta CA, Candidate-Gene Association Resource (CARe) Study, et al. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010;122(20):2009–15.
Borzecki AM, Bridgers DK, Liebschutz JM, et al. Racial differences in the prevalence of atrial fibrillation among males. J Natl Med Assoc. 2008;100(2):237–45.
Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042–6.
ACC/AHA guidelines for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association. Task Force on practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). J Am Coll Cardiol 1998;32;1486–588.
Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476.
Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982;306:1018.
Liberthson RR, Salisbury KW, Hutter Jr AM, DeSanctis RW. Atrial tachyarrhythmias in acute myocardial infarction. Am J Med. 1976;60:956.
Crenshaw BS, Ward SR, Granger CB, et al. Atrial fibrillation in the setting of acute myocardial infarction: the GUSTO-I experience. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J Am Coll Cardiol. 1997;30:406.
Wong CK, White HD, Wilcox RG, et al. New atrial fibrillation after acute myocardial infarction independently predicts death: the GUSTO-III experience. Am Heart J. 2000;140:878.
Eldar M, Canetti M, Rotstein Z, et al. Significance of paroxysmal atrial fibrillation complicating acute myocardial infarction in the thrombolytic era. SPRINT and Thrombolytic Survey Groups. Circulation. 1998;97:965.
Cameron A, Schwartz MJ, Kronmal RA, Kosinski AS. Prevalence and significance of atrial fibrillation in coronary artery disease (CASS Registry). Am J Cardiol. 1988;61:714.
Robinson K, Frenneaux MP, Stockins B, et al. Atrial fibrillation in hypertrophic cardiomyopathy: a longitudinal study. J Am Coll Cardiol. 1990;15:1279.
Zhou Z, Hu D. An epidemiological study on the prevalence of atrial fibrillation in the Chinese population of mainland China. J Epidemiol. 2008;18(5):209–16.
Zubaid M, Rashed WA, Alsheikh-Ali AA, Gulf Survey of Atrial Fibrillation Events (Gulf SAFE) Investigators, et al. Gulf Survey of Atrial Fibrillation Events (Gulf SAFE): design and baseline characteristics of patients with atrial fibrillation in the Arab Middle East. Circ Cardiovasc Qual Outcomes. 2011;4(4):477–82.
Sliwa K, Carrington MJ, Klug E, et al. Predisposing factors and incidence of newly diagnosed atrial fibrillation in an urban African community: insights from the Heart of Soweto Study. Heart. 2010;96(23):1878–82.
Oldgren J, Healey JS, Ezekowitz M, RE-LY Atrial Fibrillation Registry Investigators, et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY Atrial Fibrillation Registry. Circulation. 2014;129(15):1568–76.
Ntep-Gweth M, Zimmermann M, Meiltz A, et al. Atrial fibrillation in Africa: clinical characteristics, prognosis, and adherence to guidelines in Cameroon. Europace. 2010;12(4):482–7.
Glancy DL, O’Brien KP, Gold HK, Epstein SE. Atrial fibrillation in patients with idiopathic hypertrophic subaortic stenosis. Br Heart J. 1970;32:652.
Cecchi F, Olivotto I, Montereggi A, et al. Hypertrophic cardiomyopathy in Tuscany: clinical course and outcome in an unselected regional population. J Am Coll Cardiol. 1995;26:1529.
Gex G, Gerstel E, Righini M, et al. Is atrial fibrillation associated with pulmonary embolism? J Thromb Haemost. 2012;10:347.
Hald EM, Enga KF, Løchen ML, et al. Venous thromboembolism increases the risk of atrial fibrillation: the Tromso study. J Am Heart Assoc. 2014;3:e000483.
Weber DM, Phillips Jr JH. A re-evaluation of electrocardiographic changes accompanying acute pulmonary embolism. Am J Med Sci. 1966;251:381.
Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353:1386.
Davidson E, Weinberger I, Rotenberg Z, et al. Atrial fibrillation. Cause and time of onset. Arch Intern Med. 1989;149:457.
Buch P, Friberg J, Scharling H, et al. Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. Eur Respir J. 2003;21:1012.
Walsh JJ, Burch GE, Black WC, et al. Idiopathic myocardiopathy of the puerperium (Postpartal heart disease). Circulation. 1965;32:19.
Ansari A, Larson PH, Bates HD. Cardiovascular manifestations of systemic lupus erythematosus: current perspective. Prog Cardiovasc Dis. 1985;27:421.
Spodick DH. Arrhythmias during acute pericarditis. A prospective study of 100 consecutive cases. JAMA. 1976;235:39.
Levine HD. Myocardial fibrosis in constrictive pericarditis. Electrocardiographic and pathologic observations. Circulation. 1973;48:1268.
Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589–94.
Munger TM, Dong YX, Masaki M, et al. Electrophysiological and hemodynamic characteristics associated with obesity in patients with atrial fibrillation. J Am Coll Cardiol. 2012;60:851.
Wanahita N, Messerli FH, Bangalore S, et al. Atrial fibrillation and obesity–results of a meta-analysis. Am Heart J. 2008;155(2):310–5.
Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123(14):1501–8.
Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840.
Devereux RB, Roman MJ, Paranicas M, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271.
Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114(9):1453–68.
Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation. 2013;128(23):2470–7.
Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111(20):2684–98.
Shan H, Zhang Y, Lu Y, et al. Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc Res. 2009;83(3):465–72.
D’Alessandro A, Boeckelmann I, Hammwhöner M, Goette A. Nicotine, cigarette smoking and cardiac arrhythmia: an overview. Eur J Prev Cardiol. 2012;19(3):297–305.
Pope CA, Eatough DJ, Gold DR, et al. Acute exposure to environmental tobacco smoke and heart rate variability. Environ Health Perspect. 2001;109(7):711–6.
Felber Dietrich D, Schwartz J, Schindler C, SAPALDIA-Team, et al. Effects of passive smoking on heart rate variability, heart rate and blood pressure: an observational study. Int J Epidemiol. 2007;36(4):834–40.
Smit MD, Maass AH, De Jong AM, et al. Role of inflammation in early atrial fibrillation recurrence. Europace. 2012;14(6):810–7.
Dixit S, Pletcher MJ, Vittinghoff E, et al. Secondhand smoke and atrial fibrillation: data from the Health eHeart Study. Heart Rhythm 2015. pii: S1547-5271(15)01018-8.
Alonso A, Lopez FL, Matsushita K, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:2946.
Lubitz SA, Yin X, Rienstra M, et al. Long-term outcomes of secondary atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2015;131:1648.
Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med. 2001;135:1061.
Pires LA, Wagshal AB, Lancey R, Huang SK. Arrhythmias and conduction disturbances after coronary artery bypass graft surgery: epidemiology, management, and prognosis. Am Heart J. 1995;129:799.
Rubin DA, Nieminski KE, Reed GE, Herman MV. Predictors, prevention, and long-term prognosis of atrial fibrillation after coronary artery bypass graft operations. J Thorac Cardiovasc Surg. 1987;94:331.
Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539.
Pavri BB, O’Nunain SS, Newell JB, et al. Prevalence and prognostic significance of atrial arrhythmias after orthotopic cardiac transplantation. J Am Coll Cardiol. 1995;25:1673.
Ahmari SA, Bunch TJ, Chandra A, et al. Prevalence, pathophysiology, and clinical significance of post-heart transplant atrial fibrillation and atrial flutter. J Heart Lung Transplant. 2006;25:53.
Gialdini G, Nearing K, Bhave PD, et al. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA. 2014;312:616.
Bhave PD, Goldman LE, Vittinghoff E, et al. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am Heart J. 2012;164:918.
Woeber KA. Thyrotoxicosis and the heart. N Engl J Med. 1992;327:94.
Arnsdorf MF, Childers RW. Atrial electrophysiology in experimental hyperthyroidism in rabbits. Circ Res. 1970;26:575.
Chen YC, Chen SA, Chen YJ, et al. Effects of thyroid hormone on the arrhythmogenic activity of pulmonary vein cardiomyocytes. J Am Coll Cardiol. 2002;39:366.
Frost L, Vestergaard P, Mosekilde L. Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch Intern Med. 2004;164:1675.
Fox CS, Parise H, D'Agostino Sr RB, et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851.
Lubitz SA, Yin X, Fontes JD, et al. Association between familial atrial fibrillation and risk of new-onset atrial Epidemiology of and risk factors for atrial fibrillation. JAMA. 2010;304:2263.
Darbar D, Herron KJ, Ballew JD, et al. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol. 2003;41:2185.
Brugada R, Tapscott T, Czernuszewicz GZ, et al. Identification of a genetic locus for familial atrial fibrillation. N Engl J Med. 1997;336:905.
Chen YH, Xu SJ, Bendahhou S, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251.
Ellinor PT, Shin JT, Moore RK, et al. Locus for atrial fibrillation maps to chromosome 6q14-16. Circulation. 2003;107:2880.
Olson TM, Michels VV, Ballew JD, et al. Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA. 2005;293:447.
Gudbjartsson DF, Arnar DO, Helgadottir A, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353.
Lubitz SA, Sinner MF, Lunetta KL, et al. Independent susceptibility markers for atrial fibrillation on chromosome 4q25. Circulation. 2010;122:976.
Conen D, Tedrow UB, Cook NR, et al. Birth weight is a significant risk factor for incident atrial fibrillation. Circulation. 2010;122:764.
Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006.
Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886.
Conway DS, Buggins P, Hughes E, Lip GY. Predictive value of indexes of inflammation and hypercoagulability on success of cardioversion of persistent atrial fibrillation. Am J Cardiol. 2004;94:508.
Malouf JF, Kanagala R, Al Atawi FO, et al. High sensitivity C-reactive protein: a novel predictor for recurrence of atrial fibrillation after successful cardioversion. J Am Coll Cardiol. 2005;46:1284.
Marott SC, Nordestgaard BG, Zacho J, et al. Does elevated C-reactive protein increase atrial fibrillation risk? A Mendelian randomization of 47,000 individuals from the general population. J Am Coll Cardiol. 2010;56:789.
Al Chekakie MO, Welles CC, Metoyer R, et al. Pericardial fat is independently associated with human atrial fibrillation. J Am Coll Cardiol. 2010;56:784.
Thanassoulis G, Massaro JM, O’Donnell J, et al. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2010;3(4):345–50.
Herweg B, Dalal P, Nagy B, Schweitzer P. Power spectral analysis of heart period variability of preceding sinus rhythm before initiation of paroxysmal atrial fibrillation. Am J Cardiol. 1998;82:869.
Coumel P. Autonomic influences in atrial tachyarrhythmias. J Cardiovasc Electrophysiol. 1996;7:999.
Johnson JN, Tester DJ, Perry J, et al. Prevalence of early-onset atrial fibrillation in congenital long QT syndrome. Heart Rhythm. 2008;5:704.
Giustetto C, Schimpf R, Mazzanti A, et al. Long-term follow-up of patients with short QT syndrome. J Am Coll Cardiol. 2011;58:587.
Nielsen JB, Graff C, Pietersen A, et al. J-shaped association between QTc interval duration and the risk of atrial fibrillation: results from the Copenhagen ECG study. J Am Coll Cardiol. 2013;61:2557.
Dewland TA, Vittinghoff E, Mandyam MC, et al. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med. 2013;159:721.
Ortiz J, Niwano S, Abe H, et al. Mapping the conversion of atrial flutter to atrial fibrillation and atrial fibrillation to atrial flutter. Insights into mechanisms. Circ Res. 1994;74:882. This should be ref 100.
Hsieh MH, Tai CT, Tsai CF, et al. Mechanism of spontaneous transition from typical atrial flutter to atrial fibrillation: role of ectopic atrial fibrillation foci. Pacing Clin Electrophysiol. 2001;24:46.
Hamer ME, Wilkinson WE, Clair WK, et al. Incidence of symptomatic atrial fibrillation in patients with paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 1995;25:984.
Campbell RW, Smith RA, Gallagher JJ, et al. Atrial fibrillation in the preexcitation syndrome. Am J Cardiol. 1977;40:514.
Bauernfeind RA, Wyndham CR, Swiryn SP, et al. Paroxysmal atrial fibrillation in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1981;47:562.
Haissaguerre M, Fischer B, Labbé T, et al. Frequency of recurrent atrial fibrillation after catheter ablation of overt accessory pathways. Am J Cardiol. 1992;69:493.
Waspe LE, Brodman R, Kim SG, Fisher JD. Susceptibility to atrial fibrillation and ventricular tachyarrhythmia in the Wolff-Parkinson-White syndrome: role of the accessory pathway. Am Heart J. 1986;112:1141.
Khan AM, Lubitz SA, Sullivan LM, et al. Low serum magnesium and the development of atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2013;127:33.
Ettinger PO, Wu CF, De La Cruz Jr C, et al. Arrhythmias and the “Holiday Heart”: alcohol-associated cardiac rhythm disorders. Am Heart J. 1978;95:555.
Mukamal KJ, Psaty BM, Rautaharju PM, et al. Alcohol consumption and risk and prognosis of atrial fibrillation among older adults: the Cardiovascular Health Study. Am Heart J. 2007;153:260.
Djoussé L, Levy D, Benjamin EJ, et al. Long-term alcohol consumption and the risk of atrial fibrillation in the Framingham Study. Am J Cardiol. 2004;93:710.
Frost L, Vestergaard P. Alcohol and risk of atrial fibrillation or flutter: a cohort study. Arch Intern Med. 2004;164:1993.
Mukamal KJ, Tolstrup JS, Friberg J, et al. Alcohol consumption and risk of atrial fibrillation in men and women: the Copenhagen City Heart Study. Circulation. 2005;112:1736.
Berry JD, Prineas RJ, van Horn L, et al. Dietary fish intake and incident atrial fibrillation (from the Women’s Health Initiative). Am J Cardiol. 2010;105:844.
Frost L, Vestergaard P. n-3 Fatty acids consumed from fish and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Clin Nutr. 2005;81:50.
Brouwer IA, Heeringa J, Geleijnse JM, et al. Intake of very long-chain n-3 fatty acids from fish and incidence of atrial fibrillation. The Rotterdam Study. Am Heart J. 2006;151:857.
Mozaffarian D, Psaty BM, Rimm EB, et al. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368.
Van der Hooft CS, Heeringa J, van Herpen G, et al. Drug-induced atrial fibrillation. J Am Coll Cardiol. 2004;44:2117.
Varriale P, Ramaprasad S. Aminophylline induced atrial fibrillation. Pacing Clin Electrophysiol. 1993;16:1953.
Strickberger SA, Man KC, Daoud EG, et al. Adenosine-induced atrial arrhythmia: a prospective analysis. Ann Intern Med. 1997;127:417.
Schmidt M, Christiansen CF, Mehnert F, et al. Non-steroidal anti-inflammatory drug use and risk of atrial fibrillation or flutter: population based case-control study. BMJ. 2011;343:d3450.
De Caterina R, Ruigómez A, Rodríguez LA. Long-term use of anti-inflammatory drugs and risk of atrial fibrillation. Arch Intern Med. 2010;170:1450.
Krijthe BP, Heeringa J, Hofman A, et al. Non-steroidal anti-inflammatory drugs and the risk of atrial fibrillation: a population-based follow-up study. BMJ Open. 2014;4:e004059.
Gurwitz JH. NSAIDs and atrial fibrillation. BMJ. 2011;343:d2495.
Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:223.
Levy S, Maarek M, Coumel P, et al. Characterization of different subsets of atrial fibrillation in general practice in France: the ALFA study. The College of French Cardiologists. Circulation. 1999;99(23):3028–35.
De Vos CB, Breithardt G, Camm AJ, et al. Progression of atrial fibrillation in the REgistry on Cardiac rhythm disORDers assessing the control of Atrial Fibrillation cohort: clinical correlates and the effect of rhythm-control therapy. Am Heart J. 2012;163(5):887–93.149.
http://www.who.int/healthinfo/global_burden_disease/metrics_daly/en/. Accessed 10 Oct 2015.
Institute for Health Metrics and Evaluation. GBD Compare [online]. http://viz.healthmetricsandevaluation.org/gbd-compare/. 2013.
Rahman F, Kwan G, Benjamin E. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11:639–54.
Moran A, et al. The epidemiology of cardiovascular diseases in sub-Saharan Africa: the Global Burden of Diseases, Injuries and Risk Factors 2010 Study. Prog Cardiovasc Dis. 2013;56:234–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Soto-Ruiz, K.M. (2016). Atrial Fibrillation: Epidemiology and Demographics. In: Peacock, W., Clark, C. (eds) Short Stay Management of Atrial Fibrillation. Contemporary Cardiology. Humana Press, Cham. https://doi.org/10.1007/978-3-319-31386-3_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-31386-3_1
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-31384-9
Online ISBN: 978-3-319-31386-3
eBook Packages: MedicineMedicine (R0)