Abstract
Progression from paroxysmal to persistent atrial fibrillation (AF) is occasionally encountered in patients with previous pacemaker implantation (PMI) for the treatment of tachycardia–bradycardia syndrome (TBS). We aimed to determine the rate of its incidence occurring within the early years after PMI and the predictors. We studied TBS patients who received PMI at 5 core cardiovascular centers. The end point was a conversion from paroxysmal to persistent AF. We extracted 342 TBS patients out of 2579 undergoing PMI. During 5 ± 3.1 years of follow-up, 114 (33.3%) reached the end point. The time to the end point was 2.9 ± 2.7 years. The event rates within a year and 3 years after the PMI were 8.8% and 19.6%, respectively. In the multivariate hazard analyses, hypertension (hazard ratio [HR] 3.2, P = 0.03) and congestive heart failure (HR 2.1, P = 0.04) were found to be independent predictors of the end point occurring within a year after the PMI. Congestive heart failure (HR 1.82, P = 0.04), left atrial diameter of ≥ 40 mm (HR 4.55, P < 0.001), and the use of antiarrhythmic agents (HR 0.58, P = 0.04) were independently associated with the 3-year end point. Prediction models including combinations of those 4 parameters for the 1- and 3-year incidence both exhibited a modest risk discrimination (both c-statistics 0.71). In conclusion, early progression from paroxysmal to persistent AF was less frequent than expected in the TBS patients with PMI. Factors related to atrial remodeling and no use of antiarrhythmic drugs may facilitate the progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tachycardia–bradycardia syndrome (TBS) occasionally coexists with paroxysmal atrial fibrillation (AF) and is an indication for pacemaker implantation (PMI) if it is symptomatic [1, 2]. Given that conversion from paroxysmal AF to its persistent form occurs as the natural history of AF [3], the phenomenon is often noticed at regular PM clinics in subjects with TBS. Once the conversion to persistent AF becomes irreversible, cardiac pacing would be no longer necessary, and, at least, the atrial PM lead would become a “white elephant”. Therefore, it would be beneficial if physicians could identify beforehand patients with TBS who are likely to develop the progression from paroxysmal to persistent AF during the early period following the PMI. We therefore in the present study aimed to find out the frequency of early progression from paroxysmal to persistent AF and its predictors in patients who underwent PMI for the treatment of TBS.
Methods
Patients
This is a retrospective and multicenter study of progression from paroxysmal to persistent AF in subjects with PMI for the treatment of TBS. The participating institutions included Hiroshima University Hospital, Onomichi General Hospital, Tsuchiya General Hospital, Hiroshima City Hiroshima Citizens Hospital, and Hiroshima Red Cross Hospital and Atomic-bomb Survivors Hospital. We reviewed the databases of PMI and electronic charts to screen the subjects who newly underwent PMI between 2007 and 2016, and extracted data of consecutive patients who received PMI for the treatment of TBS. Patients were excluded if they were followed up for less than 3 years, had scant data, had atrial tachyarrhythmias other than AF that were responsible for TBS, or had symptomatic episodes of sinus bradycardia or sinus arrest without any antecedent atrial tachyarrythmia events as well as TBS episodes. We did not exclude the patients who underwent surgical or catheter ablation of AF before or after the PMI. The study protocol was approved by the research committee of each institution (E191021-4).
Definition
TBS was defined as an episode of sinus pause longer than 3 s resulting in syncope or faintness on termination of an atrial tachyarrhythmia [4]. The types of AF were defined according to the current guidelines [3]. Specifically, paroxysmal AF was defined as AF that terminates spontaneously or with intervention within 7 days of onset. Persistent AF was defined as continuous AF that is sustained beyond 7 days.
Follow-up
The patients were followed up at PM clinics over a 6-month interval. Device interrogations and 12-lead electrocardiograms were routinely performed at each clinical visit. Progression from paroxysmal to persistent AF was confirmed when AF had sustained for at least 7 days until the last interrogation, and AF was also recorded on the 12-lead electrocardiograms at that time in patients with a dual chamber PM. Among the patients with a single chamber PM, progression to persistent AF was diagnosed when AF was documented on the 12-lead electrocardiograms at 2 consecutive clinical visits for PM check [5].
Outcome measure
The end point of the present study was the progression from paroxysmal to persistent AF.
Statistical analysis
The continuous variables were summarized as the mean ± SD or median with the interquartile range, and categorical variables as proportions. A Kaplan–Meier analysis was used to assess the time required for the clinical end point. Cox proportional hazard models were used to determine the predictors of the endpoint. On the basis of the results of a landmark trial [6], the latest algorithm of atrial antitachycardia pacing, reactive antitachycardia pacing, was included in the models as well as other potential clinical parameters such as hypertension, enlarged left atrium, and congestive heart failure [5, 7]. Parameters with a statistical significance in the univariate models were further included in the multiple models. On the basis of the hazard models and HATCH score [7, 8], prediction models for the end point were created. Harrell’s concordance statistic (c-statistic) was used to investigate the model performance of the prediction models. All statistical analyses were performed with the use of JMP software version 15.0 (SAS Institute, Cary, North Carolina).
Results
Patients
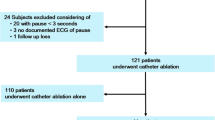
We screened 2579 patients undergoing PMI, and extracted 434 subjects who received PMI for the treatment of TBS. Among them, 92 met the exclusion criteria. We then finally analyzed 342 patients (Fig. 1). The mean age was 77 years, approximately 60% of the patients were female, one-fourth had congestive heart failure, and half were prescribed with an antiarrhythmic agent. The mean left atrial diameter and median brain natriuretic peptide level were 41 mm and 131.2 pg/ml, respectively. A single chamber PM was implanted in 8.2%, and reactive antitachycardia pacing was introduced in 5.8% of the patients. Catheter or surgical ablation was performed before the PMI in 18.4% (Table 1). Three (0.9%), 7 (2%) and 7 (2%) patients received catheter ablation within 1 year, 1–3, and 3–5 years after the PMI, respectively.
Outcomes
Out of the 342 patients included, 114 (33.3%) reached the clinical endpoint during 5 ± 3.1 years of follow-up (Fig. 2). The time to the end point was 2.9 ± 2.7 years. The event rates within 1, 3, and 5 years were 8.8%, 19.6% and 26.3%, respectively. No subjects reached the end point among the patients receiving catheter ablation after the PMI. A total of 11 (3.2%) patients died before reaching the endpoint.
Kaplan–Meier estimate of the incidence of conversion from paroxysmal to persistent atrial fibrillation. The abbreviations are the same as in Fig. 1
Predictors of transition to persistent AF
In the multivariate hazard analyses, hypertension, congestive heart failure, and brain natriuretic peptide level of ≥ 100 pg/ml were found to be independent predictors of the clinical endpoint that occurred within a year after the PMI (Table 2). Left atrial diameter of ≥ 40 mm, congestive heart failure, and brain natriuretic peptide level of ≥ 100 pg/ml had an independent positive association with, and the use of antiarrhythmic agents had an independent negative association with the endpoint occurring within 3 years after the PMI (Table 3).
Prediction models
Based on the hazard analyses, the prediction models were created for end points that occurred within a year and 3 years. Patients with all of the followings were approximately 9 times more likely than those who did not have any of the 3 parameters to have the endpoint observed within a year (c-statistic 0.71): hypertension, congestive heart failure, and left atrial diameter of ≥ 40 mm. Patients with 2 or all of the factors including congestive heart failure, left atrial diameter of ≥ 40 mm, and no use of antiarrhythmic agents were approximately 4 or 11 times more likely than those with none of those 3 parameters to have the end point occurring within 3 years, respectively (c-statistic 0.71, Fig. 3A). The risk stratification with the use of the HATCH score did not predict the 1-year end point (c-statistic 0.63). Patients with the HATCH score of 5–7 had about a 5 times higher 3-year incidence of the end point as compared to those with the score of 0 (c-statistic 0.6, Fig. 3B).
Prediction models for the 1-year and 3-year incidence of progression from paroxysmal to persistent atrial fibrillation that incorporate the relevant parameters (A) and HATCH score (B). AAD antiarrhythmic drug, CHF congestive heart failure, HT hypertension, LAD left atrial diameter, PMI pacemaker implantation
Discussion
Major findings
The major findings of the present study were twofold. (1) Progression from paroxysmal to persistent AF was observed in one-third of the pacemaker patients with TBS during the entire follow-up period, and approximately 9% developed the event within a year after the PMI. (2) Hypertension, enlarged left atrium, congestive heart failure, and no use of antiarrhythmic agents predicted the early conversion to persistent AF following the PMI.
Rate of progression to persistent AF
The subjects in whom AF coexists with sinus node dysfunction are known to have atria with extensive electrical and structural remodeling [1,2,3, 9]. The remodeled atrium, in turn, facilitates progression of AF, as shown in the present study and others [1, 3, 5, 10, 11]. From a syllogistic point of view, therefore, it is theoretically possible that the patients with both paroxysmal AF and TBS may more often develop the progression to persistent AF within the early years than those with paroxysmal AF alone. We thus expected its high rate prior to the present study. Let us consider the previous reports on this topic. There are several reports available on the conversion of paroxysmal to persistent AF, thus far [5, 7, 10, 12]. According to them, the conversion rates range from 8.6%/year [5] to 24%/401 days [12]. In the present study, the persistent AF conversion rate was 8.8% during the first follow-up year, and it is close to the lowest in that range. That was contrary to our expectation. Why is that? TBS perhaps could differ from the other forms of sinus node dysfunction (sinus bradycardia and sinus arrest) in terms of the remodeling. Studies have shown that curative AF ablation can successfully eliminate TBS [13]. Further, a study [14] showed that paroxysmal AF subjects with TBS had a significantly smaller scar area in the left atrium and had trends toward a longer atrial effective refractory period and greater conduction velocity as compared to those with the other types of sinus node dysfunction. Those studies suggest that “TBS might be associated with transient sinus node remodeling or a lesser degree of atrial and sinus remodeling compared with the other types of sinus node dysfunction [14]”.
Predictors of progression to persistent AF
Several predictors of transition from paroxysmal to persistent AF have been proposed [5, 7, 10]. They include congestive heart failure, hypertension, advanced age, transient ischemic attack or stroke, chronic obstructive pulmonary disease, bradycardia, angina, valvular deficiencies, and enlarged left atrium. The predictors we found in the present study were among them. Heart failure and hypertension are tightly associated with electroanatomical remodeling of human atria [13, 14], and even atrial enlargement itself is atrial remodeling [1, 15]. The present study, therefore, confirmed the theory again that atrial remodeling plays a crucial role in the progression from paroxysmal to persistent AF [1, 7, 12].
Unlike previous reports [5, 7, 10], the use of antiarrhythmic drugs prevented the early progression to persistent AF in the present study. This may be attributed to the following peculiarities of participants in the present study. (1) Bepridil was used in one-third of patients who were prescribed antiarrhythmic drugs. (2) All patients included were previously implanted with pacemaker, and a combination of atrial pacing and antiarrhythmic drugs might have been beneficial.
The prediction models we created and the HATCH score [7] shared only 2 components. The following may explain the reason why the performances of our models were better than the HATCH score in predicting the transition to persistent AF in our cohort. (1) The prevalence of chronic obstructive pulmonary disease, which is one of the components of the HATCH score, was much lower in the present study. (2) Left atrial dilatation is not included in the HATCH score even though it is almost an undoubted predictor of the progression to persistent AF [3, 5, 10]. (3) The participants’ background, of course, differed.
Clinical implications
To the best of our knowledge, the present study is the first to investigate the transition from paroxysmal to persistent AF in subjects implanted with PM for the treatment of TBS. The predictors of progression to persistent AF we found could be helpful in identifying TBS subjects with a high risk of progression to persistent AF ahead of PMI. However, the prediction models we created may not have the ability to contribute to the clinical decision making regarding whether or not to place an atrial lead in those patients, probably due to the lower-than-expected incidence of conversion to persistent AF.
Limitations
We carefully excluded the TBS subjects who also had clinically significant sick sinus syndrome events other than TBS events: symptomatic sinus arrest or sinus bradycardia. However, we were still unable to exclude the possibility of their coexistence in some patients. Given that a spontaneous reversion from persistent to paroxysmal AF has been reported [16], the concept of an irreversible conversion to persistent AF itself, which is the end point of the present study, might be open to question. We failed to include sufficient number of patients with reactive antitachycardia pacing. This did not allow us to assess its efficacy in TBS patients. Although the present study was a multicenter study, considering the lower-than-expected incidence of the end points, the number of participants was probably insufficient. Finally, the present study is a retrospective one.
References
John RM, Kumar S (2016) Sinus node and atrial arrhythmias. Circulation 133:1892–1900
Jackson LR 2nd, Rathakrishnan B, Campbell K, Thomas KL, Piccini JP, Bahnson T, Stiber JA, Daubert JP (2017) Sinus node dysfunction and atrial fibrillation: a reversible phenomenon? Pacing Clin Electrophysiol 40:442–450
Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen PS, Chen SA, Chung MK, Nielsen JC, Curtis AB, Davies DW, Day JD, d’Avila A, de Groot NMSN, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao HM, Verma A, Wilber DJ, Yamane T (2017) 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 14:e275–e444
Kaplan BM, Langendorf R, Lev M, Pick A (1973) Tachycardia–bradycardia syndrome (so-called “sick sinus syndrome”). Pathology, mechanisms and treatment. Am J Cardiol 31:497–508
Padfield GJ, Steinberg C, Swampillai J, Qian H, Connolly SJ, Dorian P, Green MS, Humphries KH, Klein GJ, Sheldon R, Talajic M, Kerr CR (2017) Progression of paroxysmal to persistent atrial fibrillation: 10-year follow-up in the Canadian Registry of Atrial Fibrillation. Heart Rhythm 14:801–807
Boriani G, Tukkie R, Manolis AS, Mont L, Pürerfellner H, Santini M, Inama G, Serra P, de Sousa J, Botto GL, Mangoni L, Grammatico A, Padeletti L, Investigators MINERVA (2014) Atrial antitachycardia pacing and managed ventricular pacing in bradycardia patients with paroxysmal or persistent atrial tachyarrhythmias: the MINERVA randomized multicentre international trial. Eur Heart J 35:2352–2362
de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, van den Heijkant AC, Allessie MA, Crijns HJ (2010) Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 55:725–731
Shibata N, Kondo T, Morimoto R, Kazama S, Sawamura A, Nishiyama I, Kato T, Kuwayama T, Hiraiwa H, Umemoto N, Asai T, Okumura T, Murohara T (2022) Clinical value of the HATCH score for predicting adverse outcomes in patients with heart failure. Heart Vessels 37:1363–1372
Akoum N, McGann C, Vergara G, Badger T, Ranjan R, Mahnkopf C, Kholmovski E, Macleod R, Marrouche N (2012) Atrial fibrosis quantified using late gadolinium enhancement MRI is associated with sinus node dysfunction requiring pacemaker implant. J Cardiovasc Electrophysiol 23:44–50
Kochhäuser S, Dechering DG, Trought K, Hache P, Haig-Carter T, Khaykin Y, Wulffhart Z, Pantano A, Tsang B, Eckardt L, Verma A (2016) Predictors for progression of atrial fibrillation in patients awaiting atrial fibrillation ablation. Can J Cardiol 32:1348–1354
Ukita K, Egami Y, Kawamura A, Nakamura H, Matsuhiro Y, Yasumoto K, Tsuda M, Okamoto N, Matsunaga-Lee Y, Yano M, Nishino M, Tanouchi J (2022) Impact of radiofrequency catheter ablation for atrial fibrillation in patients with left atrial enlargement. Heart Vessels 37:1899–1905
Saksena S, Hettrick DA, Koehler JL, Grammatico A, Padeletti L (2007) Progression of paroxysmal atrial fibrillation to persistent atrial fibrillation in patients with bradyarrhythmias. Am Heart J 154:884–892
Hocini M, Sanders P, Deisenhofer I, Jaïs P, Hsu LF, Scavée C, Weerasoriya R, Raybaud F, Macle L, Shah DC, Garrigue S, Le Metayer P, Clémenty J, Haïssaguerre M (2003) Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation 108:1172–1175
Chung H, Uhm JS, Sung JH, Kim JY, Pak HN, Lee MH, Joung B (2014) The type of sinus node dysfunction might predict the severity of atrial remodeling and clinical outcome after catheter ablation of atrial fibrillation. Int J Cardiol 172:487–489
Shimizu A, Centurion OA (2002) Electrophysiological properties of the human atrium in atrial fibrillation. Cardiovasc Res 54:302–314
Sugihara C, Veasey R, Freemantle N, Podd S, Furniss S, Sulke N (2015) The development of AF over time in patients with permanent pacemakers: objective assessment with pacemaker diagnostics demonstrates distinct patterns of AF. Europace 17:864–870
Funding
None declared.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oguri, N., Sairaku, A., Morishima, N. et al. Progression from paroxysmal to persistent atrial fibrillation in pacemaker patients with tachycardia–bradycardia syndrome: a multicenter study. Heart Vessels 38, 1149–1155 (2023). https://doi.org/10.1007/s00380-023-02266-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-023-02266-5