Abstract
Purpose
To evaluate the efficacy of magnetic-activated cell sorting (MACS) or testicular sperm aspiration (TESA) to improve reproductive outcomes in cases with elevated sperm DNA fragmentation undergoing assisted reproduction.
Methods
This randomized controlled trial included couples with failed IVF cycles and sperm DNA fragmentation > 30%. Sperm DNA fragmentation was assessed using the sperm chromatin structure assay (SCSA) method. Participants were randomly assigned to either the MACS or TESA group. Testicular sperm retrieval was performed for the TESA group, while MACS involved sperm selection using magnetic beads. Extended blastocyst culture, freeze all policy of blastocysts by vitrification, and frozen embryo transfer were undertaken as per clinic’s standard operating protocols. Blastocyst formation rate, implantation rate, miscarriage rate, multiple pregnancy rate, and live birth rate were analyzed and compared between MACS and TESA groups.
Results
There were no significant differences in female age, male age, or sperm DNA fragmentation index (DFI) between the MACS and TESA groups. The blastocyst conversion rate was slightly higher in the TESA group (39%) compared to the MACS group (32%). However, the MACS group had a higher implantation rate (50%) than the TESA group (35%). Miscarriage rates, multiple pregnancy rates, and live birth rates did not show statistically significant differences between the groups. A chi-squared test was conducted to compare categorical variables, and t-tests were done to compare continuous variables.
Conclusion
In cases with raised sperm DNA fragmentation, sperm selection by MACS or TESA seems to offer comparable reproductive outcomes. There seems no superiority of one intervention over the other in cases with raised sperm DNA fragmentation undergoing assisted reproduction. Both interventions seem to be beneficial for couples seeking assisted reproduction with raised sperm DNA fragmentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infertility is defined as failing to conceive while having unprotected sexual intercourse for at least 1 year. This condition can give rise to substantial distress, social stigma, and financial challenges, negatively impacting individuals’ mental and psychosocial well-being [1, 2]. Globally, approximately 17.5% of the adult population, roughly 1 in 6 people, experiences infertility. These statistics emphasize the widespread prevalence of infertility and underline its significance as a critical concern for global health [3, 4].
In recent years, the use of assisted reproductive technologies (ART) to treat infertility has grown in popularity. One of the most common ART techniques is in vitro fertilization (IVF) [5]. Intracytoplasmic sperm injection (ICSI) is a form of IVF that involves an injection of a single sperm directly into the oocyte. ICSI is often indicated in cases with severe male factor infertility, where there are abnormalities in the number or quality of sperm [6]. One of the leading causes of male factor infertility is sperm DNA fragmentation. Sperm DNA fragmentation is the presence of breaks in the DNA of sperm cells (single- and double-strand DNA breaks). Single-strand DNA breaks are exhibited as multiple break points in all regions of the genome, whereas double-strand DNA breaks are mainly localized to the sperm nuclear matrix as very few break points. These breaks can damage the genetic material of the sperm, making it less likely to fertilize an oocyte and lead to a successful pregnancy. DNA fragmentation is caused by several factors such as oxidative stress, exposure to environmental toxins, and infections. Sperm DNA fragmentation can lead to poor embryo fertilization rate, poor embryo quality, and increased rates of miscarriages [7, 8].
Several treatments are available for couples with male factor infertility due to sperm DNA fragmentation. One of the treatment options is to use advanced sperm selection techniques to select sperm cells with good DNA integrity. Two commonly used sperm selection techniques that have been prevalent in assisted reproduction to obtain sperms with good DNA integrity are magnetic-activated cell sorting (MACS) and testicular sperm aspiration (TESA) [9, 10]. MACS is a non-invasive technique that uses magnetic beads coated with antibodies to bind and separate sperm with lower levels of DNA fragmentation[10]. This technique can improve the quality of sperm used in ART and increase the chance of successful fertilization and embryo development. However, MACS may not be suitable for all men with high levels of sperm DNA fragmentation, and it is ineffective in cases where there is no viable sperm in the ejaculate or from TESA [10, 11].
Conversely, TESA is a surgical technique that can retrieve sperm from the testicles of men for various indications like azoospermia, anejaculation, and raised sperm DNA fragmentation. This method can be combined with other techniques, such as ICSI, to achieve fertilization and pregnancy. However, TESA involves surgical intervention when compared to MACS and carries some risks, such as bleeding, infection, and testicular damage [12]. The rationale for the use of TESA sperms in men with raised sperm DNA fragmentation is to avoid storage of sperm in the male reproductive tract, which would expose the sperm to reactive oxygen species and cause oxidative stress. The use of testicular sperm for raised sperm DNA fragmentation has been shown to be beneficial in optimizing reproductive outcomes in few studies [13].
Nevertheless, there are few studies which have shown that TESA may also retrieve sperm with higher levels of DNA fragmentation, which can decrease the chance of successful fertilization and embryo development [14]. Considering this controversy in outcomes with TESA sperms, there seems a need for further research to evaluate the efficacy of the use of TESA as an intervention to obtain sperms with good DNA integrity and optimize reproductive outcomes. Sperm DNA fragmentation is a crucial marker of chromatin damage, impacting the chances of a healthy offspring [15]. The extent of DNA damage plays a pivotal role in sperm’s fertilizing potential, determined by the balance between DNA damage and oocyte repair capabilities. To meet the demand for eliminating damaged sperm in assisted reproductive technology (ART), various newer techniques like TESA, MACS, and sperm microfluidics sorting have emerged, showing reduced DNA fragmentation compared to ejaculated sperm [16]. Literature regarding the effectiveness and safety of the abovementioned newer techniques in the field of assisted reproduction is limited, and hence we intended to compare the efficiency of two of the abovementioned newer techniques MACS and TESA in this study.
Nevertheless, historically clinics have used frequent ejaculation or shorter abstinence as an intervention to retain good DNA integrity of sperm, and additionally, the double density gradient method has been shown to filter sperms with good DNA integrity. Previous studies have not definitively established which method of sperm processing or active intervention on sperm is beneficial in case of raised sperm DNA fragmentation [17, 18].
This study aims to compare the efficacy of TESA or MACS, as a preferred sperm selection technique for men undergoing assisted reproduction for raised sperm DNA fragmentation. Can sperm selection by MACS or TESA help in optimizing the reproductive outcomes will be investigated in this study.
The rationale for choosing only MACS and TESA in this study, though several other methods have been available for use in cases with raised sperm DNA fragmentation, is addressed further in the discussion segment of our work.
Materials and methods
This prospective, open-label, interventional, parallel, comparative, single-center, randomized control trial was conducted at a private teaching fertility clinic (Oasis Centre for Reproductive Medicine) between April 2019 and February 2020. The study was approved by the KIMS Ethical Committee, Secunderabad, Telangana, India (reference number KIMS/EC/2019/35–1). The study was also registered with the Clinical Trials Registry (CTRI) of India (registration reference number CTRI/2019/07/020140). Written consent with a clear understanding of the risks associated with testicular sperm retrieval was obtained from all patients who had undergone TESA. A thorough discussion about the benefits and limitations of TESA and its experimental nature was done with patients. Couples recruited in both arms signed an informed consent regarding the risks and uncertainty of the outcomes from the interventions mentioned above. The study was conducted in accordance with the Declaration of Helsinki (2013) and in compliance with the protocol. The reporting of our study adhered rigorously to the guidelines set forth by Equator Network, following the CONSORT statement (Supplementary Material), which ensures transparency and completeness in reporting our research.
Study participants
Raised sperm DNA fragmentation (SDF) exceeding 30% as determined by the Sperm Chromatin Structure Assay (SCSA) method was the primary inclusion criterion for this study. SDF testing was performed on ejaculated semen samples produced with a shorter abstinence period of less than 48 h. If the SDF was more than 30%, subjects were recruited into the study and allocated to one of the study arms, MACS or TESA.
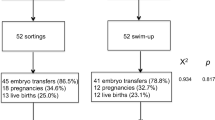
Additionally, a minimum sperm count of more than 5 million sperm per milliliter was considered for inclusion. The study population comprised of men aged between 21 and 50 years and females aged between 21 and 37 years. Inclusion was extended to couples with a documented history of at least one failed IVF cycle or two or more miscarriages in the past. In the previous failed IVF cycles, sperm processing was done by the double-density gradient technique (DDG). Men with a history of recurrent genital tract infections and individuals engaged in chronic smoking, binge alcohol consumption, or recreational drug use were considered ineligible for this study. Men with a Body Mass Index (BMI) exceeding 35, presence of clinically palpable varicocele, or cycles with donor sperm ICSI were also excluded. Additionally, females with any endometrial or uterine factors that may adversely affect embryo implantation, such as severe adenomyosis or a thin endometrium, were not eligible for participation in the study. These stringent exclusion criteria aim to ensure a focused and homogeneous study population for the investigation of sperm selection methods and reproductive outcomes. Using computer-generated sequences, a total of 150 recruited participants were block-randomized (1:1) into TESA (n = 75) and MACS (n = 75). Blinding concealment was not done in the present study (Fig. 1).
Interventions
Testicular sperm retrieval
Participants in this group were subjected to local anesthesia with 1% lidocaine injection into the scrotal skin. A sterile surgical field was prepared, and the patient’s scrotum was cleansed with an antiseptic solution. An 18-gauge needle attached to a 20 ml syringe was inserted into the testicle under aseptic precautions. Multiple punctures were made in different regions of the testis to increase the chances of obtaining sperm. Negative pressure was applied to the syringe and testicular tissue was aspirated. The aspiration process was carefully performed to minimize trauma to the testicular tissue. The aspirated testicular tissue was transferred to a sterile container containing a culture medium. The tissue was then carefully minced to release the spermatozoa. The suspension was incubated at 37 °C for a specified duration (30–60 min) to allow the spermatozoa to swim out. Sperm selection of TESA sperm for ICSI was done under 40 × magnification of ICSI micro-manipulator. Viable sperm cells demonstrated by twitch or motility with normal morphology were selected for injection.
Magnetic-activated cell sorting
On the oocyte pick-up (OPU) day, fresh ejaculate was collected from the male partner. Once the sample had liquefied, a double-density gradient washing technique was employed. Magnetic-activated cell sorting (MACS) processing was done as per the manufacturer’s instructions. After the gradient processing, a 0.1 ml pellet was obtained and resuspended in 1 ml of binding buffer. The suspension was centrifuged at 1200 rpm for 5 min. Subsequently, the resulting 0.1 ml pellet was mixed with 0.1 ml of Annexin reagent and 0.3 ml of binding buffer. The cellular suspension was then incubated at room temperature for 15 min. The sperm-micro bead suspension was loaded into a separate column pre-rinsed with 1 ml of binding buffer and attached to a Mini MACS magnet. The positive fraction containing apoptotic spermatozoa was trapped within the separation column, while the non-apoptotic spermatozoa (negative fraction) passed through the column and were collected in a separate tube. Finally, the negative fraction was resuspended in a 1:2 ratio of sperm wash media and centrifuged for 5 min at 1200 rpm. The resulting 0.5 ml pellet containing the non-apoptotic spermatozoa was utilized for intracytoplasmic sperm injection (ICSI).
Sperm chromatin structure assay (SCSA) protocol
Semen samples stored in liquid nitrogen tanks (− 196 °C) were thawed in a 37 °C water bath and placed on crushed ice. A portion of the semen was transferred to TNE buffer (0.01 M Tris–HCl:0.15 M NaCl:1 mM EDTA, pH 7.4) at 4°C, reaching a concentration of approximately 1–2 × 106 sperm/ml. Then, 200 µl of this sperm suspension was mixed with 400 µl of a solution of 0.08 N HCl:0.15 M NaCl:0.1% (v:v) Triton X-100 at 4 °C. The HCl was diluted from a 2.0 N commercial solution. After 30 s, the sperm were stained by adding 1.2 ml of a staining solution containing 6 µg/ml acridine orange (AO) in a specific ratio to DNA-P. The stained sample was analyzed using a FACS CALIBURTM flow cytometer, with 5000 sperm being analyzed at an event rate of 100–250 events/s. A new sample was prepared for equilibrium if the event rate exceeded 250 events/s. The flow cytometer was calibrated with a reference sample before analysis, and duplicate measurements were taken for each test sample. The percentage of sperm with increased red fluorescence (indicating fragmented DNA) was determined using proprietary SCSAsoft® software. Samples with a DFI difference greater than 10% between raw X and Y means were repeated, and the standard deviation between replicates was calculated.
Controlled ovarian stimulation
Antagonist protocol was the preferred regimen for all women recruited in this study. The dosage of gonadotrophin was decided based on the women’s antral follicle count (AFC), anti-Mullerian hormone (AMH) level, and BMI. Close monitoring with serial vaginal ultrasound scans (USG) and serum Estradiol levels was performed. The decision of trigger was taken when a majority of the follicles had crossed 14 mm upon USG with either hCG (Human Chorionic Gonadotrophin) 5000 IU or with Agonist (Triptorelin 0.2 mg dosage). The decision of hCG or Agonist was based on the serum Estradiol levels at trigger. As per our clinic’s SOP, oocyte retrievals from follicles greater than 14 mm should be 60–80% and the KPI (Key Performance Indicator) for mature oocytes should be 65–80%. 17-gauge single-lumen oocyte aspiration needle was used for the oocyte retrieval procedure for all patients.
Embryo culture
As per standard operating procedure, all cumulus oophorous complexes (COCs) were exposed to 80 IU hyaluronidase enzyme and subjected to a denudation process 2 h after the oocyte retrieval procedure.ICSI (intra-cytoplasmic sperm injection) was the preferred method of oocyte insemination. In the TESA and MACS groups, oocytes were injected with TESA and MACS sperm, respectively. ICSI was performed within 40 h post-hCG trigger. After ICSI and confirmation of fertilization, embryos were cultured continuously in SAGE one-step medium until day 5/6. On day 5 (116 h after insemination), the embryos were examined for the formation of blastocysts. The blastocyst was graded according to the criteria of Gardner and Lane [19]. The vitrification of day 5/6 expanded blastocysts using Kitazato vitrification media according to the manufacturer’s protocol.
Embryo transfer
The endometrium of the female partner was prepared as per the clinic’s standard operating procedures (SOP). All women were prepared using hormone replacement therapy (HRT) protocol using Estradiol valarate. Endometrial thickness of more than 7 mm, serum Estradiol of more than 300 pg/ml, and serum progesterone of less than 1.2 ng/ml were considered appropriate to start luteal phase support before embryo transfer. On the day of embryo transfer, no more than two blastocysts were thawed using Kitazato thawing media per the manufacturer’s instructions. After 2 h of warming, the embryos were examined for expansion. The expanded blastocyst was transferred under ultrasound guidance within 4 h of thawing. All blastocysts transferred to subjects had blastocoele cavity expansion of more than three, inner cell mass and trophectoderm (TE) were either A or B grade. Blastocysts with ICM or TE with Grade C were not considered for transfer. Two weeks after embryo transfer, a urine pregnancy test and blood beta HCG test were performed to confirm pregnancy.
Outcome variables
The endpoints of the study were to analyze both embryonic and reproductive outcomes. These include blastocyst conversion—The average blastocyst conversion rate was calculated by assessing the number of embryos that reached the blastocyst stage (day 5/6) divided by the total number of embryos fertilized after ICSI. Implantation rate (IR)—The implantation rate was determined by dividing the number of implanted embryos by the total number of transferred embryos in that cycle. Miscarriage rate (MR)—The miscarriage rate was calculated by dividing the number of miscarriages by the total number of embryo transfers. Multiple pregnancy rate (MPR)—The multiple pregnancy rate was determined by calculating the percentage of pregnancies that resulted in the development of multiple fetuses divided by the number of embryo transfer cycles. Live birth rate (LBR)—The live birth rate was assessed based on the intention-to-treat (ITT) population and the per embryo transfer (ET) cycle.
Data analysis
The randomization and statistical analyses were performed by a biostatistician using statistical software version 4.2.1 (R Core Team, 2022, Vienna, Austria). Categorical parameters were represented using frequencies and percentages, whereas continuous variables were expressed as means and standard deviations. A chi-squared test was conducted to compare categorical variables, and t-tests were done to compare continuous variables. Statistical significance was set at P < 0.05.
Ethical considerations
Ethical clearance was obtained from the KIMS ethical committee (KIMS/EC/2019/35–1) for this study, and the study was also registered with the Clinical Trials Registry (CTRI) of India (Registration reference number CTRI/2019/07/020140). Written consent with a clear understanding of the risks associated with testicular sperm retrieval was obtained from all patients who had undergone TESA. A thorough discussion about the benefits and limitations of TESA and its experimental nature was done with patients. Couples recruited in both arms signed an informed consent regarding the risks and uncertainty of the outcomes from the interventions mentioned above. The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and in compliance with the protocol.
Trial registration
Clinical Trial Registry-India (CTRI),
CTRI/2019/07/020140, Registered on: 10/07/2019.
Trial Registered Prospectively.
Type of Trial: Interventional.
URL
https://ctri.nic.in/Clinicaltrials/pmaindet2.php?EncHid=MzE4NDE=&Enc=&userName=TESA%20or%20MACS
Results
Table 1 shows the average age of female and male participants and the sperm DNA fragmentation index (DFI). In the MACS group, the average age of females is 34.1 years, while in the TESA group, it is 33.1 years. The average age of males is 37.01 years in the MACS group and 36.5 years in the TESA group. No significant differences in age were observed between the two groups (P > 0.05). The mean sperm DFI was similar in both groups, with values of 34 in the MACS group and 34 in the TESA group. Again, no statistically significant difference was found in sperm DFI between the groups (P = 0.8).
As shown in Table 2, the TESA group participants showed a slightly higher blastocyst conversion rate (39%) compared to the MACS group (32%) (RR, 1.22; CI, 1.00–1.50). However, the implantation rate was lower in the TESA group (35%) compared to the MACS group (50%) (RR, 0.71; CI, 0.51 to 0.98). The MR and MPR were slightly higher in the TESA group (11% and 4%, respectively) compared to the MACS group (5.3% and 8%, respectively), but the differences were not statistically significant. The live birth rate per in vitro fertilization (IVF) cycle (LBR per ITT (intention to treat)) and per embryo transfer (ET) cycle (LBR per ET cycle) were similar between the two groups, with no significant differences observed (P > 0.05).
Discussion
Male infertility treatment has been revolutionized by using testicular sperm and various advanced sperm selection techniques which have helped optimize reproductive outcomes in men undergoing assisted reproduction [20]. Among these techniques, magnetic-activated cell sorting (MACS), a recently advanced sperm selection technique, stands out as it employs magnetic beads coated with antibodies to meticulously isolate healthy sperm with intact DNA, eliminating apoptotic sperms and resulting in improved outcomes in intracytoplasmic sperm injection (ICSI) procedures. On the other hand, testicular sperm aspiration (TESA) involves the direct extraction of sperm from the testes using a fine-needle aspiration method. This approach is particularly advantageous as testicular sperm generally exhibit lower levels of DNA damage than sperm obtained through ejaculation [21]. In the context of male infertility treatment, the management of sperm DNA fragmentation plays a vital role in ensuring successful fertilization and optimal embryo development. Furthermore, the vigilant role of Sertoli cells in identifying and eliminating apoptotic sperm cells adds to the overall effectiveness of these advanced techniques, contributing to enhanced quality and viability of sperm used in male infertility treatments [7, 10, 16].
Our rationale to choose only MACS and TESA as active interventions to optimize DNA fragmentation though there are other available and safer methods reported in the literature is justified below. There seem several publications claiming optimal success with TESA sperms in men with raised DNA fragmentation [21,22,23]. However, this method involves surgical intervention and certain groups have questioned the safety of offsprings with the use of testicular sperm [24]. Nevertheless, TESA has been an active intervention in the field of assisted reproduction over three to four decades, and the outcomes seem to be safe [25]. MACS on the other hand is a relatively newer technique with encouraging results but lacks adequate data to consider its routine clinical usage. MACS technique uses ejaculated sperm which makes it easier for the patient with no surgical risks and might have a better acceptance when offered as a sperm selection technique. Therefore, we decided to compare one traditional, surgical intervention against a non-invasive newer intervention.
In the present study, TESA demonstrated a higher average blastocyst conversion rate of 39% compared to MACS, which had a conversion rate of 32%. This suggests that TESA has slightly better success in achieving blastocyst formation during the assisted reproductive process. It indicates that TESA may be more effective in supporting the development and maturation of embryos to the blastocyst stage, potentially enhancing the chances of successful implantation and subsequent pregnancy. This improved blastocyst conversion in the TESA group might be due to the better DNA integrity of testicular sperm against the ejaculated sperm. Blastocyst development is essential in assisted reproduction treatment (ART) as it indicates embryo quality and the likelihood of successful pregnancy. Stimpfei et al. [26] showed that couples dealing with male infertility due to teratozoospermia could benefit from MACS selection of spermatozoa with a higher percentage of good quality blastocysts but only when the woman is older than 30 years. The reasons for lesser blastocyst conversion in the MACS group of this study need further research.
In the present study, TESA exhibited an implantation rate of 35%, while MACS showed a higher implantation rate of 50%. This indicates that MACS had a higher success rate regarding successful embryo implantation than TESA. The higher implantation rate of MACS suggests that this technique may offer better quality embryos which might aid in better implantation rates. In studies [17, 27] using MACS in ICSI cycles, some have reported improved implantation rates because MACS helps select sperms with the least amount of DNA fragmentation. In a study by Ziarati et al. [27], the implantation rate was higher in the MACS group than in the conventional sperm selection group. TESA’s implantation rate largely depends on the severity of male factor infertility. Tournaye et al. [28] reported that using testicular sperm in ICSI resulted in an implantation rate of 17.4% per transferred embryo for patients with obstructive azoospermia. These rates can vary significantly depending on the underlying cause of male infertility. The implantation rate is a better KPI (Key Performance Indicator) to assess the effectiveness of any intervention or treatment offered in the field of assisted reproduction. Variability in implantation rates in our study between TESA and MACS groups need further extensive evaluation and a larger cohort study might help us obtain further clarity. Though the implantation is better with MACS in this study, we would not want to make a recommendation of MACS against TESA based on the inference of this study.
In the present study, TESA showed a miscarriage rate of 11% (8 out of 75 cases), while MACS had a lower miscarriage rate of 5.3% (4 out of 75 cases) which showed that MACS had a lower risk of miscarriage compared to TESA. The lower miscarriage rate associated with MACS suggests that this technique may provide more favorable conditions for successful embryo development and pregnancy maintenance. Studies by Horta et al. [29] and Gil et al. [11] did not find significant differences in implantation and miscarriage rates between MACS and standard sperm selection methods. In the study conducted by Pacheco et al. [10], the MACS group had a significantly lower miscarriage rate of 14.7% compared to the control group, which had a rate of 20.6%. Mantravadi et al. [17] found a lower miscarriage rate in the MACS group, although not statistically significant.
Comparing miscarriage rates between MACS and TESA procedures is challenging due to their different purposes and patient populations. MACS, a sperm selection process for ejaculated sperm, may reduce miscarriage rates by selecting sperm with low DNA fragmentation. TESA, surgical retrieval of testicular sperm, is used in azoospermia cases, and the miscarriage rates depend on factors like the cause of azoospermia and the woman’s age. Directly comparing miscarriage rates between MACS and TESA may not be meaningful due to inherent differences and specific circumstances of each procedure. Herein, TESA exhibited a multiple pregnancy rate of 4%, while MACS had a slightly higher rate of 8%, indicating that MACS had a slightly higher likelihood of multiple pregnancies than TESA. The higher multiple pregnancy rate associated with MACS suggests that this technique may increase the chances of multiple embryos implanting and developing into viable pregnancies. The available research [11, 17] suggests that MACS selection of spermatozoa may improve pregnancy rates compared to other sperm selection techniques, such as density gradient centrifugation and swim-up. However, there is no clear consensus on the superiority of MACS over TESA in terms of multiple pregnancy rates. Further research is needed to provide more conclusive evidence on comparing MPR between sperm selection by TESA and MACS.
In our study, TESA showed a live birth rate per intention-to-treat (ITT) analysis of 44%, while MACS had a slightly lower rate of 41.3%. The difference in live birth rates between TESA and MACS was not statistically significant. However, when analyzing the live birth rates per embryo transfer (ET) cycle, MACS exhibited a higher rate of 63% compared to TESA’s rate of 56%. This suggests that MACS had a slightly higher likelihood of resulting in a live birth per ET cycle compared to TESA. According to the study conducted by Pacheco et al. [10], the live birth rate was significantly higher in the MACS group at 47.4% compared to the control group, which had a rate of 31.2%. In a retrospective study by Javed et al. [30], the live birth rate (LBR) per embryo transfer (ET) cycle was compared between fresh and frozen TESA (testicular sperm aspiration) and PESA (percutaneous epididymal sperm aspiration) spermatozoa. The study reported a slightly higher LBR for fresh epididymal sperm (PESA) than frozen-thawed sperm and a higher LBR for fresh TESA compared to frozen-thawed TESA sperm. Another study by Mei et al. [31] compared MACS (magnetic-activated cell sorting) with a control group and reported a higher LBR for the MACS group in the first ET cycle and cumulative LBR. However, it is essential to note that the results regarding LBR per ET cycle and the comparison between TESA and MACS are inconsistent across studies. This study suggests that using MACS was associated with a significantly increased likelihood of achieving a live birth compared to the control group.
While our exploration of these two techniques yielded promising insights, it is essential to acknowledge certain constraints that require consideration. MACS and TESA are procedures used to address male factor infertility, but they have potential risks. MACS, a non-invasive sperm selection method, carries minimal risks, but there is a possibility of selecting sperm with subtle defects that may affect embryo development [32]. MACS is a relatively new intervention introduced recently into the field of assisted reproduction. There is still a need for further research, randomized control trials, and meta-analysis at this point. The efficacy and limitations of this new MACS technique need further validation. TESA, a surgical procedure, has risks such as hematoma or infection, persistent scrotal pain, tissue damage, retrieval of non-viable sperm, and standard surgical risks associated with anesthesia. These risks are generally rare, and the procedures are considered safe, but discussing them with a healthcare provider for a complete understanding is essential. The use of testicular sperm in assisted reproduction has been considered safe and has shown not to affect the fetal well-being [33]. Laboratory procedures to handle surgically retrieved sperms and optimize sperm selection are well-validated and help in optimizing reproductive outcomes [34].
To our knowledge, this study is the only randomized control trial done so far comparing TESA versus MACS where the follow-up has been till live births. Most studies involving MACS have compared the outcomes with traditional sperm processing techniques like density gradient sperm processing. This seems to be the first study that has compared MACS against TESA for reproductive outcomes. Though MACS showed better outcomes with implantation and live births, it is a newer intervention which still needs further research. Outcomes of the TESA arm are numerically low but not very significant, apart from the need for anesthesia and surgical intervention it seems to offer encouraging results. Hence, adequate caution needs to be exercised before offering either of the intervention for men with raised SDF as none of the interventions is superior against each other.
Nevertheless, our study had few limitations. One of the limitations of the study was the absence of a control arm, where the study population received no intervention for raised SDF in the subsequent cycle. This would have further helped compare the effectiveness of the interventions used in this study. Additionally, we did not consider to check the post-intervention sperm DNA fragmentation level of TESA and MACS sperms. This would have helped us estimate the efficiency of the intervention to bring down the raised SDF and also guided the embryologist about the availability of sperms with good DNA integrity. Lastly, the assessment of sperm DNA fragmentation was done by the SCSA method and not the TUNNEL assay.
Conclusion
In cases with raised sperm DNA fragmentation, sperm selection by MACS or TESA both offered comparable reproductive outcomes. There seemed no superiority of one intervention over the other. Both interventions seem to be beneficial for couples seeking assisted reproduction with raised sperm DNA fragmentation.
Data availability
The data that supports the findings of this research are not publicly available due to institutional restrictions. However, the datasets/tables/figures generated during this study or support the findings of the current study are available from the corresponding author upon reasonable request.
References
Infertility [Internet]. World Health Organization. Available from: https://www.who.int/news-room/fact-sheets/detail/infertility. Accessed 23 June 2023.
Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10.
1 in 6 people globally affected by infertility: Who [Internet]. World Health Organization. Available from: https://www.who.int/news/item/04-04-2023-1-in-6-people-globally-affected-by-infertility. Accessed 23 June 2023.
Ombelet W. Global access to infertility care in developing countries: a case of human rights, equity and social justice. Facts Views Vis Obgyn. 2011;3(4):257–66.
Jain M, Singh M. Assisted Reproductive Technology (ART) Techniques. [Updated 2022 Nov 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK576409/. Accessed 7 June 2023.
Merchant R, Gandhi G, Allahbadia GN. In vitro fertilization/intracytoplasmic sperm injection for male infertility. Indian J Urol. 2011;27(1):121–32.
Agarwal A, Majzoub A, Baskaran S, Panner Selvam MK, Cho CL, Henkel R, et al. Sperm DNA fragmentation: a new guideline for clinicians. World J Mens Health. 2020;38(4):412–71.
González-Marín C, Gosálvez J, Roy R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci. 2012;13(11):14026–52.
Hozyen M, Hasanen E, Elqusi K, ElTanbouly S, Gamal S, Hussin AG, et al. Reproductive outcomes of different sperm selection techniques for ICSI patients with abnormal sperm DNA fragmentation: a randomized controlled trial. Reprod Sci. 2022;29(1):220–8.
Pacheco A, Blanco A, Bronet F, Cruz M, García-Fernández J, García-Velasco JA. Magnetic-activated cell sorting (MACS): a useful sperm-selection technique in cases of high levels of sperm DNA fragmentation. J Clin Med. 2020;9(12):3976.
Gil M, Sar-Shalom V, Melendez Sivira Y, Carreras R, Checa MA. Sperm selection using magnetic activated cell sorting (MACS) in assisted reproduction: a systematic review and meta-analysis. J Assist Reprod Genet. 2013;30(4):479–85.
Shah R. Surgical sperm retrieval: techniques and their indications. Indian J Urol. 2011;27(1):102–9.
Esteves S, Roque M, Garrido N. Use of testicular sperm for intracytoplasmic sperm injection in men with high sperm DNA fragmentation: a SWOT analysis. Asian J Androl. 2018;20(1):1.
Zini A, Alharbi M, Hamouche F, Phillips S, Kadoch J. Use of testicular sperm in couples with SCSA-defined high sperm DNA fragmentation and failed intracytoplasmic sperm injection using ejaculated sperm. Asian J Androl. 2019;22(4):348–53.
Li F, Duan X, Li M, Ma X. Sperm DNA fragmentation index affect pregnancy outcomes and offspring safety in assisted reproductive technology. Scientific Reports. 2024;14(1):356. https://www.nature.com/articles/s41598-023-45091-6. Accessed 3 Jan 2024.
Esteves SC, Zini A, Coward RM, Evenson DP, Gosálvez J, Lewis SEM, Sharma R, Humaidan P. Sperm DNA fragmentation testing: summary evidence and clinical practice recommendations. Andrologia. 2021;53(2):e13874.
Mantravadi K, Gedela DR, Karunakaran S. Raised sperm DNA fragmentation–randomized controlled trial of MACS vs TESA to optimise reproductive outcomes. Fertil Steril. 2020;114(3):e370.
Makhseed MA, Al Salem MH, Ahmed MA. Percutaneous testicular sperm aspiration and intracytoplasmic sperm injection in obstructive and non-obstructive azoospermia: an easy alternative to TESE and MESA. Urol Int. 2002;68(2):86–90.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–8.
Javed M, L. Tan S. Advances in male infertility treatment through assisted reproductive technology [Internet]. Recent Advances in Male Reproductive System. IntechOpen; 2023. Available from: https://doi.org/10.5772/intechopen.1002435
Esteves SC, Sánchez-Martín F, Sánchez-Martín P, Schneider DT, Gosálvez J. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril. 2015;104(6):1398–405.
Greco E, Scarselli F, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Human Reproduction. 2005;20(1):226–30. https://academic.oup.com/humrep/article/20/1/226/671628. Accessed 11 Nov 2004.
Bradley CK, McArthur SJ, Gee AJ, Weiss KA, Schmidt U, Toogood L. Intervention improves assisted conception intracytoplasmic sperm injection outcomes for patients with high levels of sperm DNA fragmentation: a retrospective analysis. Andrology. 2016;4(5):903–10.
Fedder J, Loft A, Parner ET, Rasmussen S, Pinborg A. Neonatal outcome and congenital malformations in children born after ICSI with testicular or epididymal sperm: a controlled national cohort study. Human Reprod. 2013;28(1):230–40.
Jin L, Li Z, Gu L, Huang B. Neonatal outcome of children born after ICSI with epididymal or testicular sperm: a 10-year study in China. Sci Rep. 2020;10(1):5145. https://www.nature.com/articles/s41598-020-62102-y. Accessed 20 Mar 2020.
Stimpfel M, Verdenik I, Zorn B, Virant-Klun I. Magnetic-activated cell sorting of non-apoptotic spermatozoa improves the quality of embryos according to female age: a prospective sibling oocyte study. J Assist Reprod Genet. 2018;35(9):1665–74.
Ziarati N, Tavalaee M, Bahadorani M, Nasr Esfahani MH. Clinical outcomes of magnetic activated sperm sorting in infertile men candidate for ICSI. Hum Fertil (Camb). 2019;22(2):118–25.
Tournaye H, Verheyen G, Albano C, Camus M, Van Landuyt L, Devroey P, et al. Intracytoplasmic sperm injection versus in vitro fertilization: a randomized controlled trial and a meta-analysis of the literature. Fertil Steril. 2002;78(5):1030–7.
Horta F, Crosby J, Mackenna A, Huidobro C. Male factor infertility outcomes using magnetic activated cell sorting in intra citoplasmatic sperm injection cycles. Androl Open Access. 2016;5:1–6.
Javed A, Ramaiah MK, Talkad MS. ICSI using fresh and frozen PESA-TESA spermatozoa to examine assisted reproductive outcome retrospectively. Obstet Gynecol Sci. 2019;62(6):429–37.
Mei J, Chen LJ, Zhu XX, Yu W, Gao QQ, Sun HX, et al. Magnetic-activated cell sorting of non-apoptotic spermatozoa with a high DNA fragmentation index improves the live birth rate and decreases transfer cycles of IVF/ICSI. Asian J Androl. 2022;24(4):367–72.
Garrido N, Juliá María Gil. The use of non-apoptotic sperm selected by magnetic activated cell sorting (MACS) to enhance reproductive outcomes: what the evidence says. Biology. 2024;13(1):30.
Mantravadi Krishna Chaitanya, Rao Durga Gedela, Rupa Sree Y. Does testicular sperm alter reproductive and perinatal outcomes in assisted reproductive technology cycles? 10 years’ experience in an Indian clinic. J Hum Reprod Sci. 2022;15(4):388–8.
Popal W, Nagy ZP. Laboratory processing and intracytoplasmic sperm injection using epididymal and testicular spermatozoa: what can be done to improve outcomes? Clinics. 2013;68(Suppl 1):125–30.
Acknowledgements
The authors would like to thank Dr. Mehul R. Chorawala and Ms. Sakshi Srivastava (Intas Pharmaceuticals Limited, Ahmedabad, India) for providing scientific writing assistance and follow-up with the editorial office of the journal. The authors are also grateful to Dr. Vishal Dave of Intas Pharmaceuticals Limited, Ahmedabad, India for the critical review of the manuscript at various stages.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent to participate
All participants provided a written informed consent form to participate in the study.
Consent for publication
Consent for publication was obtained from all authors, the participants, or legally authorized representatives involved in this study.
Competing interest
The authors declare that they have no financial or non-financial or any other conflicts of interest related to this study. This study has no commercial interest in MACS technology and we have not received any funding or support from MACS technology company. The reason for choosing MACS is purely academic and scientific and not intended for any marketing or commercial purpose.
Additional information
The authors declare that this manuscript has not been submitted elsewhere for consideration and has not been published previously. All authors have read and approved the manuscript for submission.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mantravadi, K.C., Rao, D. In cases with raised sperm DNA fragmentation, can sperm selection by magnetic-activated cell sorting or testicular sperm aspiration help improve reproductive outcomes?. J Assist Reprod Genet 41, 1507–1515 (2024). https://doi.org/10.1007/s10815-024-03128-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-024-03128-3