Abstract
The aim of the study is to compare the reproductive outcomes of different sperm selection techniques: density gradient centrifugation (DGC), testicular sperm (Testi), physiological ICSI (PICSI), and magnetic-activated cell sorting (MACS) in abnormal sperm DNA fragmentation (SDF) ICSI patients. A randomized controlled trial included 302 patients with abnormal SDF undergoing ICSI where they were randomized into 4 groups: a control group of DGC (n= 72), Testi (n=73), PICSI (n=78), and MACS (n=79). Results showed no significant differences in the male age, female age, or SDF between the four groups. Testi group had significantly lower cleavage and blastulation rates compared to PICSI, DGC, or MACS groups (p =0.001). For the high-quality blastocysts, DGC and MACS groups had significantly higher rate than the Testi group (p =0.014). The highest pregnancy rate was scored for the PICSI group (69.6%), while the lowest pregnancy rate was scored for the DGC group (51.4%) with (p =0.025). The PICSI group showed a significantly higher implantation rate compared to the other groups (p =0.003). Regarding the ongoing pregnancy rate, the significant difference was observed between the PICSI (62.8%) and MACS (62%) vs. DGC (45.8%). Besides, no significant differences were found in the miscarriage rates between the four groups. In conclusion, PICSI and MACS along with DGC showed significant improvement in embryological and clinical outcome over testicular sperm or sperm processed by DGC alone in patients with abnormal SDF
Registration number: NCT04482517
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Routine semen analysis (SA) is the conventional way for male fertility evaluation [1, 2]. However, many consider that SA is not enough to give an accurate prediction of male fertility [1, 3, 4]. Evaluation of sperm DNA fragmentation (SDF) is looked to be controversial as a beneficial addition for better assessment and prediction of male fertility potential and the subsequent reproductive outcomes [4, 5]. Abnormal SDF is thought to affect the process of achieving pregnancy, either spontaneous or by assisted reproductive techniques [6,7,8]. Through the journey of a tiny sperm carrying a massive amount of DNA to the egg, it turns out not everything arrives intact [9]. It has been found that higher percentages of SDF are found in infertile men, even fertile men have different levels of SDF [3]. Despite some of SA parameters are correlated with SDF; the crucial issue is that SDF is an independent factor that cannot be predicted from conventional SA [2, 10, 11]. Hence, SDF testing adds a value when it is used as a complementary step to the conventional semen analysis.
There are associations between SDF and many male intrinsic factors (varicocele, immature abnormal spermatozoa, oxidative stress, chromatin remodeling, and packaging problems) [12, 13] and extrinsic factors (smoking, drugs, and pollution) [8, 14]. Abnormal SDF can affect reproductive outcomes at different level [7, 9, 15, 16]. It negatively affects the pre-implantation embryogenesis (fertilization, cleavage, and blastulation rates), post-implantation outcomes (clinical pregnancy, miscarriage, and even live birth rates), or offspring health (congenital diseases, psychiatric disorders, and childhood cancers) [7, 12, 16,17,18,19]. Intracytoplasmic sperm injection (ICSI) is the most commonly used fertilization method of oocytes even in non-male factor patients [20]. ICSI procedure bypasses the barriers of natural sperm selection, increasing the risk of fertilizing the oocyte with suboptimal spermatozoa [21, 22]. That urges the need for advanced sperm selection techniques to eliminate the negative impact of injecting sperm with abnormal SDF [4].
There is a debate for the need to do sperm selection, while others debate the method to be used for the selection [12, 22,23,24]. Some studies have reported that conventional processing methods as density gradient centrifugation (DGC) or swim-up are enough [15, 24, 25]. On the contrary, others suggested advanced selection methods that have been tested to improve SDF are the way forward [9, 12, 13, 26]. There are different sperm selection techniques available such as physiological ICSI (PICSI) that depends on dishes with hyaluronan microdots. PICSI is mimicking the nature of sperm-oocyte binding affinity between sperm head with high DNA integrity to hyaluronan [25, 27] that makes it different from the conventional ICSI, where sperm selection is based only on motility and morphology while that of PICSI, sperms are selected from those attached to hyaluronan drops present in the dish [27, 28]. Magnetic-activated cell sorting (MACS) de-selects the apoptotic spermatozoa from a sperm population where apoptotic sperm with externalized phosphatidylserine residues bind to high-affinity annexin V microbeads, followed by column separation [29, 30]. Other researchers suggested the use of testicular sperm (Testi) in ICSI patients with abnormal SDF [31]. Testi has been suggested based upon the topographic mapping that has shown different SDF levels through male genital tract [32], where the lowest SDF level was in the testis and increased along the way to be the highest in the ejaculated sperm [31, 32]. Despite the invasiveness of the testicular sperm extraction procedures—in contrast to the other techniques—it is clinically supported not only by decreasing SDF levels, but also by improving the subsequent ICSI pregnancy outcomes [31,32,33,34].
Our study aimed to compare the embryological and clinical outcomes after using different sperm selection techniques including DGC, PICSI, MACS, or Testi for men with abnormal SDF levels undergoing ICSI.
Materials and Methods
This study is approved by Ganin Fertility Center ethics committee in February 2, 2017, in accordance with the declaration of Helsinki as a statement of ethical principles of medical research containing human subjects, under the identifier number: GFC-130720. Patients were informed with all study details and signed their consent before inclusion.
Study Design and Randomization
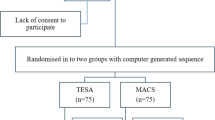
A randomized controlled trial was performed on 320 couples from March 2017 to December 2018 at Ganin Fertility Center in Egypt. We used IBM SPSS Software Version 22 to generate a random sequence and allocated cases between the four groups: DGC as a control (n= 72), PICSI (n= 78), MACS (n= 79), or Testi (n=73) using random numbers Rv.uniform and arithmetic Trunc (1) functions from compute variable dialog box. Eighteen cases were excluded after randomization for mismatching the inclusion criteria: as 9 cases had leukocytospermia on the day of ICSI and 9 produced a smaller number of MII oocytes.
Eligibility Criteria
Couples were eligible to participate if the following criteria were met: the female age ≤ 37 years and had ≥ 5 COCs on the trigger day with at least one mature oocyte developed to a blastocyst with fresh embryo transfer. Sexual abstinence up to 2 days to limit SDF variations [35, 36], semen samples with at least 1 million total progressive motile sperm, males had abnormal SDF levels ≥ 20.3% determined by TUNEL assay according to a previously published ROC analysis [37]. We measured the SDF up to 3 weeks before ICSI to limit the variation between the tested samples and those used for sperm selection and ICSI [38]. We excluded patients with endometriosis, recurrent implantation failure (RIF), recurrent miscarriage (RM), leukocytospermia (>1 million leukocytes/ml ejaculate), varicocele, cryopreserved semen samples, sexually transmitted diseases (STDs), sperm/oocytes donation, or gestational carrier.
Sperm DNA Fragmentation Test, Sperm Processing, and Selection Techniques
Sperm DNA fragmentation assessment was done by a validated TUNEL assay using Apodirect kit (BD Pharmingen, San Diego, CA) and BD Accuri C6 benchtop flow cytometer [39]. For DNA fragmentation index calculation, 10,000 sperm were counted and analyzed per sample with BD flow cytometer software and a cut-off value of 20.3% [37].
On the day of ICSI, semen samples were collected and assessed by microscopic examination for count and motility within 20–30 min of collection according to the World Health Organization (WHO) guidelines 2010 [40]. Leukocytospermia is confirmed using peroxidase test LeucoScreen (FertiPro, Belgium) with a lower reference limit of 1 million/ml [40]. The control group was processed using double-layer DGC (90%,45%) (Irvine Scientific, California, USA) followed by sperm wash using modified sperm washing media (Irvine Scientific, USA).
For PICSI (Cooper Surgical, USA), hyaluronan dots were hydrated with multipurpose handling media (MHM) (Irvine Scientific, USA), and sperm was selected after being bound to the dots; incubation was at 30°C for 10 min. While in the MACS, semen was incubated with annexin V microbeads and binding buffer for 20 min followed by column separation (Miltenyi Biotec, Bergisch Gladbach, Germany) that resulted in the elution of the non-apoptotic sperm population. Both PICSI and MACS sperm selection techniques were preceded by DGC as a routine semen processing method.
Testicular sperm was sampled by micro-TESE, a surgical biopsy done using the assistance of a microscopic examination of the testis under general anesthesia, or TESA performed by aspirating fluid and tissue through a needle with negative pressure from the testis. Samples from both techniques were processed by erythrocyte lysis buffer wash before sperm collection.
Controlled Ovarian Stimulation and Oocyte Pick Up
We used long protocol for ovarian stimulation in all cases in the study; stimulation was started on day 3 of the menstrual cycle with combined oral contraceptive pills, Gynera® (Bayer, Germany), for 12–21 days and 20 IU of Lucrin® (Abbvie, Spain) overlapped with Gynera® at the last 5 days of stimulation. From day 3 of the next cycle, downregulation was confirmed by endocrine profile and transvaginal ultrasound, after which ovarian stimulation was started using Gonal-F 150 IU® (Merck, Italy) or 150 IU Fostimon® (IBSA, Switzerland), combined with 75 IU Menopur® (Ferring, Germany). These doses were adjusted in the following days according to each patient’s response. When at least two follicles reached approximately 19mm or more, intramuscular injection of 10,000 IU of HCG Pregnyl® (Organon, the Netherlands) was applied. Oocyte pick up was done under general anesthesia after 36 h of HCG using transvaginal follicle aspiration needle and ultrasound guidance.
ICSI, Embryo Culture, and Transfer
ICSI was done for all patients at 38–40 h, followed by embryo culture in continuous single culture media CSC (Irvine Scientific, USA) supplemented with 10% serum substitute supplement (SSS) (Irvine Scientific, USA) with incubation conditions of 6% CO2, 5% O2, and 37°C. Blastocysts were graded by two different experienced embryologists according to Gardner’s criteria, and all cases had high-quality blastocyst transfer on day 5 or day 6 [41]. Embryo transfer was done on day 5 (118–120-h post-ICSI) or day 6 (142–144-h post ICSI) using Wallace® classic embryo replacement catheter (Cooper Surgical, USA) under transabdominal ultrasound guidance. And the blastocysts were placed around 8–10 mm from the fundus. Patients were prescribed for progesterone injection 100 mg Progynova (Ferring, Germany) until the day of pregnancy test and continued until the 10th week of pregnancy.
Outcome Measures and Statistical Analysis
All embryological parameters (cleavage rate on day 3, blastulation on day 5/6, and high-quality blastocyst rates) and clinical outcomes (clinical pregnancy, implantation, ongoing pregnancy, and miscarriage rates) for the four groups were recorded. The cleavage rate is the number of cleaved embryos on day 3/number of MII oocytes. Blastulation rate is the number of blastocysts on days 5 and 6/number of cleaved embryos; the blastocyst is considered to be of high quality if graded higher than 3BB. The clinical pregnancy rate is defined as the presence of fetal heartbeats at 6 to 7 weeks of gestation, and pregnancy is considered ongoing if exceeded 20 weeks of gestation. Implantation rate is defined as the number of gestational sacs with fetal heartbeat, confirmed by ultrasound, at 6 weeks of gestation over the number of embryos transferred. The miscarriage rate is the proportion of miscarried cases within 20 weeks of gestation over the number of the clinically pregnant cases [42]. Results were analyzed using IBM SPSS Software Version 22 for Microsoft windows. Comparison of numerical variables between the study groups was done using one-way analysis of variance (ANOVA) with post hoc multiple 2-group comparisons. The differences were considered significant if the p-value was ≤ 0.05.
Results
There were no significant differences between all study groups in the female age, BMI, hormonal profile, number of retrieved oocytes, and metaphase II oocytes (MII) (Table 1). In addition, there were no significant differences in the male age, SDF level, semen parameters, abstinence days, or smoking status (Table 2).
Looking into the pre-implantation embryological parameters, Testi had the lowest cleavage rate of 65.2%, which was significantly different compared to any of the other groups PICSI, MACS, or DGC (p < 0.05) (Table 3). Again, there was a significant difference in the blastulation rate of Testi (40.2%) compared to any other group, PICSI (58.7%), MACS (61.6%), or DGC (64.8%), while there was no significant difference between the other groups (Table 3). Regarding the high-quality blastocyst rate, DGC and MACS groups had a significantly higher percentage than the Testi group (p <0.05), but when comparing PICSI to either MACS, DGC, or Testi, we found no significance (Table 3).
For the clinical outcomes, PICSI (69.6%) and MACS (67.1%) groups had significantly higher clinical pregnancy rates compared to the DGC group (51.4%) (p < 0.05) (Table 4). Comparing the implantation rates, the PICSI group had a significantly higher implantation rate than the other groups (p < 0.05). PICSI group had an implantation rate of 59.3% that was almost double that of the DGC group 29.4% (Table 4). Also, the results showed a higher ongoing pregnancy rate in the PICSI and MACS groups than the DGC group (p < 0.05) (Table 4). Finally, for the miscarriage rates, although the DGC had the highest rate, there were no significant differences among all the groups (Table 4).
Data are presented as mean ± standard deviation, otherwise stated. N number of patients, DGC density gradient centrifugation, PICSI physiological ICSI, MACS magnetic-activated cell sorting, Testi testicular sperm, BMI body mass index, MII metaphase II oocytes. p-values represent the comparison between the four groups. p≤ 0.05 is considered significant
Data were presented in mean ± standard deviation, otherwise stated. N number of patients, SDF sperm DNA fragmentation, DGC density gradient centrifugation, PICSI physiological ICSI, MACS magnetic-activated cell sorting, Testi testicular sperm. p-values represent the comparison between the four groups. p≤ 0.05 is considered significant
Data are presented in mean ± standard deviation, DGC density gradient centrifugation, PICSI physiological ICSI, MACS magnetic-activated cell sorting, Testi testicular sperm. a* is a significant difference between DGC and Testi groups. The other groups have no significant differences. The mentioned p-values represent the comparison between the four groups. p≤ 0.05 is considered significant
Data are presented in numbers and percentages, otherwise stated. DGC density gradient centrifugation, PICSI physiological ICSI, MACS magnetic activated cell sorting, Testi testicular sperm. ET is number of embryos transferred/patient. b* is a significant difference between DGC and PICSI, c* is a significant difference between DGC and MACS, d* is a significant difference between PICSI and MACS, and e* is a significant difference between PICSI and Testi. The other groups have no significant values. The mentioned p-values represent the comparison between the four groups. p≤ 0.05 is considered significant
Discussion
The findings of this study showed significant improvement in clinical outcomes in the form of implantation, clinical pregnancy, and ongoing pregnancy rates in cases with abnormal SDF. Selecting the most “competent sperm” by PICSI or MACS showed better clinical outcomes than DGC alone, as it is the routine way for sperm processing during conventional ICSI. The use of PICSI or MACS has been introduced as sperm selection techniques as many suggested that either would reduce sperm head DNA fragmentation [43,44,45,46]. These findings support the use of SDF assessment to identify the cases that might benefit from using specific sperm selection techniques to improve the clinical outcomes in ICSI patients.
Our results indicated that using Testi when SDF is abnormal had the lowest cleavage, blastulation, and high-quality blastulation rates compared to the results of sperm selection from the ejaculate. So, Testi may not have the ability to select the most “competent sperm” compared to ejaculate processing and selection. Meanwhile, selecting sperm by PICSI or MACS have shown comparable results to Testi in both pre-implantation embryological assessment as well as clinical outcomes, except the implantation rate in favor of PICSI. When comparing PICSI vs. MACS, PICSI had higher implantation rate than MACS, while both had higher clinical pregnancy and ongoing pregnancy rates compared to the DGC group. DGC showed better results for pre-implantation embryonic parameters only when compared to Testi without reflection on the clinical outcomes. The reason might be due to the sole dependence on morphological assessment, which did not provide a complete assessment of the embryos.
In the DGC group, sperm selection depended on sedimentation only that seems to be not enough to select the best sperm out of the ejaculate. Data from previous studies showed that DGC decreased the level of SDF in normozoospermic, oligozoospermic, and teratozoospermic semen samples than unprocessed ones (p<0.05) [24, 47]. However, De Martin et al. suggested that DGC is not the best choice for ICSI patients and it cannot select sperm free of DNA fragmentation [48]. In our study, combining DGC with PICSI or MACS improved the clinical outcomes. In vivo, sperm passes through a cascade of unique selection mechanisms [21]. During ICSI, those mechanisms of natural selection are bypassed, which might lead to the selection of sperm of rather lower fertilization potential which might have some pre- and post-implantation consequences [21].
Testicular sperm had been suggested to be used in case of abnormal SDF, as it might have lower levels of SDF than the ejaculated sperm [31, 32]. A systematic review and meta-analysis by Esteves et al. reported significantly higher clinical pregnancy and live birth rates when using testicular than ejaculated sperm in cases with abnormal SDF [31]. It is important to realize that this review lacks preimplantation embryo assessment such as cleavage or blastulation rates. Also, it was criticized for combining case series with cohort studies in different patient populations and using different SDF assays [31].
Contrary to the above suggestions, some researchers have concerns about the motility and the fertilizing capacity of testicular sperm, which might have a negative impact on the reproductive outcomes [12, 49, 50]. Others reported a higher risk of using chromosomally abnormal sperm for ICSI using a testicular source since it has been reported that testicular sperm has higher aneuploidy rate than ejaculated sperm [12, 51, 52]. Cheung et al. revisited this issue in their recent study [53]. They assessed the chromosomal content of the ejaculated and testicular sperm by fluorescence in situ hybridization (FISH) and next-generation sequencing (NGS) techniques in abnormal SDF patients [53]. And, as an opposite notion, they reported higher aneuploidy rate in ejaculated sperm than the testicular sperm. This study needs more conformational studies as it had a very limited number of samples [53]. Our results showed no superiority of testicular over ejaculated sperm processed with DGC in all embryological parameters but showed a higher tendency in implantation, pregnancy, and ongoing pregnancy rates with lower miscarriage rate than the DGC group. However, when comparing PICSI or MACS to Testi, both PICSI and MACS had significantly higher cleavage and blastulation rates on the embryological level. While PICSI had a significantly higher implantation rate than the Testi group, both PICSI and MACS had a higher tendency in clinical pregnancy and ongoing pregnancy rates.
Our results disagreed with Bradley et al. [33]. They compared TESE, PICSI, and no intervention groups. Their study reported a higher pregnancy rate (49.5%, 37.6%, 27.5%) implementing the intervention of TESE than PICSI or no intervention, respectively [33]. In addition, higher live birth rate (43.7%, 32.7%, 24.9%) in TESE, PICSI, and no intervention, respectively [33]. Bradley’s study was retrospective with a relatively small sample size. For the comparison between MACS and Testi, we did not find any published studies so far.
These results imply that testicular sperm was not better neither at the level of embryological nor the clinical parameters than ejaculated sperm selected using PICSI or MACS, although it was superior to ejaculated sperm processed with DGC alone. Sperm selected from the testicular source depends only on the morphology and sometimes on the motility of the sperm without being a “hand-picked” sperm. However, the use of Testi might be the right choice for (I) abnormal SDF cases when the semen parameters are not suitable for PICSI or MACS techniques (in our study semen samples with at least 1 million total motile progressive count are considered suitable), (II) leukocytospermic semen samples >1 million, and (III) in case of PICSI or MACS unavailability since they are not registered in many countries yet. On the other hand, PICSI and MACS as selection techniques are combining the sperm morphology and motility with either selecting individual sperm with high DNA integrity according to the hyaluronan binding as in the case of PICSI or a population of non-apoptotic sperm resulting from MACS elution. Once our findings have been confirmed with larger prospective statistically powered studies, we would support the use of an ejaculated source combined with sperm selection technique (PICSI/MACS) rather than an invasive procedure of testicular source to obtain sperm with lower SDF.
The use of advanced sperm selection techniques for ejaculated sperm in ICSI is still debatable. PICSI was studied by Miller et al. in a large randomized controlled trial with 2772 couples in 16 fertility clinics. They found that PICSI had a significantly lower miscarriage rate than normal ICSI (4.3% vs 7.0%, p=0·003), but they did not find superiority from using PICSI than ICSI in terms of live birth rate (27.4% vs 25.2%, p=0.18) [25]. It is worth noting that despite Miller’s study included a high number of patients, the authors included unindicated cases with almost normal semen parameters in both groups: PICSI and ICSI. Furthermore, their study implied that SDF is expected to be normal since they scored more than 72% of hyaluronan binding scores in most of their cases where more than 65% is considered normal [54, 55]. So, and as it was commented by Repping, their results are not provided with the number or the quality of the embryos generated [56]. Parmegiani also commented on the same study explaining that it seems strange that the investigators discouraged the use of PICSI despite showing a significant reduction in the miscarriage rate, which was the same as our correspondence [57]. Miller replied that they are preparing an update by considering the level of SDF [58]. In support of our argument, Worrilow et al. and Parmegiani et al. recommended the use of PICSI dishes as they reported improvements in reproductive outcomes compared to ICSI, especially the implantation and miscarriage rates [55, 59]. On another front, Gil et al. in their meta-analysis have found that MACS showed significant improvement in pregnancy rate compared to DGC (45.4% vs 30.1%, p=0.004), with no significant differences regarding the implantation and miscarriage rates [60].
Comparing PICSI to MACS, Troya et al. reported higher clinical pregnancy rates using MACS compared to PICSI and ICSI (58.1%, 40.4%, and 27.3%, respectively, p =0.019), in addition to a significantly higher fertilization rate of the MACS group [43]. Our group had found no significant differences between PICSI and MACS in patients with abnormal SDF; however, the study reported significant improvement in good quality blastocysts, clinical and ongoing pregnancy rates after using MACS in females <30 years old [61], which may be explained by the oocyte contribution to a cascade of events during fertilization, including sperm chromatin decondensation and DNA repair [61, 62]. Our study agreed with the two previous studies, as PICSI and MACS groups showed improvement in clinical pregnancy, ongoing pregnancy, and implantation rates compared to DGC. In addition, PICSI has shown a significantly higher implantation rate than the MACS group. However, there were no significant differences between PICSI and MACS neither in all pre-implantation embryo development parameters nor in the pregnancy, ongoing, or the miscarriage rates.
To the best of our knowledge, this study is the first registered randomized controlled trial comparing four different sperm selection techniques in terms of embryological and clinical outcomes in ICSI patients with abnormal SDF. Our study is limited by the following: (1) it is relatively small sample size, and (2) it is not statistically powered. It would be better if the embryo grading was supported by the morphokinetics using time-lapse technology, the genetic content of the embryos generated from each selection technique was investigated through PGT-A, and the outcomes were extended to the live birth rate. This will be considered in our future research plans.
Conclusion
Sperm DNA fragmentation management helps in improving the clinical outcomes in ICSI patients. Application and mastering of different advanced sperm selection techniques help in that management when SDF is increased to the abnormal levels. Testicular sperm seems to be a good option and shows improved outcomes when compared to simple DGC in such cases. Using PICSI or MACS combined with DGC results in higher reproductive outcomes than Testi or sperm processed with DGC alone. This conclusion needs to be affirmed by multicentric statistically powered studies with larger sample size.
References
Cho CL, Agarwal A. Role of sperm DNA fragmentation in male factor infertility: a systematic review. Arab J Urol Arab Association of Urology; 2018;16:21–34. Available from: https://doi.org/10.1016/j.aju.2017.11.002
Wang C, Swerdloff RS. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. 2014;102:1502–7.
Panner Selvam MK, Agarwal A. A systematic review on sperm DNA fragmentation in male factor infertility: laboratory assessment. Arab J Urol Arab Association of Urology; 2018;16:65–76. Available from: https://doi.org/10.1016/j.aju.2017.12.001
Kim GY. What should be done for men with sperm DNA fragmentation? Clin Exp Reprod Med. 2018;45:101–9.
Baldi E. Genetic Damage in Human Spermatozoa [Internet]. 2014. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/978-1-4614-7783-9. Accessed 20 Dec 2019.
Kerkeni L, Ruano P, Delgado LL, Picco S, Villegas L, Tonelli F, et al. Sperm DNA fragmentation and its relation with fertility. Intech. 2016:13 Available from: https://www.intechopen.com/books/advanced-biometric-technologies/liveness-detection-in-biometrics.
Simon L, Zini A, Dyachenko A, Ciampi A, Carrell D. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J Androl. 2017;19:80–90.
Zini A, Editors AA. A clinician’s guide to sperm DNA and chromatin damage. A Clin Guid. 2018.
Colaco S, Sakkas D. Paternal factors contributing to embryo quality. J Assist Reprod Genet. 2018;35:1953–68.
Aydos OS, Yükselten Y, Kaplan F, Sunguroğlu A, Aydos K. Analysis of the correlation between sperm DNA integrity and conventional semen parameters in infertile men. Turk Urol Derg. 2015;41:191–7.
Minh Tam T. does sperm DNA fragmentation correlate with semen parameters? 2019; 390–396.
Cho CL, Agarwal A, Majzoub A, Esteves SC. Use of sperm DNA fragmentation testing and testicular sperm for intracytoplasmic sperm injection. Transl Androl Urol. 2017;6:S688–90.
Bungum M. Sperm DNA integrity assessment: a new tool in diagnosis and treatment of fertility. Obstet Gynecol Int. 2012;2012:1–6.
Rubes J, Selevan SG, Evenson DP, Zudova D, Vozdova M, Zudova Z, et al. Episodic air pollution is associated with increased DNA fragmentation in human sperm without other changes in semen quality. Hum Reprod. 2005;20:2776–83.
Avalos-Durán G, Del Ángel AMEC, Rivero-Murillo J, Zambrano-Guerrero JE, Carballo-Mondragón E, Checa-Vizcaíno MÁ. Physiological ICSI (PICSI) vs. conventional ICSI in couples with male factor: a systematic review. J Bras Reprod Assist. 2018;22:139–47.
Osman A, Alsomait H, Seshadri S, El-Toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod BioMed Online. Reproductive Healthcare Ltd.; 2015;30:120–127. Available from: https://doi.org/10.1016/j.rbmo.2014.10.018
Sedó CA, Bilinski M, Lorenzi D, Uriondo H, Noblía F, Longobucco V, et al. Effect of sperm DNA fragmentation on embryo development: clinical and biological aspects. J Bras Reprod Assist. 2017;21:343–50.
Majzoub A, Agarwal A, Esteves SC. Understanding sperm DNA fragmentation. Transl Androl Urol. 2017;6:S535–8.
Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. American Society for Reproductive Medicine; 2010;93:1027–1036. Available from: https://doi.org/10.1016/j.fertnstert.2009.10.046
Committees P, Society A, Technology R. Intracytoplasmic sperm injection (ICSI) for non–male factor indications: a committee opinion. Fertil Steril. American Society for Reproductive Medicine; 2020;114:239–245. Available from: https://doi.org/10.1016/j.fertnstert.2020.05.032
Sakkas D, Ramalingam M, Garrido N, Barratt CLR. Sperm selection in natural conception: what can we learn from Mother Nature to improve assisted reproduction outcomes? Hum Reprod Update. 2015;21:711–26.
Oseguera-López I, Ruiz-Díaz S, Ramos-Ibeas P, Pérez-Cerezales S. Novel techniques of sperm selection for improving IVF and ICSI outcomes. Front Cell Dev Biol. 2019;7.
Lewis SEM. The place of sperm DNA fragmentation testing in current day fertility management. Middle East Fertil Soc J; 2013;18:78–82. Available from: https://doi.org/10.1016/j.mefs.2013.01.010
Jayaraman V, Upadhya D, Narayan PK, Adiga SK. Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J Assist Reprod Genet. 2012;29:557–63.
Miller D, Pavitt S, Sharma V, Forbes G, Hooper R, Bhattacharya S, et al. Physiological, hyaluronan-selected intracytoplasmic sperm injection for infertility treatment (HABSelect): a parallel, two-group, randomised trial. Lancet 2019;393:416–422. Available from: https://doi.org/10.1016/S0140-6736(18)32989-1, 2019
Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril. 2008;89:823–31.
Parmegiani L. PICSI and Sperm slow, two ready-to-use systems designed for sperm-hyaluronic acid binding selection. Curr Trends Clin Embryol. 2017;32:310–5.
Hydro C, Summary E, Of F, Potential THE, Ferreres XR, Font AR, et al. Intracytoplasmic sperm injection – factors affecting fertilization. Intech. 2013;32:137–44 Available from: http://www.intechopen.com/books/trends-in-telecommunications-technologies/gps-total-electron-content-tec- prediction-at-ionosphere-layer-over-the-equatorial-region%0AInTec%0Ahttp://www.asociatiamhc.ro/wp-content/uploads/2013/11/Guide-to-Hydropower.pdf.
Cakar Z, Cetinkaya B, Aras D, Koca B, Ozkavukcu S, Kaplanoglu İ, et al. Does combining magnetic-activated cell sorting with density gradient or swim-up improve sperm selection? J Assist Reprod Genet. 2016;33:1059–65.
Agarwal A, Sharma R, Beydola T. Sperm preparation and selection techniques. Med Surg Manag Male Infertil. 2014:244–4.
Esteves SC, Roque M, Bradley CK, Garrido N. Reproductive outcomes of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with high levels of DNA fragmentation in semen: systematic review and meta-analysis. Fertil Steril. 2017;108:456–467.e1.
Xie P, Keating D, Parrella A, Cheung S, Rosenwaks Z, Goldstein M, et al. Sperm Genomic Integrity by TUNEL Varies throughout the Male Genital Tract. J Urol. 2020;203:802–8.
Bradley CK, McArthur SJ, Gee AJ, Weiss KA, Schmidt U, Toogood L. Intervention improves assisted conception intracytoplasmic sperm injection outcomes for patients with high levels of sperm DNA fragmentation: a retrospective analysis. Andrology. 2016;4:903–10.
Greco E, Scarselli F, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod. 2005;20:226–30.
Agarwal A, Gupta S, Du Plessis S, Sharma R, Esteves SC, Cirenza C, et al. Abstinence time and its impact on basic and advanced semen parameters. Urology. Elsevier Inc.; 2016;94:102–110. Available from: https://doi.org/10.1016/j.urology.2016.03.059
Comar VA, Petersen CG, Mauri AL, Mattila M, Vagnini LD, Renzi A, et al. Influence of the abstinence period on human sperm quality: analysis of 2,458 semen samples. J Bras Reprod Assist. 2017;21:306–12.
Hassanen E, Elqusi K, Zaki H, Henkel R, Agarwal A. TUNEL assay: establishing a sperm DNA fragmentation cut-off value for Egyptian infertile men. Andrologia. 2019;51:e13375.
Sergerie M, Laforest G, Boulanger K, Bissonnette F, Bleau G. Longitudinal study of sperm DNA fragmentation as measured by terminal uridine nick end-labelling assay. Hum Reprod. 2005;20:1921–7.
Ribeiro S, Sharma R, Gupta S, Cakar Z, De Geyter C, Agarwal A. Inter- and intra-laboratory standardization of TUNEL assay for assessment of sperm DNA fragmentation. Andrology. 2017;5:477–85.
World Health Organization. WHO laboratory manual for the Examination and processing of human semen. Eng. 2010;238:42.
Schoolcraft WB, Gardner DK, Lane M, Schlenker T, Hamilton F, Meldrum DR. Blastocyst culture and transfer: analysis of results and parameters affecting outcome in two in vitro fertilization programs. Fertil Steril. 1999;72:604–9.
Anshina MB, Dolgushina NV, Koloda YUА, Korsak VS, Savina VM, Smirnova. The international glossary on infertility and fertility care. Russ J Hum Reprod. 2019;25:6–15.
Troya J, Zorrilla I. Annexin V-MACS in infertile couples as method for separation of sperm without DNA fragmentation. JBRA Assist Reprod. 2015;19:66–9. https://doi.org/10.5935/1518-0557.20150015.
Chi H, Kwak S, Kim S, Kim Y, Park J, Yoo C, et al. Efficient isolation of sperm with high DNA integrity and stable chromatin packaging by a combination of density-gradient centrifugation and magnetic- activated cell sorting. Clin Exp Reprod Med. 2016;43:199–206. https://doi.org/10.5653/cerm.2016.43.4.199.
Degheidy T, Abdelfattah H, Seif A, Albuz FK, Gazi S, Abbas S. Magnetic activated cell sorting : an effective method for reduction of sperm DNA fragmentation in varicocele men prior to assisted reproductive techniques. 2015;892–896.
Parmegiani L, Sc B, Cognigni GE, Bernardi S, Sc B, Troilo E, et al. ‘“ Physiologic ICSI ”’: hyaluronic acid ( HA ) favors selection of spermatozoa without DNA fragmentation and with normal nucleus , resulting in improvement of embryo quality. Fertil Steril. Elsevier Ltd; 2010;93:598–604. Available from: https://doi.org/10.1016/j.fertnstert.2009.03.033
Ricci G, Perticarari S, Boscolo R, Montico M, Guaschino S, Presani G. Semen preparation methods and sperm apoptosis: swim-up versus gradient-density centrifugation technique. Fertil Steril American Society for Reproductive Medicine; 2009;91:632–638. Available from: https://doi.org/10.1016/j.fertnstert.2007.11.068
De Martin H, Miranda EP, Cocuzza MS, Monteleone PAA. Density gradient centrifugation and swim-up for ICSI: useful, unsafe, or just unsuitable? J Assist Reprod Genet. 2019:10–2.
Sullivan R, Mieusset R. The human epididymis: its function in sperm maturation. Hum Reprod Update. 2016;22:574–87.
Belleannée C, Calvo E, Thimon V, Cyr DG, Légaré C, Garneau L, et al. Role of microRNAs in controlling gene expression in different segments of the human epididymis. PLoS One. 2012;7:e34996.
Mehta A, Esteves SC, Schlegel PN, Niederberger CI, Sigman M, Zini A, et al. Use of testicular sperm in nonazoospermic males. Fertil Steril American Society for Reproductive Medicine; 2018;109:981–987. Available from: https://doi.org/10.1016/j.fertnstert.2018.04.029
Moskovtsev SI, Alladin N, Lo KC, Jarvi K, Mullen JBM, Librach CL. A comparison of ejaculated and testicular spermatozoa aneuploidy rates in patients with high sperm DNA damage. Syst Biol Reprod Med. 2012;58:142–8.
Cheung S, Schlegel PN, Rosenwaks Z, Palermo GD. Revisiting aneuploidy profile of surgically retrieved spermatozoa by whole exome sequencing molecular karyotype. PLoS One. 2019;14:1–15.
Worrilow KC, Huynh HT, Bower JB, Anderson AR, Schillings W, Crain JL. PICSITM vs. ICSI: statistically significant improvement in clinical outcomes in 240 in vitro fertilization (IVF) patients. Fertil Steril. 2007;88:S37.
Worrilow KC, Eid S, Woodhouse D, Perloe M, Smith S, Witmyer J, et al. Use of hyaluronan in the selection of sperm for intracytoplasmic sperm injection (ICSI): significant improvement in clinical outcomes-multicenter, double-blinded and randomized controlled trial. Hum Reprod. 2013;28:306–14.
Repping S. Evidence-based medicine and infertility treatment. Lancet. 2019;393:380–2.
Parmegiani L. Hyaluronan-selected sperm should not be considered an add-on In Lancet 2019;394:1319:1320. Available from: https://doi.org/10.1016/S0140-6736(19)31340-6
Miller D. Hyaluronan-selected sperm should not be considered an add-on In – Authors’ reply. Lancet. 2019;394:1321.
Parmegiani L, Cognigni GE, Ciampaglia W, Pocognoli P, Marchi F, Filicori M. Efficiency of hyaluronic acid (HA) sperm selection. J Assist Reprod Genet. 2010;27:13–6.
Gil M, Sar-Shalom V, Melendez Sivira Y, Carreras R, Checa MA. Sperm selection using magnetic activated cell sorting (MACS) in assisted reproduction: a systematic review and meta-analysis. J Assist Reprod Genet. 2013;30:479–85.
Hasanen E, Elqusi K, Eltanbouly S, Hussin AE, Alkhadr H, Zaki H. PICSI vs . MACS for abnormal sperm DNA fragmentation ICSI cases : a prospective randomized trial. J Assist Reprod Genet 37, 2605–2613 (2020). https://doi.org/10.1007/s10815-020-01913-4.
Gou LT, Lim DH, Ma W, Aubol BE, Hao Y, Wang X, et al. Initiation of parental genome reprogramming in fertilized oocyte by splicing kinase SRPK1-catalyzed protamine phosphorylation. Cell. Elsevier; 2020;180:1212-1227.e14. Available from: https://doi.org/10.1016/j.cell.2020.02.020
Availability of Data and Material
Data are available upon request
Code Availability
Not applicable.
Author information
Authors and Affiliations
Contributions
Manar Hozyen: study design, contributed to laboratory work, analysis and interpretation of results, and manuscript preparation
Eman Hasanen: contributed to patient recruitment and data collection and helped in manuscript preparation
Khaled Elqusi: contributed to patient recruitment and data collection
Salma ElTanbouly: contributed to patient recruitment and data collection
Samar Gamal: contributed to patient recruitment and data collection
Abdul Ghafar Hussin: contributed to laboratory work and data collection
Hanaa Alkhader: contributed to laboratory work and data collection
Hosam Zaki: approved the study design, analysis, and interpretation of results; critically reviewed the manuscript; and gave the final approval for submission.
Corresponding author
Ethics declarations
Ethics Approval
This study was reviewed, discussed, and approved by Ganin Fertility Center ethics committee in February 2, 2017, in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standard. The committee approved the study before starting it under the identifier number, GFC-130720, and assured that the research plans are reasonable and participants are adequately protected.
Consent to Participate
Informed consents were obtained from all individual participants included in the study before their inclusion.
Consent for Publication
Patients signed informed consent regarding publishing their data.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hozyen, M., Hasanen, E., Elqusi, K. et al. Reproductive Outcomes of Different Sperm Selection Techniques for ICSI Patients with Abnormal Sperm DNA Fragmentation: a Randomized Controlled Trial. Reprod. Sci. 29, 220–228 (2022). https://doi.org/10.1007/s43032-021-00642-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00642-y