Abstract
Purpose
The DNA fragmentation in sperm is associated with reduced outcome in assisted reproduction. Using YoPro1 as the staining dye and flow cytometry and sorting (FACS), the number of spermatozoa with DNA fragmentation can be lowered to 5%. Can the cumulative outcome of ICSI be improved using FACS?
Methods
A prospective, randomized, double-blind clinical trial was conducted in 104 infertile couples with male infertility based on abnormal conventional semen analysis results. Cumulative ongoing pregnancy rate was the primary outcome parameter. In 52 cases, semen was processed for ICSI using swim-up. In another 52 cases, spermatozoa with fragmented DNA were removed with FACS.
Results
The cumulative pregnancy rate at 12 weeks of gestation (51.9% versus 46.2%) and live birth rate (42.3% versus 34.6%) were higher and the miscarriage rate was lower (27.8% versus 35.3%) after FACS-sorting as compared with swim-up. An interim analysis scheduled before initiation of the study after 100 cases demonstrated that the aim of a 20% gain in pregnancy rate could not be achieved. For that reason, the prospective study was stopped prematurely.
Conclusions
A trend towards consistently better results was achieved by removing spermatozoa with fragmented DNA. The fragmentation of the DNA in sperm is the end stage of apoptosis. Sorting of spermatozoa may be improved by selecting parameters of processes active more upstream of apoptosis, such as chromatin decondensation.
Trial registration
NCT02166567. June 14, 2014.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Semen analysis is essential for the diagnosis of infertility and for deciding about the most appropriate treatment. Although conventional semen analysis has been shown to be of low prognostic accuracy [1] with a very broad confidence interval [2], it is still the predominantly used diagnostic method in evaluating male fertility. The results of conventional semen analysis often influence the decision if an assisted reproduction technology (ART) should be performed and have an impact on the choice of IVF and ICSI. The lack of prognostic accuracy of conventional semen analysis might be one of the reasons why ICSI is used more often than IVF [3].
Sperm function might impact on the outcome of ART beyond fertilization and fragmentation of the genomic material of the sperm has moved into the focus of science in recent years [4]. Before and after ejaculation, spermatozoa encounter the risk of apoptotic or necrotic cell death. The former process involves initial fragmentation of the DNA mediated by enzymes like caspases and nucleases and the release of reactive oxygen species (ROS) from the mitochondria followed by increased permeability of the cellular membrane and progressive fragmentation of the genetic material. Based on indirect evidence, mainly cohort studies, enhanced fragmentation levels of the DNA in spermatozoa are associated with reduced outcomes in infertility treatments (see recent reviews [5, 6]) and with a higher incidence of miscarriage [6,7,8]. Several DNA fragmentation diagnostic systems have been proposed, including the single-cell gel electrophoresis assay (COMET), sperm chromatin structure assay (SCSA), acridine orange test (AOT), terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay, and sperm chromatin dispersion test (SCD). As none of these test systems has undergone thorough validation processes in sufficiently large patient cohorts, formal acceptance of any of these test systems by the World Health Organization or any other overarching regulatory body is still pending.
At present, most evidence on the involvement of the fragmentation of sperm DNA derives from epidemiological studies comparing the number of spermatozoa with damaged DNA in infertile men with those of healthy controls. Higher numbers of spermatozoa with damaged DNA were shown in populations of men with health hazards, such as intensive cigarette smoking [9], obesity [10], and diabetes mellitus [11]. All findings provide indirect evidence for a connection between the extent of DNA-damaged spermatozoa and male infertility. In order to elucidate the real impact of DNA fragmentation in a human sperm well-designed prospective studies are required, comparing the outcome of ART performed with normal sperm or with sperm containing elevated levels of fragmented DNA. Various sorting systems have been proposed to separate DNA-damaged spermatozoa from healthy ones in ART. These include the separation of pre-apoptotic spermatozoa using Annexin V-coated magnetic beads [12] or binding to hyaluronic acid [13]. Despite initial enthusiasm, neither method has produced significant benefit for the outcome of ART [14, 15].
We established a method for sorting of DNA-intact spermatozoa through excluding membrane-damaged spermatozoa by staining with the dye YoPro1 in combination with flow cytometry and cell sorting (FACS) [16]. We could demonstrate that with this accurate technology TUNEL-positive spermatozoa can be excluded reliably in a way that their abundance was lowered to less than 5%. We now present the results of a randomized clinical trial in which the results of the conventional swim-up method were compared with the novel sorting technique based on YoPro1 staining [16].
Material and methods
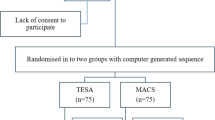
A prospective randomized clinical trial was initiated to demonstrate the superiority of deselecting spermatozoa with fragmented DNA using FACS and YoPro1 in comparison to conventional sperm preparation using the swim-up technology [17] in a cohort of infertile couples treated with ICSI. The study was double-blinded: neither the treating physicians nor the treated couple was informed about the method of sperm preparation. Allocation to either treatment was based on two computer-generated lists (stratified in two groups of 100 cases each). The study was presented to and approved by the local ethics committee (EKBB178/12) and monitored by the clinical trial unit (CTU) of the University Hospital of Basel.
The primary outcome parameter was the cumulative ongoing pregnancy rate (at 12 weeks of gestation). Secondary outcome parameters were the number of diploidic fertilized oocytes, the number of cryopreserved zygotes, the number of blastocyst embryos, the implantation rate, the number of miscarriages, the live birth rate, and the quality of life assessment of the toddlers. This study was entirely performed in one single university-based institution in Switzerland.
The study was designed to demonstrate that the ongoing pregnancy rate (at week 12 of pregnancy) after ICSI can be improved significantly by sorting out spermatozoa with fragmented DNA using the YoPro1 dye from the sperm sample used for ICSI. The comparator was conventional swim-up [17].
Inclusion criteria
The inclusion criteria were couples diagnosed with male infertility as given by at least one abnormal parameter assessed by conventional semen analysis (concentration, progressive motility, morphology) [2]. The diagnostic accuracy of the spermatology laboratory is verified by both an internal (ISO 17025) and an external quality control system (QuaDeGa). The level of DNA fragmentation was not measured prior to recruitment and was not a selection criterion for recruitment to the study. The age of the female partners was 21 to 40 years, normal ovarian reserve was determined in a preceding menstrual cycle with normal range basal FSH concentration < 10 IU/l and AMH concentration > 10 pmol/l. The inclusion criteria also stipulated normal uterine morphology without fibroids or endometrial abnormalities, as given by vaginal ultrasound.
Exclusion criteria
Infertile men with azoospermia or cryptozoospermia were excluded from participation. Other exclusion criteria were two preceding unsuccessful treatments with ICSI, simultaneous participation to another clinical study, and chronic infection with HIV and hepatitis B and C.
Management of the infertile couples before and after treatment with ICSI
All infertile couples underwent a routine evaluation of their fertility status within 12 months before their participation to the study. All female partners were examined during early follicular phase to determine ovarian reserve and just prior to ovulation to evaluate uterine morphology. The male partners underwent a physical examination and semen analysis together with a hormonal assessment. After signing an informed consent, ovarian hyperstimulation was performed with gonadotropins using the GnRH-antagonist protocol. Ovulation was uniformly induced with human chorionic gonadotropin and oocytes were collected 37 h after ovulation induction. At the time of the study, restrictive Swiss legislation stipulated that not more than three zygotes can be cultured up to the blastocyst stage and that all blastocysts (not more than three) must be transferred back to the uterus [18]. If more than three zygotes arose after ICSI, they were cryopreserved through vitrification. All transfers of embryos in the blastocyst stage were carried out under transabdominal ultrasound guidance. All pregnancies were diagnosed in the institution and followed up to the 12th week of gestation. Thereafter, they were referred back to their local gynecologist or obstetrician. A written report of the delivery and of the health status of the newborns was obtained in all cases.
For the thawing of cryopreserved zygotes and subsequent transfer at blastocyst stage, the patients were sequentially treated with estradiol valerate and vaginally administered micronized progesterone. In addition, patients were supplemented prior to fresh and frozen transfer with folic acid. The follow-up was identical to the fresh cycles.
Sperm preparation for ICSI
Semen samples were collected in 110 ml sterile plastic containers (BD Falcon, Franklin Lakes, NJ, USA) and allowed to liquefy at 37 °C for 30 min. In ART, semen may be prepared either with density gradient centrifugation or with swim-up. We selected the swim-up method, because swim-up is used as a first wash in FACS thereby allowing direct comparison of swim-up alone with additional sorting with FACS. For the swim-up procedure 1 or 2 ml of the freshly collected ejaculate were washed twice with culture medium and centrifuged for 10 min at 460g. The supernatant was discarded and the spermatozoa pellet covered with the culture medium. The sample was incubated for 1 hour (37 °C, 5% CO2) for the viable spermatozoa to migrate into the overlaid culture medium. The supernatant (0.5 ml) was then carefully separated and both the concentration and motility of the swim-up spermatozoa were determined.
The details and validation of the sorting with FACS are described elsewhere [16]. In brief, YoPro1 (Molecular Probes, Eugene, OR, USA) was diluted 1:10 in sterile water and kept at 4 °C until used. One μl of the diluted YoPro1-solution (final concentration 0.2 μM) was added together with 1 μl/ml of the Hoechst dye (Hoechst 33342, BD Biosciences, San Jose, CA, USA) to an aliquot containing spermatozoa prepared with swim-up. The Hoechst dye was used to exclude debris. During staining, the samples were kept for 15 min in the incubator at 37 °C, 5% CO2 or at room temperature in the dark. The stained sample was then analyzed by FACS using a BD Influx flow cytometer (BD Biosciences) equipped with a flow hood. The FACS equipment was installed in the spermatology laboratory and used for the sorting of human spermatozoa only. Prior to each sorting, the lasers were aligned using calibration beads provided by BD (8 peak beads). Stained spermatozoa were separated in the FACS device with a solid-state laser (100 mW) for excitation at 355 nm, a blue laser (200 mW) for excitation at 488 nm, and a red laser (120 mW) for excitation at 640 nm. The analyses were performed with acquisition software (BD Biosciences). For acquisition, the threshold was set using a forward scatter trigger to eliminate debris and noise. The following settings were used for sorting 100 μm nozzle, sample pressure 19.5–20.5 psi, and event rate 1000–9000 events/s. Sorting was continued until 60,000 to 200,000 spermatozoa negative for YoPro1 but positive for the Hoechst dye were collected thereby not exceeding the total sorting volume of 0.5 ml. The degree of DNA fragmentation in the sorted sample was not measured.
Infant and toddler quality of life questionnaire
The study protocol included an investigation into the outcome of the children at the age of 1 to 2 years, which were born after pregnancies achieved during the fresh cycle. To that purpose, all parents were contacted by regular mail and they were given a validated questionnaire (ITQOL-97), using either the German or the French version [19]. This questionnaire assesses the quality of life in infants and toddlers from 2 months to 5 years of age.
Case numbers
The statistical power calculation of the study was based on a higher proportion of blastocyst embryos arising from ICSI and from the culture of not more than three zygotes per treatment cycles. Previous experience has demonstrated that the number of spermatozoa with fragmented DNA can be reduced from 25 to 5% with FACS using the YoPro1 dye [16]. We calculated a subsequent increase of the blastocyst rate from 45 to 65% in the sorting group, allowing for an estimated 20% rise of the cumulative ongoing pregnancy rate. Based on this study objective, we calculated that 200 treatments with ICSI would be required to demonstrate a statistically significant benefit of FACS compared with conventional swim-up with an alpha error rate of 5% and an accuracy of > 80%. An interim analysis was scheduled to take place after the recruitment of the first 100 treatments to evaluate whether the study objective (e.g., + 20% blastocyst rate) could be reached.
Statistical analysis
We performed a Kolmogorov-Smirnov (KS) goodness-of-fit analysis of the clinical characteristics of both experimental groups. All statistical analyses were carried out using the Chi-squared test (χ2) and the Mann-Whitney U test, as appropriate, and the results are given by the mean values either together with the standard deviation (SD) or with the 95% confidence intervals. The level of statistical significance was set at the 5% level.
Results
A total of 111 infertile couples were recruited to participate in the study (Fig. 1), but 7 were excluded later because they did not completely fit all inclusion criteria. Ultimately, in 52 cases, semen was prepared with swim-up, and in another 52 cases, semen was sorted with FACS. In the sorted group, 36 couples were diagnosed with primary infertility (69.2%) and 39 in the swim-up group (75.0%, p = 0.231). Before deciding for ICSI, 17 of the couples treated with FACS-sorting had already undergone one or more intrauterine inseminations (32.7%), whereas in the swim-up group 13 had previously undergone intrauterine inseminations (25.0%, p = 0.454). The clinical characteristics of both groups are summarized in Table 1: the BMI of the female partners was somewhat lower in the FACS-sorted group (p = 0.038), as was the testosterone concentration in the male partner (p = 0.019).
In the sorted group, the mean percentage of YoPro1-positive spermatozoa discarded after sorting was 55% (ranging between 29 and 85%). The number of collected oocytes, the number of fertilized oocytes, and the number of embryos, both in the freshly stimulated treatments and in the thawing cycles, are listed in Table 2 and in Fig. 2. In the freshly stimulated treatments, neither the number of oocytes collected nor the number of zygotes after ICSI were significantly different in both groups. Both in the freshly stimulated treatments and in the thawing cycles, more blastocysts developed from similar numbers of treated oocytes or zygotes after sorting with FACS, but none of the differences reached statistical significance.
The absolute numbers and percentages of oocytes, zygotes, and embryos obtained after ICSI are given, both after sorting with FACS and after conventional swim-up. During embryo culture, the percentages indicate the number of embryos resulting from cultured zygotes. All listed blastocyst embryos were transferred. None of the observed differences were statistically significant (chi-squared analysis)
The outcome after embryo transfer in both treatment groups is summarized in Table 3 and Fig. 1. Although, after FACS-sorting, more pregnancies were observed, more children were born, and fewer miscarriages occurred than after swim-up; the differences between both groups were statistically not significant. In addition, the cumulative outcome results were similar in both groups.
Adverse events during pregnancy and delivery
One case with ovarian hyperstimulation syndrome was observed in the swim-up group. Four pregnancies in the FACS-sorting group ended prematurely: two twin pregnancies in the 37th week of gestation and one in the swim-up group (one twin pregnancy in the 36th week of gestation). One pregnancy in the swim-up group ended as a late miscarriage in the 20th week of gestation due to severe pre-eclampsia and placental insufficiency.
Children’s outcome
Two twins in the FACS-sorting group were diagnosed with cleft lip at birth (one on one side, one bilateral). Another child born at term in the FACS-sorting group was treated for respiratory distress syndrome. Nine of 13 women responded the ITQOL-97-questionnaire in the FACS-sorting group (69.2%), as did nine of 12 women in the swim-up group (75.0%). Two children were reported to suffer of some allergy in the FACS-sorting group (“nuts,” “proteins”). One parent in the swim-up group reported an earlier severe health problem and uncertainties about future health of its child, without providing further details.
Discussion
We sought to improve the outcome of ART by removing spermatozoa with fragmented DNA from the sample used in ICSI. Higher numbers of spermatozoa with fragmented DNA have been related to lower pregnancy and live birth rates in ART [5, 6] and to a higher incidence of miscarriages [6,7,8]. A validation study has demonstrated that by using FACS technology in combination with YoPro1 as a staining dye the number of DNA-fragmented spermatozoa in the sample can consistently be reduced to less than 5%, as given by TUNEL [16]. We designed a single-center, prospective, randomized, double-blind clinical trial to demonstrate the superiority using sorted spermatozoa with intact DNA in improving the cumulative pregnancy rate after ICSI. We considered an improvement of the ongoing pregnancy rate of 20% to be necessary to justify the use of the sorting equipment. The interim analysis after 104 cases demonstrated a higher cumulative ongoing pregnancy rate (+ 5.7%) and a higher cumulative live birth rate (+ 7.7%) with the sorting technology. The differences in outcome were more pronounced at the embryo growth level. There were more embryos growing on day 3 after thawing of zygotes in the sorted group (+ 15.3%) than in the swim-up group resulting in a higher pregnancy rate (+ 6.1%), but these differences were statistically not significant. As the interim goal set at the onset of the study was not reached, we decided not to continue the trial.
Despite the tremendous opportunities given by flow cytometry, FACS has been used clinically for sex sorting only [20] and has not yet been used in prospective trials in ART so far. To assess the safety and the impact of the FACS technology on the offspring, the developmental outcome of the children was evaluated as a part of the study. Two dizygotic twins were born with cleft palate after sorting with FACS. Whereas cleft palate occurs more commonly among relatives, we cannot completely exclude that this malformation might be related to the sorting procedure. Approximately 2 years after birth of the children, we sent an infant-toddler quality of life questionnaire to the families, which did not reveal any major abnormalities or differences between both treatment groups.
Although the outcome of the present study does not yet justify the use of the sophisticated and costly FACS equipment in clinical ART, the results of this prospective clinical trial provide valuable information about the impact of DNA fragmentation on sperm functionality. Quantification of the degree of DNA fragmentation in spermatozoa for diagnostic purposes in male infertility is based on the concept that good sperm is ejaculated with intact genetic material and that their genetic material is progressively damaged through the detrimental action of both aging and toxicants, consisting of ROS, leukocytes, or both [21]. However, recent studies could show that the condition of spermatozoa within the ejaculate is much more complex and variable. Double staining with both TUNEL and the nuclear stain propidium iodide (PI) did reveal at least two populations of spermatozoa with fragmented DNA. The first population consists of a staining dimer containing dead sperm with fragmented DNA, thought to arise from testicular pathology, while the second PI-stained population is supposed to contain dead and living spermatozoa with fragmented DNA—which inversely correlates with fertility [22]. These findings indicate that damage to the genome of spermatozoa in the semen may result from events occurring both during spermatogenesis and thereafter. An even more complex picture of various subgroups of damaged spermatozoa was proposed by studies on the steric hindrance of differently labeled antibodies used in TUNEL to the more or less condensed DNA of spermatozoa in the seminal plasma [23]. The results of the study demonstrated that accessibility of the DNA to the labeling agent was influenced by the moment at which apoptosis occurred during sperm maturation. When apoptosis occurs at an early stage, i.e., during testicular transit, the DNA is less condensed and more accessible to staining agents. When damage occurred after ejaculation, the chromatin of the sperm already had become more condensed [23].
During maturation of human sperm, over 85% of histones are replaced by protamines leading to an enormous compaction of the sperm nucleus. Whereas most of compaction occurs during the final stages of testicular spermatogenesis, some compaction is still ongoing during the epididymal transit. Some parts of the genome remain decondensed: the remaining 15% of nucleosomes predominantly localizes at genomic regions characterized by the presence of multiple CpG dinucleotides that lack methylation, constituting the so-called CpG islands (CGIs) that are frequently located in promoters of mammalian genes needed for embryonic development [24,25,26]. The distribution of histones and protamines in the nuclei of spermatozoa of infertile men has been demonstrated to be different from that of fertile men [27, 28]. Decondensed chromatin renders the genomic material of immature spermatozoa more vulnerable to damaging agents, such as oxidative radicals [21, 29]. Multicolor FC has demonstrated that fragmentation of the DNA in spermatozoa is directly associated with chromatin decondensation [30].
In conclusion, the nearly complete removal of the DNA-damaged spermatozoa based on enhanced permeability of their membranes was insufficient to significantly improve treatment results in ART. Current data suggest that DNA damage is the result of a detrimental process, which is initiated much more upstream during the substitution of histone by protamine needed for chromatin condensation. Chromatin decondensation, as evidenced by higher nuclear histone content and visualized with the CMA3-fluorochrome, is associated with male subfertility [31] and with a higher incidence of miscarriage [32]. The trend towards better results achieved with the removal of DNA-fragmented spermatozoa presented here is encouraging. Future developments in the diagnostics of sperm dysfunction should focus less on the endpoint of apoptosis, i.e., DNA fragmentation and membrane permeability, but rather on events occurring upstream, such as chromatin remodeling and histone replacement.
References
Bonde JP, Ernst E, Jensen TK, Hjollund NH, Kolstad H, Henriksen TB, et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet. 1998;352:1172–7.
Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45.
De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2014: results generated from European registries by ESHRE: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod. 2018;33:1586–601.
Muratori M, De Geyter C. Chromatin condensation, fragmentation of DNA and differences in the epigenetic signature of infertile men. Best Pract Res Clin Endocrinol Metab. 2018;33(1):117–26.
Osman A, Alsomait H, Seshadri S, El-Toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod BioMed Online. 2015;30:120–7.
Simon L, Emery BR, Carrell DT. Review: diagnosis and impact of sperm DNA alterations in assisted reproduction. Best Pract Res Clin Obstet Gynaecol. 2017;44:38–56.
Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod. 2008;23:2663–8.
Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27:2908–17.
Taha EA, Ez-Aldin AM, Sayed SK, Ghandour NM, Mostafa T. Effect of smoking on sperm vitality, DNA integrity, seminal oxidative stress, zinc in fertile men. Urology. 2012;80:822–5.
Dupont C, Faure C, Sermondade N, Boubaya M, Eustache F, Clément P, et al. Obesity leads to higher risk of sperm DNA damage in infertile patients. Asian J Androl. 2013;15:622–5.
Roessner C, Paasch U, Kratzsch J, Glander HJ, Grunewald S. Sperm apoptosis signalling in diabetic men. Reprod BioMed Online. 2012;25:292–9.
Said TM, Agarwal A, Zborowski M. Utility of magnetic cell separation as a molecular sperm preparation technique. J Androl. 2008;29:134–42.
Ye H, Huang GN, Gao Y, Liu DY. Relationship between human sperm-hyaluronan binding assay and fertilization rate in conventional in vitro fertilization. Hum Reprod. 2006;21:1545–50.
Gil M, Sar-Shalom V, Melendez Sivira Y, Carreras R, Checa MA. Sperm selection using magnetic activated cell sorting (MACS) in assisted reproduction: a systematic review and meta-analysis. J Assist Reprod Genet. 2013;30:479–85.
McDowell S, Kroon B, Ford E, Hook Y, Glujovsky D, Yazdani A. Advanced sperm selection techniques for assisted reproduction. Cochrane Database Syst Rev. 2014;CD010461.
Ribeiro SC, Sartorius G, Pletscher F, de Geyter M, Zhang H, de Geyter C. Isolation of spermatozoa with low levels of fragmented DNA with the use of flow cytometry and sorting. Fertil Steril. 2013;100:686–94.
De Geyter C, Bals-Pratsch M, Doeren M, Yeung CH, Grunert JH, Bordt J, et al. Human and bovine cervical mucus penetration as a test of sperm function for in-vitro fertilization. Hum Reprod. 1988;3:948–54.
De Geyter C. Assisted reproductive medicine in Switzerland. Swiss Med Wkly. 2012;142:w13569.
Raat H, Landgraf JM, Oostenbrink R, Moll HA, Essink-Bot ML. Reliability and validity of the Infant and Toddler Quality of Life Questionnaire (ITQOL) in a general population and respiratory disease sample. Qual Life Res. 2007;16:445–60.
De Geyter C, Sterthaus O, Miny P, Wenzel F, Lapaire O, De Geyter M, et al. First successful pregnancy in Switzerland after prospective sex determination of the embryo through the separation of X-chromosome bearing spermatozoa. Swiss Med Wkly. 2013;143:w13718.
Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 2010;16:3–13.
Muratori M, Marchiani S, Tamburrino L, Cambi M, Lotti F, Natali I, et al. DNA fragmentation in brighter sperm predicts male fertility independently from age and semen parameters. Fertil Steril. 2015;104:582–90.
Ribeiro SC, Muratori M, De Geyter M, De Geyter C. TUNEL labeling with BrdUTP/anti-BrdUTP greatly underestimates the level of sperm DNA fragmentation in semen evaluation. PLoS One. 2017;12:e0181802.
Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–8.
Brykczynska U, Hisano M, Erkek S, Ramos L, Oakeley EJ, Roloff TC, et al. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biol. 2010;17:679–87.
Erkek S, Hisano M, Liang C-Y, Gill M, Murr R, Dieker J, et al. Molecular determinants of nucleosome retention at CpG-rich sequences in mouse spermatozoa. Nat Struct Mol Biol. 2013;20:868–75.
Hammoud SS, Nix DA, Hammoud AO, Gibson M, Cairns BR, Carrell DT. Genome-wide analysis identifies changes in histone retention and epigenetic modifications at developmental and imprinted gene loci in the sperm of infertile men. Hum Reprod. 2011;26:2558–69.
Azpiazu R, Amaral A, Castillo J, Estanyo JM, Guimera’ M, Ballesca’ JL, et al. High-throughput sperm differential proteomics suggests that epigenetic alterations contribute to failed assisted reproduction. Hum Reprod. 2014;29:1225–37.
Ni K, Spiess AN, Schuppe HC, Steger K. The impact of sperm protamine deficiency and sperm DNA damage on human male fertility: a systematic review and meta-analysis. Andrology. 2016;4:789–99.
Muratori M, Tamburrino L, Marchiani S, Cambi M, Olivito B, Azzari C, et al. Investigation on the origin of sperm DNA fragmentation: role of apoptosis. Immaturity and Oxidative Stress Mol Med. 2015;21:109–22.
Manochantr S, Chiamchanya C, Sobhon P. Relationship between chromatin condensation, DNA integrity and quality of ejaculated spermatozoa from infertile men. Andrologia. 2012;44:187–99.
Sakkas D, Urner F, Bizzaro D, Manicardi G, Bianchi PG, Shoukir Y, et al. Sperm nuclear DNA damage and altered chromatin structure: effect on fertilization and embryo development. Hum Reprod. 1998;13(Suppl 4):11–9.
Acknowledgments
We are grateful to the work of the monitoring group of the Clinical Trial Unit of the Basler University Hospital (www.dkf.unibas.ch) and to Ms. Hanna Flükiger for coordinating all activities related to this study.
Funding
This study was funded by the Repronatal Foundation, Basel, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was presented to and approved by the local ethics committee (EKBB178/12) and monitored by the clinical trial unit (CTU) of the University Hospital of Basel.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Geyter, C., Gobrecht-Keller, U., Ahler, A. et al. Removal of DNA-fragmented spermatozoa using flow cytometry and sorting does not improve the outcome of intracytoplasmic sperm injection. J Assist Reprod Genet 36, 2079–2086 (2019). https://doi.org/10.1007/s10815-019-01571-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01571-1