Abstract
Ovarian age is classically considered the main cause of female reproductive infertility. In women, the process proceeds as an ongoing decline in the primordial follicle stockpile and it is associated with reduced fertility in the mid-thirties, irregular menstruation from the mid-forties, cessation of fertility, and, eventually, menopause in the early fifties. Reproductive aging is historically associated with changes in oocyte quantity and quality. However, besides the oocyte, other cellular as well as environmental factors have been the focus of more recent investigations suggesting that ovarian decay is a complex and multifaceted process. Among these factors, we will consider mitochondria and oxidative stress as related to nutrition, changes in extracellular matrix molecules, and the associated ovarian stromal compartment where immune cells of both the native and adaptive systems seem to play an important role. Understanding such processes is crucial to design treatment strategies to slow down ovarian aging and consequently prolong reproductive lifespan and, more to this, alleviaingt side effects of menopause on the musculoskeletal, cardiovascular, and nervous systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well established that neuroendocrine factors and processes such as implantation, placentation, and delivery may reduce female reproductive performance with age; however, the close temporal relationship between the loss of female reproductive potential and functional ovarian decline identifies the main regulator of reproductive aging as the ovary.
Although in the last 150 years much has been understood on the two main functions of the mammalian ovary, i.e., the formation of fertilizable oocytes capable to develop into an embryo and the production of hormones regulating various biological processes, the ovary has retained its role as a mysterious organ in many respects. Among these, ovarian aging has been the subject of scientific inquiry for decades. The failure of this organ represents one of the earliest phenomena characterizing natural female aging and raises intriguing questions regarding the relationship between reproductive and organismal aging.
Many studies established that ovarian functional decline is related to the gradual loss of resting follicles and reduced ability to produce oocytes competent for fertilization and embryo development. It is likely that ovary aging depends on multiple intraovarian and extraovarian factors whose respective contribution have not been, however, fully characterized. Reproductive aging is also associated with changes in oocyte quality, namely, a marked increase in the incidence of aneuploidy up to 60%, miscarriages, and birth defects [1].
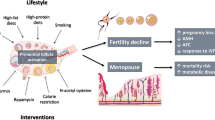
In the present work, we will first review briefly some of these factors and related molecular mechanisms including follicular dynamics, granulosa cell, and oocyte apoptosis, genetic and epigenetic factors, and then we will focus on specific aspects that have been studied in most recent years as mitochondria, oxidative stress, and changes in cellular and extracellular compartments of the ovarian stroma (Fig. 1).
Ovary reserve and follicular dynamics
Historically, since the early fifties of the past century, the notion has been accepted that in mammals, females are born with a finite endowment of primordial follicles (PMFs) which are progressively depleted over the course of their lives [2]. Such follicle stockpile is assembled in the fetal or early postnatal ovaries from local pregranulosa cells and primary oocytes arrested to the diplotene stage of the meiotic prophase I. These latter are generated from precursor germ cells termed primordial germ cells (PGCs) originating outside the gonadal anlages and migrating into the forming ovaries [3].
After established, each PMF has three possible developmental fates: (i) to remain quiescent, (ii) to die directly from the quiescent state, or (iii) to be recruited through a process called follicle activation into a growing follicle pool, which contributes to cyclic endocrine secretion and, for some follicles, ovulation. Activated PMFs develop through primary and secondary stages before acquiring an antral cavity. At the antral stage, most follicles undergo atretic degeneration, whereas a few of them reach the preovulatory stage under the cyclic hypothalamic gonadotropin-releasing hormone (GnRH)-dependent stimulation of the pituitary gonadotropin FSH and LH that occurs after puberty. These antral follicles are the major source of the cyclic secretion of ovarian estrogens in women of reproductive age. Among them, in response to preovulatory gonadotropin surges during each reproductive cycle, only one becomes the dominant Graafian follicle. This, then, ovulates to release the mature oocyte able to be fertilized, whereas the remaining theca and granulosa cells undergo transformation to become the corpus luteum.
Conventional models characterize reproductive aging as being dependent on the remaining PMF stockpile. For decades, research on reproductive aging has been focusing on the so-called quantitative aspect of ovarian aging, which has led to mathematical models predicting follicle loss on the basis of chronological age [4]. Regardless of models, it is a fact that through a combination of recruitment toward dominant follicle development and atresia or ovulation, the ovary PMF stockpile is depleted over the course of life. In this context, studies aimed to investigate whether decline of hypothalamic-pituitary–gonadal axis activities has a causative role in ovary aging indicate that hypothalamic responsivity decreases in tandem with declining ovarian function. Actually, it appears that with age the number of developing follicles becomes insufficient to produce the hormonal support necessary to stimulate hypothalamic GnRH production and the subsequent pituitary preovulatory FSH and LH surge. These diminished responsiveness and concurrent decline in ovarian functions lead to eventual reproductive failure [5,6,7]. In agreement, later onset of menopause and the maintenance of the hypothalamic-pituitary–gonadal axis homeostasis were associated with lower human mortality and prolonged longevity [8].
Therefore, under traditional thinking, the most convenient approach for increasing ovarian lifespan would be to preserve PMF stockpile or to slow the rate of PMF reserve depletion.
Because the possibility to directly repopulate the ovary with transplanted PMFs in a mouse model has proven not feasible [9], the extreme way to maintain the ovary young should be the cryopreservation in young age of ovarian cortices, where PMF reside. Although the feasibility of freezing and thawing ovarian tissue is widely documented and live births after transplantation of frozen-thawed ovarian cortex have been reported [10], these techniques are applied only in a few specialized centers. Moreover, so far, they have been limited almost exclusively to cancer patients, since considerably invasive and costly.
In a theoretically more simple way, if ovary age depends exclusively on the PMF stockpile, ovary youth might be maintained by sustaining PMF survival and/or slowing down their activation. New findings about crucial players of these processes in model animals have allowed testing such possibilities. Numerous activators (BMP4/7, GDF-9, KIT-ligand, FGF2/7, insulin, GREM1/2, and LIF) and suppressors (AMH, LHX8, PTEN, Tsc1m/TORC1, FOXO3a, YAP/Hippo signaling, and FOXL2) have been reported to be related to PMF recruitment. Likewise, relevant regulators of survival/apoptosis of pregranulosa cells and oocytes enclosed in PMF have been identified. These factors will not be discussed here; interested readers can refer to several available reviews [11,12,13,14,15,16,17]. However, only a few well-documented reports are available on interventions that increase or sustain PMF numbers acting on their recruitment or pregranulosa cell and oocyte survival/apoptosis and that significantly extend functional reproductive lifespan and delay the onset of aging-associated health problems [18,19,20,21,22,23].
Finally, revaluating old hypotheses about the origin of germ cells from the ovarian epithelium [24, 25], in 2004 Jonathan Tilly and colleagues claimed that follicular renewal may be possible in postnatal ovaries from resident stem cell precursors of pregranulosa cells and oocytes (oogonia stem cells (OSCs)) [26]; subsequently, other papers supported such a possibility [27,28,29]. Some results obtained by Tilly’s and other groups also suggested that OSCs might be expanded in vitro and used to repopulate PMF depleted ovaries [30]. More recently, to explain PMF pool depletion throughout age and the consequent ovary function decline, Tilly’s group proposed that the well-described decrease in ovarian E2 production, as females age, may underlie OSC dysfunction and a corresponding loss of oogenic support as mechanisms that contribute to aging-associated ovarian failure [31]. This implies that promoting the differentiation of OSCs that express E2 receptor-α (ERα) by estrogens might contribute to maintain adequate numbers of ovarian follicles during reproductive life. However, further investigations are necessary in order to consider this hypothesis, since pharmacological treatments with estrogens must be carefully evaluated before being used.
Genetic and epigenetic factors
There is little doubt that ovarian aging has a genetic basis. In fact, the age of menopause in women is an inheritable trait and the age at which the ovarian failure occurs has a strong genetic component. In addition, genetic analyses in women with primary ovarian insufficiency (POI), the most frequent cause of early menopause, have revealed a role of several genes known to be related to crucial processes of oogenesis, for example, ovary sex differentiation (WNT4), DNA repair during meiotic recombination in fetal oocytes (MSH4, MSH5, DMC1), PMF assembly (FIGLA), the transition from primordial to growing follicles (NOBOX, FOXO3, PTEN), the hormone-dependent phase of follicular growth (FSHR), the maintenance of meiotic arrest in oocytes of growing follicles (GPR3), and granulosa cell function (FOXO1) (for reviews, see [32, 33]).
Beside genome, epigenome is likely to contribute to decreased fertility in aging females given its role in controlling gene expression and chromatin structure. In particular, the epigenetic characteristics of an oocyte are important because their alteration could compromise the events of early development of the embryo, which then manifest themselves as subsequent evolutionary outcomes in the offspring. Most of the studies on oocyte and embryo epigenetics have been done on mouse models, since such studies are extremely difficult to perform in humans. The few investigations carried out in humans confirm anyway that changes in epigenetics and epigenetic-related enzymes in oocytes and embryos of women with advanced age include alterations in DNMT levels [34, 35], DNA methylation levels [36, 37], and acetylation patterns of the histone [38].
Oxidative stress and mitochondria
Evidence is accumulating that decrease of PMF pool is actually circumstantial to ovary aging, but it is not the main cause. In fact, in women, in the presence of normal follicular dynamics and regular menstrual cycle, the ovaries begin to show an accelerated decline in fertility long before; about 10–15 years, the PMF stockpile reaches its minimal levels. Moreover, data from assisted reproduction technologies (ART) clearly demonstrate that the age-associated decline in fertility is primarily attributable to defects at the level of the oocytes [39].
In this regard, the most relevant theory for ovarian aging, first proposed by Tarin, implies a reduced ability of oocytes and granulosa cells to counteract reactive oxygen species (ROS), which are among the most important physiological inducers of cellular injury associated with aging.
As reported above, oocytes enclosed in PMFs are arrested at the diplotene stage of the first meiotic prophase from fetal stages or soon after birth, until PMF activation and meiosis resumption at the periovulation time. During this dormant period, which in women may last 30–45 years, oxidative stress may be generated in oocytes and/or surrounding follicular cells. The external and internal factors leading to oxidative stress, mainly excessive ROS production, might be various and the severity of damage produced likely dependent on genetically programmed defense mechanisms. Alterations in these mechanisms can be caused by mutations/deletions of both nuclear and mitochondrial genes. In this context, if mitochondrial DNA mutations occur in the oocyte, mitochondrial replacement therapy might solve the problem although efficacy and safety of such treatment are controversial [41]. OSCs have been proposed as the most suitable possible donators of mitochondria to the aged oocytes [42].
Oxidative damage to the ovary is generally caused by lipid peroxidation, which seriously influences folliculogenesis, meiosis, and ovulation, and eventually leads to ovarian aging [43, 44]. In this regard, increased ROS levels in oocytes have been reported to result in telomere shortening and reduce their developmental competence [45,46,47]. Telomere shortening and dysfunction could cause defects in meiosis [48, 49]. Furthermore, high ROS decrease communication between oocytes and GCs, affecting preovulatory oocyte maturation [50].
Interestingly, recent data support the hypothesis that in various tissue types, the aging process is regulated by a continuous cross talk between ROS and sirtuins, NAD + -dependent enzymes with deacetylase, and/or mono-ADP-ribosyl transferase activity. For example, through deacetylation of the FOXO3A transcription factor, SIRT1 stimulates the expression of catalase and manganese superoxide dismutase (MnSOD) and ROS detoxification. The expression of sirtuins has been observed in mouse oocytes and embryos and reproductive defects in both sexes have been described in SIRT-null animals that exhibit sterility or altered gametogenesis and offspring with reduced vitality [51]. A study focused on prevention of aging by dietary anti-oxidant strategies has provided indirect evidence for a crucial role of SIRT1 activity in the regulation of the ovarian aging process [52]. Sirtuin expression has also been found to be downregulated in aged oocytes [53]; in particular SIRT3 seems to be involved in protecting oocytes against stress conditions during in vitro fertilization, meiosis resumption, and completion [54]. In order to counteract these alteration of sirtuins levels, observed in advance reproductive age, dietary supplementation with different compound have been proposed. Very recently, Miao et al. [55] reported that in vivo supplementation of nicotinamide mononucleotide (NMN) improved the quality of oocytes from naturally aged mice by recovering NAD + levels. NMN supplementation not only increased ovulation of aged oocytes but also enhances their meiotic competence and fertilization ability. Moreover, single-cell transcriptome analysis performed by the authors showed that the beneficial effect of NMN on aged oocytes was mediated by restoration of mitochondrial function, eliminating the accumulated ROS. In vivo oral administration of melatonin, a potent anti-oxidant released by the pineal gland known to prevent age-related oxidative stress and reproductive system disorders, has been found to improve the quality of maternally aged oocytes by maintaining anti-oxidant metabolite supply [56]. Also the treatment with curcumin, a polyphenol extract of Curcuma longa, delays the process of oocyte aging, maintaining elevated anti-Müllerian hormone and estrogen and diminished FSH serum levels [57]. Lastly, the flavonol compound quercetin reduces in vitro, in aged oocytes, ROS via SIRT3-mediated acetylation of SOD2, thus promoting IVM and subsequent formation of blastocysts both in mice and humans [58]. All these studies support the notion that ovarian functions and female health are tightly linked and provide incentive to pursue strategies to prevent or delay ovarian failure with the aim to improve life quality of women in advanced age.
The molecular fingerprint recently obtained by the ovarian gene expression profile in non-human primates provides a clear picture of the ovary aging. Germ line genes, oocyte-specific genes, and intraovarian signaling pathway appeared downregulated. In particular, downregulation of genes related to mitochondrial electron transport chain was also observed [59].
The quantity as well as quality of GCs may also play a significant role in maintaining oocyte quality and the endocrine ovarian function. For instance, analysis of mitochondrial ultrastructure in GCs from premenopausal and younger women revealed a greater degree of vacuolization and crista malformation in the older granulosa cells, which correlated with a reduction in both superoxide dismutase (SOD1-2) and catalase activity [60]. Increases in mitochondrial DNA deletion mutations have also been documented in GCs of women older than 38 years of age [61], and upregulation of the mitochondrial gene glutathione S-transferase theta 1 (GSTT1) in GCs with age has been shown [62]. More recently, global alterations in gene transcription and methylation with age have also been revealed in GCs of women affected by polycystic ovary syndrome (PCOS) characterized, besides cystic ovarian morphology, by anovulatory infertility and hormone disorders [63].
Many published data showed that ovary aging can be alleviated with a number of treatments using anti-oxidants (i.e., vitamins C and E, coenzyme Q10, folic acid, resveratrol), agents affecting the cell response against oxidative stress (i.e., growth hormone (GH)), or those combining both of these activities (e.g., melatonin) [33, 44]. Interestingly, a study in the mouse showed that dietary restrictions sustain female fertile potential with age through significant improvements in oocyte chromosomal dynamics and identifies peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), a master regulator of mitochondrial biogenesis/function and oxidative stress, as regulator of oocyte quality [64]. Although the common wisdom is that dietary restriction negatively affects fertility, these data demonstrate that it can also have a positive impact. As a matter of fact, several rodent studies clearly established that moderate dietary restriction extends functional ovarian lifespan in mammals [64, 65].
The implication of mitochondria in the aging of the oocyte, however, includes different aspects, not only those related to oxidative stress and sirtuins. In fact, there is also a close relationship between mitochondrial DNA (mtDNA), the amount of mitochondria present in the ooplasm, and oocyte quality. During aging, rearrangements of mtDNA can be observed in mature eggs and it is estimated that in 50% of human oocytes, during IVF, mutation and deletion processes occur at the mtDNA level [66]. These mutations could have repercussions on the developing embryo, but the link between the mtDNA rearrangements and aging remains controversial [67].
In order for a correct distribution of mitochondria to occur during the formation of blastomeres in embryonic development, it is necessary the presence of a sufficient and correct amount of mitochondria in the oocyte at the time of fertilization. This is shown by experiments on mice in which mitochondria were transferred from competent to incompetent oocytes, obtaining embryos with reduced fragmentation and higher implantation rate [68]. Although this technique of mitochondrial manipulation has been authorized in UK (3 February 2015), it use is still raises concerns in the scientific and clinical world.
Changes of the ovarian stroma
The ovary contains different extracellular matrices (ECM) that include the follicular basal lamina, the cumulus-oocyte complex, and the stroma that together account for the ovarian ECM and its related proteome or “matrisome” [69]. The main ovarian structure, the follicle, is surrounded by the ovarian stroma and develops by moving from the more rigid and collagen-rich ovarian cortex to the softer and more pliant medulla and eventually back to the ovarian periphery for ovulation. It is important to say that the stromal cells are fundamental as the stromal ECM, since the former decide the characteristics of the latter. In the ovary, the cell compartment consists mainly of fibroblasts, macrophages, mast cells, endothelial cells, smooth muscle cells, pericytes, and various leukocytes that, depending on the context, may arrive from the bloodstream, such as eosinophils, monocytes, and lymphocytes. About a decade ago it was first hypothesized that mechanobiology of the ovarian ECM could have a significant impact on both follicle growth and oocyte quality [70]. This hypothesis was proven also by studying in vitro systems that tried to recapitulate the in vivo microenvironment by using cells coming from the stromal compartment [71]. Thus, understanding how the ovarian stroma, formed by cells and ECM, changes during the lifetime is of critical importance and it might shed light on the cause of the precocious decline of this organ and eventually lead to discover new markers to predictively recognize it and, hopefully, counteract it in the near future. In 2016, Briley and colleagues established that fibrosis is an early hallmark of ovarian aging, and this alteration may contribute to the age-associated decline in oocyte quality [72]. This study evidenced an increased level of collagen type I and type III by picro-sirius staining and hydroxyproline assay during aging in mouse.
The ovary, through the processes of folliculogenesis, as previously described, and also ovulation, undergoes repeated cycles of connective tissue remodeling and wound healing which require the action of several molecules produced locally including growth factors and cytokines and complex interplay between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) [73, 74]. This continuous remodeling that takes place since puberty is thought to be one of the causes of fibrosis due to an increased synthesis of collagen and a decreased deposition of hyaluronan both in mouse and in human samples [73, 75]. Alternatively, or in combination with altered ECM production, there may be an age-associated change in the homeostasis of ECM through an imbalance of the activities of MMPs and TIMPs [74].
Recently, Ouni and colleagues [76] performed an accurate analysis on mechanical matrisome of human ovarian biopsies at different ages until menopause. By using picro-sirius staining, polarized light microscopy, and multiplex immunofluorescence they evaluated the characteristic and amount of various ECM components involved either in stiffness, as collagen, or in muco-elasticity, as elastin, EMILIN-1, fibrillin-1, and glycosaminoglycans (GAGs). They found that, differently to what it was described in mouse and human [73, 75], collagen and GAGs did not differ but, with aging, the relative amount of thick collagen fibers diminished. It has to be pointed out that in the previous investigations only hyaluronan, among GAGs, was analyzed [73, 75]. Conversely, the amount of elastin, fibrillin-1, and EMILIN-1 declined by age. In a subsequent study, the same group performed biophysical and biomechanical analyses evaluating fiber morphology and orientation, pore geometry, topography and surface roughness, and elastic and viscoelastic properties [77]. The results showed significant differences from reproductive age to menopause. With age, the single collagen fibers become thicker, consistent with previous data, but bundle, i.e., group of fibers, were straighter and their diameter thinner, this probably because the activity of LOX, the cross-linker of collagen fibers, is estrogen regulated. The pore size and distribution in aged tissues resulted in a less diffusible space. Mechanical property evaluation indicated that menopausal samples were more rigid and the surface was rougher compared with reproductive age samples [77]. Mostly in line with these results, a recent study on young and aged porcine ovaries showed in the latter a significant increment of collagen, GAGs, and laminins, and a decreased amount of elastin and fibronectin by using immunohistochemical, ELISA, and gene expression analyses [78]. It should be taken into account that the materials used in human studies were biopsies of ovarian cortex and then a restricted part of the whole organ; this could explain some discrepancies with studies performed in mouse and porcine, where the entire organ, composed of cortex and medulla, was used. However, in conclusion, the overall picture is quite consistent and increases our knowledge on ovarian ECM composition that, together with its biophysical and biomechanical properties, changes over time.
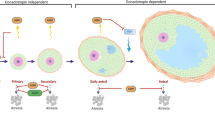
Starting at puberty, the cyclic remodeling of the ovarian ECM due to follicle maturation and atresia determines a gradually increase/variation in immune cell populations [79]. In parallel with the age-associated fibrosis of ECM, stromal cells change in number and functions and increase the expression of genes involved in immune cell recruitment and in inflammation. For the innate immune system, the most abundant cells in the ovary are macrophages [80, 81]. This cell type has the main function to phagocyte and degrade foreign antigen but it is also involved in matrix degradation and remodeling as well as in secretion of cytokines, chemokines, and growth factor. It is known that macrophages can be classically activated into M1 and alternately activated into M2 phenotype. The former are present in the acute stages of inflammation and produce proinflammatory cytokines such as IL-6 and TNFα, reported to be abundant in aged ovaries [72]. M2 macrophages are active during the later stages of infection and primarily serve to remodel the ECM and facilitate tissue repair, and could cause fibrosis if the injury lasts longer and do not resolve. Interestingly, Zhang and colleagues, by using transcriptomic and cytofluorimetric analyses, found that with advanced age the overall number of macrophages does not change, but does change the ratio M1/M2, resulting in more M2 macrophages [79]. This is line with the fact that aging is mainly related to a process of chronic and sterile inflammation called inflammaging. Moreover, they found an increase in eosinophils, another cell type typical of type 2 inflammation that releases IL4 and IL13 [82], cytokines known to alternatively activate M2 macrophages. Numerous studies have revealed a strong association between low-grade inflammation and oxidative stress; they seem to accompany one another and promote each other in many chronic diseases and in the process of aging [83]. Interestingly, low molecular weight hyaluronan, a degradation product mainly produced by ROS and known to characterize tissue inflammation, has been recently demonstrated to induce a type 2 inflammatory response in ovarian stromal cells in vitro [84]. Furthermore, besides M1 and M2 cell phenotypes, a unique population of multinucleated, giant macrophages was identified in the ovaries of reproductively elderly females (Fig. 2) [69]. Similar cells are rare in the body and are found in some cases at sites of infections or implant. In the aged ovary, they may be involved in resolving fibrotic regions of the tissue [72, 85, 86] and their presence indicates a massive tissue waste problem.
Macrophage fusion into multinucleated giant cells is a hallmark of the ovarian stroma during reproductive aging. In these micrographs, two serial sections from an ovary of a 9-month-old 129/Sv female mouse stained with hematoxylin/eosin (A) and with PAS/Alcian (B) are shown (scale bar = 200 μm). (i), (ii), and (iii) are magnifications of three different areas in A and B, showing giant cells that stain light brown in hematoxylin/eosin (yellow dashed line) and dark red/violet in PAS/Alcian (red dashed line) (scale bar = 25 μm) (our unpublished observations)
The modulation of proinflammatory pathways may be an important therapeutic avenue for prolonging reproductive lifespan. In fact, in a KO mouse model of the proinflammatory cytokine IL-1α, the ovarian lifespan, pregnancy rate, and litter size were all increased relative to controls [87]. Recently, Lliberos et al. [88] reported that the decrease in PMF numbers over the reproductive lifespan was associated with an increase in the intraovarian percentage of CD4 + T cells, B cells, and macrophages. Serum concentration and intraovarian mRNA levels of several proinflammatory cytokines, including IL-1α/β, TNF-α, and IL-6; inflammasome-related genes like the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3); and the adaptor molecule apoptosis-associated speck-like protein containing a CARD (ASC), were significantly increased with age. Similar results have been obtained in other organ as brain [89] and kidney [90]. Based on the increase of CD4 + T and B cells, it can be speculated that also the adaptive immune system can somehow become activate in the aged ovary and this could be due to degradative events on proteins, as collagen, thus producing immunogenic forms potentially able to stimulate those immune cells in the presence of macrophages. Therefore, defining the inflammatory milieu of the aging ovary and determining the causes of inflammation remain a big challenge and an interesting topic to investigate. Further studies are necessary to unravel the mechanisms of this complex scenario.
Conclusions
The ovary is the main regulator of female fertility, and its biological clock is set to ensure reproductive success during a definite life phase. The today’s tendency to postpone childbearing to the fourth decade of life has made reproductive aging an age-related disease that requires particular consideration in our health care systems. It is well established that ovarian functional decline is related to the gradual loss of resting follicles and decreased biologic competence of aged oocytes. Although clear perturbations in the dynamic of follicle growth do not seem to occur, the oocytes that reach ovulation during reproductive aging exhibit cellular and chromosomal defects that seriously hinder the reproductive process, beside improper epigenetic modifications. When the concept of oocyte aging as the main determinant of fertility decline has become clear, researchers have begun to expand investigations into the entire ovarian microenvironment looking for age-related changes with potential effects on follicle and oocyte competency. It has been proposed that stromal inflammation together with energy perturbations associated to mitochondrial dysfunction might be the cause and the effect of increased production of toxic metabolic byproducts such as ROS, which can seriously damage biomolecules and impair key regulatory mechanisms of oogenesis. Change in ovarian ECM microenvironment is emerging to have a significant impact on follicle and oocyte quality. Greater understanding of these issues could be helpful in creating innovative strategies for counteracting the effects exerted on fertility by age or age-like insults (i.e., xenobiotics and anti-cancer drugs). Therapies based on anti-oxidants, mitochondrial metabolites, and mesenchymal stem cells (MSCs) used as anti-inflammatory “medicinal signaling cells” are particularly promising. Some clues about the first two have been given in the present work while numerous recent reviews about the basis, prospects, and limitations in the use of MCSs for ovarian rejuvenation are available in the literature [91,92,93,94].
References
Touati SA, Wassmann K. How oocytes try to get it right: spindle checkpoint control in meiosis. Chromosoma. 2016;125:321–35.
Zuckerman S, Zuckerman S, Zuckerman SLZ, Zuckerman LM. The number of oocytes in the mature ovary. Recent Prog Horm Res. 1951. p. 63–109.
De Felici M. The formation and migration of primordial germ cells in mouse and man. Results Probl Cell Differ. 2016;58:23–46.
Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23:699–708.
Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JKH. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78:29–35.
Mills RH, Romeo HE, Lu JKH, Micevych PE. Site-specific decrease of progesterone receptor mRNA expression in the hypothalamus of middle-aged persistently estrus rats. Brain Res. 2002;955:200–6.
Santoro N, Banwell T, Tortoriello D, Lieman H, Adel T, Skurnick J. Effects of aging and gonadal failure on the hypothalamic-pituitary axis in women. Am J Obstet Gynecol. 1998;178:732–41.
Yonker JA, Chang V, Roetker NS, Hauser TS, Hauser RM, Atwood CS. Hypothalamic–pituitary–gonadal axis homeostasis predicts longevity. Age. 2013;35:129–38.
Park M-R, Choi Y-J, Kwon D-N, Park C, Bui H-T, Gurunathan S, et al. Intraovarian transplantation of primordial follicles fails to rescue chemotherapy injured ovaries. Sci Rep. 2013;3:1384.
Donnez J, Dolmans M-M. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32:1167–70.
Marcozzi S, Rossi V, Salustri A, De Felici M, Klinger FG. Programmed cell death in the human ovary. Minerva Ginecol. 2018;70:549–60.
Gebel J, Tuppi M, Chaikuad A, Hötte K, Schröder M, Schulz L, et al. p63 uses a switch-like mechanism to set the threshold for induction of apoptosis. Nat Chem Biol. 2020;16:1078–86.
Kerr JB, Hutt KJ, Michalak EM, Cook M, Vandenberg CJ, Liew SH, et al. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of Puma and Noxa. Mol Cell. 2012;48:343–52.
McLaughlin EA, McIver SC. Awakening the oocyte: controlling primordial follicle development. Reproduction. 2009;137:1–11.
Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30:438–64.
Reddy P, Zheng W, Liu K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab. 2010;21:96–103.
Hussein MR. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update. 2005;11:162–78.
Masciangelo R, Hossay C, Chiti MC, Manavella DD, Amorim CA, Donnez J, et al. Role of the PI3K and Hippo pathways in follicle activation after grafting of human ovarian tissue. J Assist Reprod Genet. 2020;37:101–8.
Kawamura K, Ishizuka B, Hsueh AJW. Drug-free in-vitro activation of follicles for infertility treatment in poor ovarian response patients with decreased ovarian reserve. Reprod Biomed Online. 2020;40:245–53.
Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–8.
Pelosi E, Omari S, Michel M, Ding J, Amano T, Forabosco A, et al. Constitutively active Foxo3 in oocytes preserves ovarian reserve in mice. Nat Commun. 2013;4:1843.
Maidarti M, Clarkson YL, McLaughlin M, Anderson RA, Telfer EE. Inhibition of PTEN activates bovine non-growing follicles in vitro but increases DNA damage and reduces DNA repair response. Hum Reprod. 2019;34:297–307.
Zhang H, Risal S, Gorre N, Busayavalasa K, Li X, Shen Y, et al. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 2014;24:2501–8.
Kingery HM. Oogenesis in the white mouse. J Morphol. 1917;30:261–315.
Simkins CS. Development of the human ovary from birth to sexual maturity. Am J Anat. 1932;51:465–505.
Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–50.
Bukovsky A, Gupta SK, Virant-Klun I, Upadhyaya NB, Copas P, Van Meter SE, et al. Study origin of germ cells and formation of new primary follicles in adult human and rat ovaries. In: Hou SX, Singh SR, editors. Germline Stem Cells. Totowa: Humana Press; 2008. p. 233–65.
Zou K, Hou L, Sun K, Xie W, Wu J. Improved efficiency of female germline stem cell purification using fragilis-based magnetic bead sorting. Stem Cells Dev. 2011;20:2197–204.
Park E-S, Tilly JL. Use of DEAD-box polypeptide-4 (Ddx4) gene promoter-driven fluorescent reporter mice to identify mitotically active germ cells in post-natal mouse ovaries. Mol Hum Reprod. 2015;21:58–65.
Ding X, Liu G, Xu B, Wu C, Hui N, Ni X, et al. Human GV oocytes generated by mitotically active germ cells obtained from follicular aspirates. Sci Rep. 2016;6:28218.
Satirapod C, Wang N, MacDonald JA, Sun M, Woods DC, Tilly JL. Estrogen regulation of germline stem cell differentiation as a mechanism contributing to female reproductive aging. Aging (Albany NY). 2020;12:7313–33.
Nobuhiro S, Stephanie AP, Aleksandar R. Candidate genes for premature ovarian failure. Curr Med Chem. 2007;14:353–7.
Tesarik J, Galán-Lázaro M, Mendoza-Tesarik R. Ovarian aging: molecular mechanisms and medical management. Int J Mol Sci. 2021;22:1371.
Steuerwald NM, Bermúdez MG, Wells D, Munné S, Cohen J. Maternal age-related differential global expression profiles observed in human oocytes. Reprod Biomed Online. 2007;14:700–8.
Ratnam S, Mertineit C, Ding F, Howell CY, Clarke HJ, Bestor TH, et al. Dynamics of Dnmt1 methyltransferase expression and intracellular localization during oogenesis and preimplantation development. Dev Biol. 2002;245:304–14.
Md BY, Russanova VR, Gravina S, Hartley S, Mullikin JC, Ignezweski A, et al. DNA methylome and transcriptome sequencing in human ovarian granulosa cells links age-related changes in gene expression to gene body methylation and 3′-end GC density. Oncotarget. 2015;6:3627–43.
Kawai K, Harada T, Ishikawa T, Sugiyama R, Kawamura T, Yoshida A, et al. Parental age and gene expression profiles in individual human blastocysts. Sci Rep. 2018;8:2380.
van den Berg IM, Eleveld C, van der Hoeven M, Birnie E, Steegers EAP, Galjaard R-J, et al. Defective deacetylation of histone 4 K12 in human oocytes is associated with advanced maternal age and chromosome misalignment. Hum Reprod. 2011;26:1181–90.
Coccia ME, Rizzello F. Ovarian reserve. Ann N Y Acad Sci. 2008;1127:27–30.
Tarin JJ. Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Mol Hum Reprod. 1996;2:717–24.
Zhang C, Tao L, Yue Y, Ren L, Zhang Z, Wang X, et al. Mitochondrial transfer from induced pluripotent stem cells rescues developmental potential of in vitro fertilized embryos from aging females†. Biol Reprod. 2021;104:1114–25.
Truman AM, Tilly JL, Woods DC. Ovarian regeneration: The potential for stem cell contribution in the postnatal ovary to sustained endocrine function. Mol Cell Endocrinol. 2017;445:74–84.
Lim J, Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary1. Biol Reprod. 2011;84:775–82.
Yang L, Chen Y, Liu Y, Xing Y, Miao C, Zhao Y, et al. The role of oxidative stress and natural antioxidants in ovarian aging. Front Pharmacol. 2021;11:2364.
Keefe DL, Franco S, Liu L, Trimarchi J, Cao B, Weitzen S, et al. Telomere length predicts embryo fragmentation after in vitro fertilization in women—toward a telomere theory of reproductive aging in women. Am J Obstet Gynecol. 2005;192:1256–60.
Pollack AZ, Rivers K, Ahrens KA. Parity associated with telomere length among US reproductive age women. Hum Reprod. 2018;33:736–44.
Yamada-Fukunaga T, Yamada M, Hamatani T, Chikazawa N, Ogawa S, Akutsu H, et al. Age-associated telomere shortening in mouse oocytes. Reprod Biol Endocrinol. 2013;11:108.
Liu L, Franco S, Spyropoulos B, Moens PB, Blasco MA, Keefe DL. Irregular telomeres impair meiotic synapsis and recombination in mice. PNAS. 2004;101:6496–501.
Liu L, Blasco MA, Keefe DL. Requirement of functional telomeres for metaphase chromosome alignments and integrity of meiotic spindles. EMBO Rep. 2002;3:230–4.
Cajas YN, Cañón-Beltrán K, Ladrón de Guevara M, Millán de la Blanca MG, Ramos-Ibeas P, Gutiérrez-Adán A, et al. Antioxidant nobiletin enhances oocyte maturation and subsequent embryo development and quality. Int J Mol Sci. 2020;21:5340.
Kawamura Y, Uchijima Y, Horike N, Tonami K, Nishiyama K, Amano T, et al. Sirt3 protects in vitro-fertilized mouse preimplantation embryos against oxidative stress-induced p53-mediated developmental arrest. J Clin Invest. 2010;120:2817–28.
Liu J, Liu M, Ye X, Liu K, Huang J, Wang L, et al. Delay in oocyte aging in mice by the antioxidant N-acetyl-L-cysteine (NAC). Hum Reprod. 2012;27:1411–20.
Yang Q, Dai S, Luo X, Zhu J, Li F, Liu J, et al. Melatonin attenuates postovulatory oocyte dysfunction by regulating SIRT1 expression. Reproduction. 2018;156:81–92.
Tatone C, Di Emidio G, Vitti M, Di Carlo M, Santini S, D’Alessandro AM, et al. Sirtuin functions in female fertility: possible role in oxidative stress and aging. Oxid Med Cell Longev. 2015;2015:e659687.
Miao Y, Cui Z, Gao Q, Rui R, Xiong B. Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes. Cell Rep. 2020;32(5):107987. https://doi.org/10.1016/j.celrep.2020.107987.
Zhang H, Li C, Wen D, Li R, Lu S, Xu R, et al. Melatonin improves the quality of maternally aged oocytes by maintaining intercellular communication and antioxidant metabolite supply. Redox Biol. 2022;49:102215.
Azami SH, Nazarian H, Abdollahifar MA, Eini F, Farsani MA, Novin MG, et al. The antioxidant curcumin postpones ovarian aging in young and middle-aged mice. Reprod Fertil Dev. 2020;32:292–303.
Cao Y, Zhao H, Wang Z, Zhang C, Bian Y, Liu X, et al. Quercetin promotes in vitro maturation of oocytes from humans and aged mice. Cell Death Dis. 2020;11:1–15.
Wang S, Zheng Y, Li J, Yu Y, Zhang W, Song M, et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell. 2020;180:585-600.e19.
Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, Caserta D, et al. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol Hum Reprod. 2006;12:655–60.
Seifer DB, DeJesus V, Hubbard K. Mitochondrial deletions in luteinized granulosa cells as a function of age in women undergoing in vitro fertilization. Fertil Steril. 2002;78:1046–8.
Ito M, Muraki M, Takahashi Y, Imai M, Tsukui T, Yamakawa N, et al. Glutathione S-transferase theta 1 expressed in granulosa cells as a biomarker for oocyte quality in age-related infertility. Fertil Steril. 2008;90:1026–35.
Yu Y-Y, Sun C-X, Liu Y-K, Li Y, Wang L, Zhang W. Genome-wide screen of ovary-specific DNA methylation in polycystic ovary syndrome. Fertil Steril. 2015;104:145-153.e6.
Selesniemi K, Lee H-J, Muhlhauser A, Tilly JL. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. PNAS. 2011;108:12319–24.
Nelson JF, Gosden RG, Felicio LS. Effect of dietary restriction on estrous cyclicity and follicular reserves in aging C57BL/6J mice1. Biol Reprod. 1985;32:515–22.
Jacobs L, Gerards M, Chinnery P, Dumoulin J, de Coo I, Geraedts J, et al. mtDNA point mutations are present at various levels of heteroplasmy in human oocytes. Mol Hum Reprod. 2007;13:149-154*.
May-Panloup P, Boucret L, Chao de la Barca J-M, Desquiret-Dumas V, Ferré-L’Hotellier V, Morinière C, et al. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update. 2016;22:725–43.
Perez GI, Trbovich AM, Gosden RG, Tilly JL. Mitochondria and the death of oocytes. Nature. 2000;403:500–1.
Ewald CY. The matrisome during aging and longevity: a systems-level approach toward defining matreotypes promoting healthy aging. Gerontology. 2020;66:266–74.
Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;28:3–6.
Tingen CM, Kiesewetter SE, Jozefik J, Thomas C, Tagler D, Shea L, et al. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction. 2011;141:809–20.
Briley SM, Jasti S, McCracken JM, Hornick JE, Fegley B, Pritchard MT, et al. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction. 2016;152:245–60.
Mara JN, Zhou LT, Larmore M, Johnson B, Ayiku R, Amargant F, et al. Ovulation and ovarian wound healing are impaired with advanced reproductive age. Aging (Albany NY). 2020;12:9686–713.
Curry TE, Osteen KG. Cyclic changes in the matrix metalloproteinase system in the ovary and uterus. Biol Reprod. 2001;64:1285–96.
Amargant F, Manuel SL, Tu Q, Parkes WS, Rivas F, Zhou LT, et al. Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices. Aging Cell. 2020;19:e13259.
Ouni E, Bouzin C, Dolmans MM, Marbaix E, Pyrdit Ruys S, Vertommen D, et al. Spatiotemporal changes in mechanical matrisome components of the human ovary from prepuberty to menopause. Hum Reprod. 2020;35:1391–410.
Ouni E, Peaucelle A, Haas KT, Van Kerk O, Dolmans M-M, Tuuri T, et al. A blueprint of the topology and mechanics of the human ovary for next-generation bioengineering and diagnosis. Nat Commun. 2021;12:5603.
Pennarossa G, De Iorio T, Gandolfi F, Brevini TAL. Impact of aging on the ovarian extracellular matrix and derived 3D scaffolds. Nanomaterials. 2022;12:345.
Zhang Z, Schlamp F, Huang L, Clark H, Brayboy L. Inflammaging is associated with shifted macrophage ontogeny and polarization in the aging mouse ovary. Reproduction. 2020;159:325–37.
Best CL, Pudney J, Welch WR, Burger N, Hill JA. Localization and characterization of white blood cell populations within the human ovary throughout the menstrual cycle and menopause. Hum Reprod. 1996;11:790–7.
Wu R, Van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Hum Reprod Update. 2004;10:119–33.
Goh SYP, Henderson NC, Heredia JE, Eagle AR, Odegaard JI, Lehwald N, et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci U S A. 2013;110(24):9914–9. https://doi.org/10.1073/pnas.1304046110.
Martínez de Toda I, Ceprián N, Díaz-Del Cerro E, De la Fuente M. The role of immune cells in oxi-inflamm-aging. Cells. 2021;10:2974.
Rowley JE, Amargant F, Zhou LT, Galligos A, Simon LE, Pritchard MT, et al. Low molecular weight hyaluronan induces an inflammatory response in ovarian stromal cells and impairs gamete development in vitro. Int J Mol Sci. 2020;21:1036.
McNally AK, Anderson JM. Macrophage fusion and multinucleated giant cells of inflammation. In: Dittmar T, Zänker KS, editors. Cell Fusion in Health and Disease. Dordrecht: Springer, Netherlands; 2011. p. 97–111.
Foley KG, Pritchard MT, Duncan FE. Macrophage-derived multinucleated giant cells: hallmarks of the aging ovary. Reproduction. 2021;161:V5-9.
Uri-Belapolsky S, Shaish A, Eliyahu E, Grossman H, Levi M, Chuderland D, et al. Interleukin-1 deficiency prolongs ovarian lifespan in mice. PNAS. 2014;111:12492–7.
Lliberos C, Liew SH, Zareie P, La Gruta NL, Mansell A, Hutt K. Evaluation of inflammation and follicle depletion during ovarian ageing in mice. Sci Rep. 2021;11:278.
Youm Y-H, Grant RW, McCabe LR, Albarado DC, Nguyen KY, Ravussin A, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18:519–32.
Song F, Ma Y, Bai X-Y, Chen X. The expression changes of inflammasomes in the aging rat kidneys. J Gerontol A Biol Sci Med. 2016;71:747–56.
Liao Z, Liu C, Wang L, Sui C, Zhang H. Therapeutic role of mesenchymal stem cell-derived extracellular vesicles in female reproductive diseases. Front Endocrinol. 2021;12:711.
Igboeli P, El Andaloussi A, Sheikh U, Takala H, ElSharoud A, McHugh A, et al. Intraovarian injection of autologous human mesenchymal stem cells increases estrogen production and reduces menopausal symptoms in women with premature ovarian failure: two case reports and a review of the literature. J Med Case Rep. 2020;14:108.
Chang Z, Zhu H, Zhou X, Zhang Y, Jiang B, Li S, et al. Mesenchymal stem cells in preclinical infertility cytotherapy: a retrospective review. Stem Cells Int. 2021;2021:e8882368.
Zhao Y, Chen S, Su P, Huang F, Shi Y, Shi Q, et al. Using mesenchymal stem cells to treat female infertility: an update on female reproductive diseases. Stem Cells Int. 2019;2019:e9071720.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Camaioni, A., Ucci, M.A., Campagnolo, L. et al. The process of ovarian aging: it is not just about oocytes and granulosa cells. J Assist Reprod Genet 39, 783–792 (2022). https://doi.org/10.1007/s10815-022-02478-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02478-0