Abstract
Different populations of the same species may have different physiological responses to environmental factors due to the adaptation to their environment. We tested interactive effects of temperatures (10,15, 20, 25, and 30 ℃) and nutrients (low nutrients: 5 μM NO3− and 0.5 μM PO4− (LN); medium nutrients: 50 μM NO3− and 5 μM PO4− (MN); high nutrients: 500 μM NO3− and 50 μM PO4− (HN)) in three different Ulva prolifera strains (one Chinese and two Korean strains). The results showed that all three strains of Ulva survived within the temperature range of 10 to 30 ℃. The photosynthetic rates of all strains increased with increasing temperature from 10 to 30 ℃ under MN. However, at the higher temperature (30 ℃) there was a significant reduction in the photosynthetic rate under HN in all three strains. A positive relationships between tissue nitrogen (N) and chlorophyll or soluble protein were observed in all three strains. The Chinese strain showed the lowest C:N ratio but the highest photosynthetic rate and tissue N contents. Our results show that the bloom forming Chinese strain may have higher nutrient uptake and assimilation ability, leading to higher photosynthetic activity. The Ulva strains may have lower tolerance to higher temperature at high nutrients conditions. These results suggest that the physiological responses of U. prolifera to different temperature and nutrients conditions can be population-specific.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ocean temperatures have increased over the past 4 decades and are expected to continuously increase. The regional, seasonal and diurnal changes of temperature are expected to increase by 3–7 ℃ by the end of the century (Stocker et al. 2014). The increase of temperature impacts marine life at all levels of biological communities, and also interacts with other environmental factors, such as salinity, nutrients, light, etc. (Lüning 1990; Hurd et al. 2014; Bindoff et al. 2019; Samanta et al. 2019a). Higher temperatures pose a serious threat to marine macroalgal survival, growth and reproduction (Lüning 1990; Davison 1991; Martínez et al. 2012; Wernberg et al. 2016; Han et al. 2023; Xing et al. 2023). Increases in temperature will influence the activity of enzymes involved in nutrients assimilation and carbon fixation (Davison 1991; Berges et al. 2002; Hurd et al. 2014). Additionally, different populations of the same species may exhibit different temperature tolerance due to the adaptations to surrounding environments at their origins. For example, Chinese strains Ulva show a higher hypo-salinity tolerance (20 psu) than Korean strains under the same condition (Lüning 1990; Bao et al. 2022, 2023). Consequently, the responses of macroalgae to changing temperatures may be population-specific (Poloczanska et al. 2013; Ji et al. 2016; Wiens 2016).
Another major environmental issue in coastal areas is eutrophication (Lohman and Priscu 1992; Fei 2012; Latimer et al. 2014). Nitrogen (N), as the primary component of numerous molecules, is integral to most biological processes such as the enzyme RUBISCO with carbon assimilation process (Dawes and Koch 1990). Nitrogen assimilation with nitrate reductase (NR) is also a central physiological process that primarily relies on nitrogen to synthesis amino acids and proteins in algae (Kim et al. 2012; Coelho et al. 2013). The NR activity can reflect the state of nitrogen metabolism, which is regulated by nitrogen availability (Kim et al. 2008, 2009, 2013; Jaime et al. 2014; Feng et al. 2021). Similarly, phosphorus (P) is involved in ATP generation, and enzymes involved in phosphorylation (Davison and Pearson 1996). Many studies have indicated that the moderate nutrients are crucial for the growth, metabolism and the activity of enzymes (e.g. NR, etc.) of algae to maintain healthy marine ecosystems (Hou and Hou 2013; Kittiwanich et al. 2016). However, elevated concentrations of N and P have the potential to break ecosystem balance by promoting the growth and biomass accumulation of some opportunistic species, such as Ulva prolifera in the Yellow Sea (Ye et al. 2011; Luo et al. 2012; Hurd et al. 2014; Li et al. 2016; Samanta et al. 2019b; Saldarriaga-Hernandez et al. 2020).
Generally, U. prolifera is a non-toxic species. However, the massive biomass accumulation during blooms leads to serious environmental damage to local ecosystems and causes economic losses to the working waterfront, tourism industry and recreational activities (Xu et al. 2014). Toxic compounds such as hydrogen sulphide (H2S) and ammonia (NH3) can also be produced after algal biomass decomposition (Nelson et al. 2008). Ulva species have a better capacity for growth and nutrients uptake than many other macroalgal species because of the simplicity of thallus with a high surface area to volume ratio (Taylor et al. 2001; Nelson et al. 2008; Lamb et al. 2018). Ulva prolifera can assimilate inorganic nitrogen and phosphorus at a higher rate in comparison to many other Ulva species (e.g., U. linza) (Luo et al. 2012; Fan et al. 2014). Thus, the uncontrolled proliferation of U. prolifera could result in reduced species diversity and community stability due to the resources competition (e.g. light, nutrients etc.) and the environment alteration in seawater (Lamb et al.2018; Choi et al. 2020; Huo et al. 2021). The growth and photosynthetic rate of U. conglobata (Zou and Gao 2014) and U. linza (Lee and Kang 2020) were accelerated under elevated temperatures at high nutrients levels.
Many studies reported independent or combined impacts of environmental variables on Ulva spp. Different populations of the same species may have different physiological responses to environmental conditions due to acclimation or genetic adaptation (Martins 2016; Figueira et al. 2021). The adaptation characteristics are genetically determined and may not be obliterated by the acclimation to different conditions in laboratory condition (Russell and Bolton 1975). Ulva prolifera blooms have frequently happened in the Yellow Sea, China, since 2008 but not in Korea. Temperature and eutrophication have been proposed to be the most important environmental factors for Ulva blooms. Therefore, the Chinese strains may have higher temperature tolerance and nutrient uptake efficiency. Thus, in the present study, three strains of U. prolifera (one Chinese strain, and two Korean strains) were used to examine their physiological responses to different levels of nutrients and temperatures in laboratory conditions.
Materials and methods
Sample preparation
The two Korean strains of Ulva prolifera were collected from Anmogseom, Jawoldo, Incheon, Korea (37°15′ 38"N; 126°18′ 57"E) in June, 2018 (UP-JW-ST) and Samsan-myeon, Kanghwado, Incheon, Korea (37°35′ 34"N; 126°27′ 26"E) in July, 2021, (UP-GH-ST) respectively. Ulva blooms have not been reported on both sites previously. The Chinese strain of U. prolifera was collected from Rudong, Jiangsu Province, China (31°54′ 45"N; 121°22′ 09"E) in May, 2011(UP-CN-ST) (Fig. 1), where U. prolifera blooms in the Yellow Sea originated from since 2008 (Zhang et al. 2013a). For species identification, DNA was extracted from Ulva samples and used to amplify ITS gene, which was sequenced and compared against NCBI database using blast. The qualified sequences were copied to the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov/). The accession numbers for three strains are OR434229 (Chinese Strain), OR434228 (Korean strain 2018) and OR434227 (Korean strain 2021), respectively. All these strains belong to the same species. All three strains were propagated vegetatively in 50 ± 10 μmol photons m−2 s−1 photosynthetically active radiation (PAR), 12 h light:12 h dark photoperiod, 15 ℃ temperature and 30 psu salinity at the Marine Ecology and Green Aquaculture (MEGA) Laboratory, Incheon National University, Korea. To obtain the experimental seedlings, all three strains were separately cultivated in von Stosch enriched (VSE) (Ott 1965) seawater medium at 25 ℃, 100 ± 10 μmol photons m−2 s−1 and 12 h light: 2 h dark of photoperiod. The medium was renewed every 3 days. The released zoids of U. prolifera were collected and cultivated for several days. Algal seedlings with the length of 2–3 cm were selected for the experiment.
Experimental design
Five levels of temperature (10, 15, 20, 25, and 30 ℃) and three levels of nutrients (low nutrients: 5 μM NO3− and 0.5 μM PO4− (LN); medium nutrients: 50 μM NO3− and 5 μM PO4− (MN); high nutrients: 500 μM NO3− and 50 μM PO4− (HN)) were used. The temperatures and nutrients conditions were determined based on the studies in different regions/seasons where U. prolifera blooms occur (Wu et al. 2015; Gao et al. 2017). The Ulva samples (about 0.25 g per replicate) were randomly selected and cultivated in 500 mL sterilized artificial seawater and at 30 psu of salinity enriched with VSE. The concentrations of nitrogen (NaNO3) and phosphorus (NaHPO4) were adjusted to the experimental levels. Each treatment had three replicates. Other environmental conditions were same as mentioned in “seedlings” cultivation. The growth medium enriched with N and P was replaced every 3 days. The samples were cultivated with continuous aeration for 15 days.
Measurement of growth rate
The relative growth rate (RGR) of U. prolifera was measured every 3 days when seawater medium was renewed. The increased algal biomass in each flask was reduced to the initial weight (0.25 g). RGR was calculated as follows:
where Wt2 and Wt1 are the fresh weight at day t2 and t1, respectively. t represents the cultivation period.
Determination of photosynthesis
Net photosynthetic rates of U. prolifera were measured using an optical oxygen electrode (ProODO-BOD, YSI, USA) at the end of the experiment. Approximately 0.25 g thalli from each flask was transferred to a BOD bottle containing 100 mL growth medium. The PAR and temperature were same as the experimental conditions. The content of dissolved oxygen was recorded per 30 s. The increase rates in light condition represent the net photosynthetic rate. The photosynthesis value was described as mg O2 L−1 g−1 FW h−1.
Measurement of pigments and soluble protein
Chlorophyll a and b, and carotenoids were measured based on the method of Wellburn (1994). Briefly, at the end of the experiment, about 0.02 g FW of algal samples were extracted with 5 mL methanol (100%) for 24 h (4 ℃) and kept in dark for complete extraction of photosynthetic pigments (Gao and Xu 2008). Absorbances at 470, 653, and 666 nm were measured to estimate the contents of chlorophyll a, and b, and carotenoids.
Soluble protein of U. prolifera was measured following the method of Bradford (1976). Briefly, about 0.05 g FW samples were harvested from each flask and extracted in 1 mL potassium phosphate buffer (0.1 M, pH 7.0). The extracted solution was centrifuged (20 min, 12,000 × g, 4 ℃). The supernatant was mixed with Bradford’s reagent and the absorbance was measured at 595 nm after 5 min reaction. The contents of soluble protein were calculated based on a standard curve of bovine serum albumin (BSA) and the values expressed as mg g−1 FW.
Assay of tissue carbon and nitrogen contents
To measure the contents of tissue carbon (C) and nitrogen (N) in U. prolifera, about 0.1 g FW samples were weighed from each treatment at the end of the experiment. The samples were dried in an oven at 60 ℃ until constant weight and then ground into less than 120 μm grain size by MM400 Ball Mill (Retsch, Germany). About 2–3 mg dry weight (DW) sample was used to make a capsule and used for tissue analysis. The contents of tissue C and N were analyzed using a CHN analyzer (Thermo Scientific Flash 2000 CHNS/O Analyzers, USA).
Data analysis
The results were expressed as the mean ± standard deviation (n = 3). Statistical analyses were conducted using Origin 9.0 and SPSS 25.0 software. Both Shapiro–Wilk test (P > 0.05) and Levene’s test (P > 0.05) were used to test for normal distribution and the homogeneity of variances. Three-way analysis of variance (Three-way ANOVA) was used to analyze the interactive effects of population, temperature and nutrients levels on growth rate, net photosynthetic rate, pigments contents, soluble protein, tissue carbon (C) and nitrogen (N), and the C:N ratio. Tukey’s honest significant difference (Tukey’s test) was used to determine the difference among each treatment at a 95% confidence level. Linear regression analysis was conducted to determinate the relationship between tissue N and chlorophylls, and between tissue N and soluble protein.
Results
Morphology and growth rates
When U. prolifera was cultivated under different temperature and nutrients conditions, strain-specific physiological responses were observed (Fig. 2). For example, the Chinese strain was darker green in color with the highest pigment content and highest branch density compared to other strains under the same condition (Figs. 2A and 5A, B, C). The branch length, however, was the shortest in the Chinese strain in comparison to both Korean strains (Fig. 2). The Korean strain (2021) of U. prolifera showed the brightest green in color with the lower pigment contents (Figs. 2C and 5G, H, I).
The relative growth rates (RGR) in all three strains of U. prolifera were significantly affected by population (P < 0.001), temperature (P < 0.001), nutrients (P < 0.001), the interaction of two of them (P < 0.001), and the interaction of all (P < 0.001) (Table S1). Elevated nutrients increased RGR despite temperatures in all three strains (P < 0.05) (Fig. 3). The Chinese strain showed the lowest RGR in comparison to both Korean strains under the same conditions, especially under HN. As for the Chinese strain, the highest RGR was observed at 15 ℃ and HN (16.3% day−1) (Fig. 3A). For the Korean strain 2018, the higher RGRs (about 35% day−1) were observed within a wide range of temperatures from 15 to 25 ℃ with HN, which was significantly higher than those at 10 and 30 ℃ with HN (P < 0.05) (Fig. 3B). The highest RGR of the Korean strain 2021 was observed at 15 ℃ and HN (> 40% day−1) and rapidly decreased at 10 ℃ (Fig. 3C). The RGR was decreased as temperature increased from 20 to 30 ℃ under HN (P < 0.05) (Fig. 3C).
Change in relative growth rate (RGR) in one Chinese strain (A) two Korean strains (B and C) of Ulva prolifera cultivated under various experimental conditions for 3, 6, 9 12 days. The error bars represent the standard deviation (n = 3). Low nutrients = 5 μM nitrate and 0.5 μM phosphate (LN); medium nutrients = 50 μM nitrate and 5 μM phosphate (MN); high nutrients = 500 μM nitrate and 50 μM phosphate (HN)
Photosynthetic rates
The net photosynthetic rates (NPRs) in all three strains of U. prolifera were significantly affected by population (P < 0.001), temperature (P < 0.001), nutrients (P < 0.001), the interaction of two of them (P < 0.001), and the interaction of all (P < 0.001) (Table S2). The NPRs of all three strains were enhanced by increasing nutrients levels irrespective of temperature (Fig. 4). Generally, in all three strains, the NPRs reached the highest value at 20 ℃ under LN. The NPRs increased with increasing temperature from 10 to 25 ℃ under MN, and there was no further increase at 30 ℃ (Fig. 4). At HN, however, the NPRs varied with increasing temperature (Fig. 4). The NPRs in the Chinese strain was ranged from 25.0 mg O2 L−1 FW h−1 at 15 ℃ to 39.3 mg O2 L−1 FW h−1 at 20 ℃ with HN (Fig. 4A). In terms of the Korean strain 2018, the NPR was highest at 25 ℃ with 27.5 mg O2 L−1 FW h−1 and lowest at 10 ℃ with 18.6 mg O2 L−1 FW h−1 under HN (P < 0.05) (Fig. 4B). As for the Korean strain 2021, the NPR was significantly increased with increasing temperature from 10 to 20 ℃ under HN, and the highest NPR was 32.6 mg O2 L−1 FW h−1 at 20 ℃ with HN (Fig. 4C).
Change in net photosynthetic rate in one Chinese strain (A) and two Korean strains (B and C) of Ulva prolifera cultivated under various experimental conditions. The error bars represent the standard deviation (n = 3). Low nutrients = 5 μM nitrate and 0.5 μM phosphate (LN); medium nutrients = 50 μM nitrate and 5 μM phosphate (MN); high nutrients = 500 μM nitrate and 50 μM phosphate (HN)
Pigment contents
The pigments contents in all three strains of U. prolifera were significantly affected by population (P < 0.001), temperature (P < 0.001), nutrients (P < 0.001), the interaction of two of them (P < 0.001), and the interaction of all (P < 0.001) (Table S3). The Chinese strain had higher pigment contents in comparison to both Korean strains under the same conditions (Fig. 5). For the Chinese strain, At LN, the content of chlorophyll a was lowest at 15 ℃ (0.89 mg g−1) and the highest at 30 ℃ (1.48 mg g−1) (P < 0.05) (Fig. 5A). The content of chlorophyll a was also significantly increased with increasing nutrients (P < 0.05) (Fig. 5A). Under HN, the contents of chlorophyll a increased from 3.8 mg g−1 at 10 ℃ to 5.0 mg g−1 at 30 ℃ (Fig. 5A). For the Korean strain 2018, unlike the Chinese strain, chlorophyll a content was significantly increased by HN in comparison to the LN and MN groups despite temperature (P < 0.05) (Fig. 5D). The chlorophyll a content ranged from 0.87 mg g−1 at 10 ℃ to 2.3 mg g−1 at 30 ℃ under HN (P < 0.05) (Fig. 5D). For the Korean strain 2021, the patterns were similar to the Chinese strain under LN and MN. Differently, the content of chlorophyll a was the highest at 20 ℃ under HN with 2.47 mg g−1 and decreased to 2.12 mg g−1 at 30 ℃ with HN (Fig. 5G). The trends of chlorophyll b and carotenoid contents in each strain under different conditions were similar to the trend of chlorophyll a in each strain (Fig. 5B, E, H, and C, F, I). For example, in the Chinese strains, the lowest and highest chlorophyll b and carotenoid were observed at 15 ℃ and LN, and at 30 ℃ and HN, respectively (Fig. 5B, C). In the Korean strain 2018, the highest chlorophyll b and carotenoid contents were 1.41 mg g−1 and 0.6 mg g−1, respectively, at 30 ℃ and HN (Fig. 5E, E). In the Korean strain 2021, the highest chlorophyll b and carotenoids contents were 1.44 mg g−1 and 0.60 mg g−1, respectively, at 20 ℃ and HN (Fig. 5H, I).
Change in chlorophyll a, chlorophyll b and carotenoids contents in one Chinese strain (A, B, C) and two Korean strains (D, E, F, G, H, I) of Ulva prolifera cultivated under various experimental conditions (n = 3). The error bars represent the standard deviation (n = 3). Low nutrients = 5 μM nitrate and 0.5 μM phosphate (LN); medium nutrients = 50 μM nitrate and 5 μM phosphate (MN); high nutrients = 500 μM nitrate and 50 μM phosphate (HN)
Soluble protein
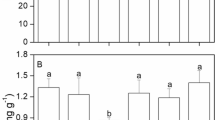
Soluble protein (SP) in all three strains was significantly influenced by population (P < 0.001), temperature (P < 0.001) and nutrients (P < 0.001), the interaction between population and temperature (P < 0.001), between population and nutrients (P < 0.001), and the interaction of all (P < 0.001) (Table S4). SP in the Chinese strain was higher in comparison to the SPs in both Korean strains under the same conditions (Fig. 6). For the Chinese strain, elevated nutrients enhanced the accumulation of SP. The lowest content of SP was observed at 15 ℃ with LN (Fig. 6A). The combination of higher temperature (> 20 ℃) and HN induced the highest SP (about 63 mg g−1) among all the conditions (Fig. 6A). In Korean strain 2018, only HN significantly enhanced the contents of SP in comparison to LN and MN at the same temperature (P < 0.05) (Fig. 6B). The lowest content of SP was 15.0 mg g−1 at 25 ℃ under both LN and MN conditions (P > 0.05). The higher SPs was about 35 mg g−1 with a wide range of temperatures from 10 to 30 ℃ with HN (Fig. 6B). As for Korean strain 2021, the content of SP was the lowest at 15 ℃ and LN, and increased with increasing temperature under LN and MN (Fig. 6C). The highest content of SP was 21.5 mg g −1 at 15 ℃ with HN (Fig. 6C).
Change in soluble protein in one Chinese strain (A) and two Korean strains (B and C) of Ulva prolifera cultivated under various experimental conditions (n = 3). The error bars represent the standard deviation (n = 3). Low nutrients = 5 μM nitrate and 0.5 μM phosphate (LN); medium nutrients = 50 μM nitrate and 5 μM phosphate (MN); high nutrients = 500 μM nitrate and 50 μM phosphate (HN)
Tissue carbon and nitrogen contents, and C:N ratio
The contents of tissue carbon (C) and nitrogen (N) in all three strains of U. prolifera were significantly influenced by population (P < 0.001), temperature (P < 0.001), nutrients (P < 0.001) and the interaction of two of them (P < 0.05), and the interaction of all (P < 0.001) (Table S5). The tissue C and N of the Chinese strain was higher than both Korean strains at the same conditions. Tissue C was the highest at 30 ℃ and HN condition in the Chinese strains (Fig. 7A). Higher nutrients promoted the accumulation of tissue N despite the temperature in all three strains (P < 0.05) (Fig. 7B, E, H). The contents of tissue N were the lowest at 15 ℃ and LN, 1.40% DW (Chinese), 0.88% DW (Korean 2018) and 1.06% DW (Korean 2021), respectively (Fig. 7B; E, H). Under HN, tissue N contents were increased with increasing temperatures from 15 to 30 ℃ in both the Chinese and the Korean 2018 strains (Fig. 7B, E). The highest tissue N contents in the Chinese strain and the Korean strain 2018 were observed at 30 ℃ and HN with the values of 5.49 and 4.88% DW, respectively (Fig. 7B, E). Korean strain 2021 had the highest tissue N contents, 4.82% DW at 15 ℃ and HN, which was significantly decreased at 10 and 30 ℃ with HN (P < 0.05) (Fig. 7H).
Change in tissue carbon, nitrogen, and C:N ratio in one Chinese strain (A, B, C) and two Korean strains (D, E, F, G, H, I) of Ulva prolifera cultivated under various experimental conditions (n = 3). The error bars represent the standard deviation (n = 3). Low nutrients = 5 μM nitrate and 0.5 μM phosphate (LN); medium nutrients = 50 μM nitrate and 5 μM phosphate (MN); high nutrients = 500 μM nitrate and 50 μM phosphate (HN)
C:N ratios in all three strains were influenced by population (P < 0.001), temperature (P < 0.001), nutrients (P < 0.001), the interaction of two of them (P < 0.001), and the interaction of all (P < 0.001) (Table S5). The C:N ratio of the Chinese strain was lower than two Korean strains at the same conditions, especially at the HN condition. For the Chinese strain, a higher C:N ratio was observed at 15 ℃ under LN (26.4) or MN (21.2) (P < 0.05) (Fig. 7C). Under HN, temperature has no effects on C:N ratio in both Chinese strain and the Korean strain 2018 (P > 0.05) (Fig. 7C, F). At LN, the highest C:N ratio (38.4) of the Korean strain 2018 was observed at 15 ℃, and significantly higher than that at 10 ℃ (30.1) and 30 ℃ (32.6) (P < 0.05) (Fig. 7F). For the Korean strain 2021, the higher C:N ratios were found at 15 ℃ under LN (32.6) and MN (20.4), respectively (P < 0.05) (Fig. 7I). Under HN, the C: N ratio in the Korean strain 2021 increased with the increasing temperature from 15 ℃ (7.37) to 30 ℃ (9.96), respectively (P < 0.05) (Fig. 7I).
Linear regression analysis
Linear regression analysis was conducted to determinate the relationships between tissue nitrogen and chlorophylls, and between tissue nitrogen and soluble protein. Both chlorophylls and soluble protein had a significant positive relationship with tissue nitrogen in all three strains (Fig. 8). The highest positive relationship between tissue nitrogen and chlorophylls (or soluble protein) was observed in the Chinese strain.
Discussion
The primary objective of this study was to analyze the effects of temperature and nutrients on physiological responses of three populations of Ulva prolifera that inhabited different environmental conditions. The Korean strains were from the coasts of Anmogseom and Samsan-myeon, Incheon. Eutrophication and Ulva blooms have never been reported in these sites. However, the Chinese strain was collected from Rudong, Jiangsu Province, China, one of the world’s largest Neopyropia yezoensis farming areas. The world largest Ulva blooms in the Yellow Sea originated from this site mainly due to the abundant nutrients and availability of substrates for Ulva’s attachment (Zhang et al. 2013a; Wu et al. 2017, 2018a; Wang et al. 2019). In the present study, U. prolifera showed population-specific responses to different temperatures and nutrients in terms of growth rate, photosynthesis, pigments and soluble protein, tissue N, and C: N ratio. Among three strains, the Chinese strain showed the highest photosynthetic and nutrients assimilation capacity. Han et al. (2022) reported that U. prolifera populations from different bloom-forming sites, Qinhuangdao, Rudong and Qingdao, showed some differences at the molecular and physiological levels. For example, genetic diversity of U. prolifera was significantly higher in the Yellow Sea green tides populations (Rudong and Qingdao) than the population from Qinhuangdao, China. The growth rates of U. prolifera from Rudong was lower than that from Qinhuangdao at the same temperature (10–25 ℃) and light (100 μmol photons m−2 s−1). Different populations showed different morphological characters and physiological responses, which are correlated with the various environmental conditions at their origins (Zhang et al. 2013b).
Thalli with dark green in color and higher branch densities were observed in the population from Rudong in the previous and the present studies. In the Southern Yellow Sea, the filamentous morpho-type of U. prolifera is dominant, while tubular or vesicular thalli are dominant in the Northern Yellow Sea (Zhang et al. 2013b). The phenotypic difference is considered a physiological response to various environmental conditions, such as nutrients and light, etc. (Zhang et al. 2013b; Gao et al. 2016). Long term adaptation may even cause ecotypic differentiation (Kim et al. 2012; Salo et al. 2014), which may contribute to the population specific responses of U. prolifera to temperature and nutrients in this study.
In the present study the photosynthetic rate of the Chinese strain was higher than both Korean strains at the same conditions. This may be due to the higher pigment and protein contents in the Chinese strain. Lee and Kang (2020) reported that elevated nutrients and higher temperatures enhanced the nutrient uptake and increased photosynthesis in Ulva. Nitrogen enrichment likely influences biosynthesis of pigments (Figueroa et al. 2009), protein (Kim et al. 2007; Ribeiro et al. 2013), and N uptake (Corey et al. 2013; Hurd et al. 2014). Similarly, the higher temperature and/or nutrients increased pigments contents in all three strains, especially the Chinese strain. Chlorophylls are related to the light-harvesting process, and the increase of pigments results in increasing photosynthetic rate (Falkowski and Raven 2013). In addition, the contents of soluble protein can be related to nutrient assimilation and be involved in osmoregulation (Li et al. 2020; Ma et al. 2021). Certain proteins (e.g. heat shock proteins) respond to thermal shock against cellular damage as a symbol of the stress tolerance (Kim et al. 2013; Hurd et al. 2014; Ma et al. 2021). Thus, higher photosynthetic capacity and temperature tolerance in the Chinese strain may be due to higher concentration of pigments and soluble proteins. However, the higher temperature (> 25 ℃) reduced the photosynthetic rate under HN in all three strains. This result suggests that the combined HN and higher temperature (30 ℃) exhibited a higher temperature sensitivity in U. prolifera. Zou and Gao (2014) reported that U. conglobata thalli exhibited a higher temperature sensitivity under the higher temperature (25 ℃) and HN (200 μM NO3−) conditions. In U. linza, the higher temperature (25 ℃) decreased the photosynthetic rate under high NH4+ (120 μM) (Lee and Kang 2020).
Tissue nitrogen (N) can reflect water column nutrient availability and the nutrient utilization capacity of macroalgae (Yu et al. 2014; Polo et al. 2015; Park et al. 2021). The C:N ratio indicates the nutrient availability for macroalgae (Hurd et al. 2014; Yu et al. 2014; Kim et al. 2015; Wu et al. 2018b; Mawi et al. 2020). Nutrient enrichment in seawater increased tissue N, which led to the decrease of C:N ratio in all three strains. U. prolifera assimilates and stores more nitrogen in its tissue when nitrogen is enriched (Kang and Chung 2017; Ober and Thornber 2017; Reidenbach et al. 2017). Previous studies have shown positive effects of higher nutrients on chlorophyll a and b synthesis in Ulva spp. (Altamirano et al. 2000; Stengel et al. 2014). The higher nutrient assimilation and storage ability enhanced the biosynthesis of chlorophylls and soluble proteins, and therefore enhanced photosynthesis (Gao et al. 2016; Bao et al. 2023). Similar results were also found in the present study. All three strains had positive relationships between tissue N and chlorophylls or soluble protein, with the most outstanding positive relationship observed in the Chinese strain, suggesting that the Chinese strains has the highest N use efficiency. The eutrophic seawater in Rudong may have affected the nutrients assimilation potential in the Chinese strain.
In the present study, the bloom forming Chinese strain showed a lower growth rate (16% day−1) than the non-bloom forming Korean strains 2018 (35% day−1) and 2021 (> 40% day −1). One possible explanation for this result is strain-specific physiological responses. The short and many branches in the Chinese strain have a higher surface area to volume ratio, accelerating nutrient uptake in comparison to the Korean strains with long and fewer branches. Seaweed with higher tissue C and N contents has higher C and N storage capacity. In the Chinese strain, therefore, the carbohydrates produced by photosynthesis and nitrogen assimilated may be internally stored rather than used for growth. However, the Korean strains showed an inverse pattern and the stored nitrogen and carbon may have mostly used for growth. Consequently, the RGRs of the Chinese strain were substantially lower than those of the Korean strains. The strain-specific physiological responses may be caused by the genetic adaptation differences and/or environmental factors at origins. Further studies should be conducted to determine if there is an underlying genetic reason for these physiological responses.
In summary, high temperature and nutrient supply interactively affect photosynthesis and nutrients assimilation in different Ulva populations, In the current study, the combined HN and higher temperature (> 25 ℃) exhibited a higher temperature sensitivity in all three strains. Among all three strains, the bloom forming Chinese strain showed higher nutrient uptake and assimilation ability, resulting in the higher photosynthesis rate. These findings indicate that the physiological responses of U. prolifera to different temperatures and nutrients are population specific. The different life stages (gametophytes vs. sporophytes) of Ulva should also be tested to see if life stage affects the population specific responses.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Altamirano M, Flores-Moya A, Conde F, Figueroa FL (2000) Growth seasonality, photosynthetic pigments, and carbon and nitrogen content in relation to environmental factors: a field study of Ulva olivascens (Ulvales, Chlorophyta). Phycologia 39:50–58
Bao ML, Park JS, Xing QK, He PM, Zhang JH, Yarish C, Yoo HI, Kim JK (2022) Comparative analysis of physiological responses in two Ulva prolifera strains revealed the effect of eutrophication on high temperature and copper stress tolerance. Front Mar Sci 9:863918
Bao ML, Xing QK, Park JS, He PM, Zhang JH, Yarish C, Kim JK (2023) Temperature and high nutrients enhance hypo-salinity tolerance of the bloom forming green alga, Ulva prolifera. Harmful Algae 123:102402
Berges JA, Varela DE, Harrison PJ (2002) Effects of temperature on growth rate, cell composition and nitrogen metabolism in the marine diatom Thalassiosira pseudonana (Bacillariophyceae). Mar Ecol Prog Ser 225:139–146
Bindoff NL, Cheung WWL, Kairo JG, Arístegui J, Guinder VA, Hallberg R, Hilmi N, Jiao N, Karim MS, Levin L, O’Donoghue S, Purca Cuicapusa SR, Rinkevich B, Suga T, Tagliabue A, Williamson P (2019) Changing ocean, marine ecosystems, and dependent communities. In: Pörtner H-O, Roberts DC, Masson-Delmotte V, Zhai P, Tignor M, Poloczanska E, Mintenbeck K, Alegría A, Nicolai M, Okem A, Petzold J, Rama B, Weyer NM (eds) IPCC special report on the ocean and cryosphere in a changing climate. Cambridge University Press, Cambridge, pp 447–587
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Choi SK, Oh HJ, Yun SH, Lee HJ, Lee K, Han YS, Kim S, Park SR (2020) Population dynamics of the ‘golden tides’ seaweed, Sargassum horneri, on the southwestern coast of Korea: The extent and formation of golden tides. Sustainability 12:2903
Coelho C, Marangon J, Rodrigues D, Moura JJG, Romão MJ, Paes de Sousa PM, Correia dos Santos MM (2013) Induced peroxidase activity of haem containing nitrate reductases revealed by protein film electrochemistry. Electroanal Chem 693:105–113
Corey P, Kim JK, Duston J, Garbary DJ, Prithiviraj B (2013) Bioremediation potential of Palmaria palmata and Chondrus crispus (Basin Head): Effect of nitrate and ammonium ratio as nitrogen source on nutrient removal. J Appl Phycol 25:1349–1358
Davison IR (1991) Environmental effects on algal photosynthesis: Temperature. J Phycol 27:2–8
Davison IR, Pearson GA (1996) Stress tolerance in intertidal seaweeds. J Phycol 32:197–211
Dawes CJ, Koch EW (1990) Physiological responses of the red algae Gracilaria verrucosa and G. tikvahiae before and after nutrient enrichment. Bull Mar Sci 46:335–344
Falkowski PG, Raven JA (2013) Aquatic photosynthesis. Princeton University Press, Princeton, New Jersey
Fan X, Xu D, Wang YT, Zhang XW, Cao SN, Mou SL, Ye NH (2014) The effect of nutrients concentrations, nutrients ratios and temperature on photosynthesis and nutrients uptake by Ulva prolifera: implications for the explosion in green tides. J Appl Phycol 26:537–544
Fei X (2012) Solving the coastal eutrophication problem by large scale seaweed. Hydrobiologia 512:145–151
Feng LN, Shi XY, Chen YH, Tang HJ, Wang LS (2021) Effects of temperature on the nitrate reductase activity and growth of Ulva prolifera. J Phycol 57:955–966
Figueira TA, Martins NT, Ayres-Ostrock L, Plastino EM, Enrich-Prast A, de Oliveira VP (2021) The effects of phosphate on physiological responses and carbohydrate production in Ulva fasciata (Chlorophyta) from upwelling and non-upwelling sites. Bot Mar 64:1–11
Korbee N (2009) Effects of nutrient supply on photosynthesis and pigmentation in Ulva lactuca (Chlorophyta): Responses to short-term stress. Aquat Biol 7:173-183
Gao KS, Xu JT (2008) Effects of solar UV radiation on diurnal photosynthetic performance and growth of Gracilaria lemaneiformis (Rhodophyta). Eur J Phycol 43:297–307
Gao G, Zhong ZH, Zhou XH, Xu JT (2016) Changes in morphological plasticity of Ulva prolifera under different environmental conditions: A laboratory experiment. Harmful Algae 59:51–58
Gao G, Liu YM, Li XS, Feng ZH, Xu ZG, Wu HY, Xu JT (2017) Expected CO2-induced ocean acidification modulates copper toxicity in the green tide alga Ulva prolifera. Environ Exp Bot 135:63–72
Han HB, Li Y, Ma XJ, Song W, Wang ZL, Fu MZ, Zhang XL (2022) Population differentiation in the dominant species (Ulva prolifera) of green tide in coastal waters of China. Acta Oceanol Sin 41:108–114
Han S, Song HI, Park JS, Kim YJ, Umanzor S, Yarish C, Kim JK (2023) Sargassum horneri and Ascophyllum nodosum extracts enhance thermal tolerance and antioxidant activity of Neopyropia yezoensis. J Appl Phycol 35:201-207
Hou X, Hou HJ (2013) Roles of manganese in photosystem II dynamics to irradiations and temperatures. Front Biol 8:312–322
Huo YZ, Kim JK, Yarish C, Augyte S, He PM (2021) Responses of the germination and growth of Ulva prolifera parthenogametes, the causative species of green tides, to gradients of temperature and light. Aquat Bot 170:103343
Hurd CL, Harrison PJ, Bischof K, Lobban CS (2014) Seaweed ecology and physiology. Cambridge University Press, New York, USA
Ji Y, Xu ZG, Zou DH, Gao KS (2016) Ecophysiological responses of marine macroalgae to climate change factors. J Appl Phycol 28:2953–2967
Jaime A, Helder TM, Carvalho LF, Esteves E, Rocha C (2014) Macroalgae mitigation potential for fish aquaculture effluents: an approach coupling nitrogen uptake and metabolic pathways using Ulva rigida and Enteromorpha clathrata. Environ Sci Pollut 21:13324–13334
Kang JW, Chung IK (2017) The effects of eutrophication and acidification on the ecophysiology of Ulva pertusa Kjellman. J Appl Phycol 29:2675–2683
Kim JK, Kraemer GP, Neefus CD, Chung IK, Yarish C (2007) Effects of temperature and ammonium on growth, pigment production and nitrogen uptake by four species of Porphyra (Bangiales, Rhodophyta) native to the New England coast. J Appl Phycol 19:431-440
Kim JK, Kraemer GP, Yarish C (2008) Physiological activity of Porphyra in relation to zonation. J Exp Mar Biol Ecol 365:75–85
Kim JK, Kraemer GP, Yarish C (2009) Research note: comparison of growth and nitrate uptake by New England Porphyra species from different tidal elevations in relation to desiccation. Phycol Res 57:152–157
Kim JK, Kraemer GP, Yarish C (2012) Metabolic plasticity of nitrogen assimilation by Porphyra umbilicalis enables broad intertidal distribution. J Ocean Univ China 11:517–526
Kim JK, Kraemer GP, Yarish C (2013) Effects of emersion on nitrogen release and physiological function in the intertidal genus Porphyra. PLoS One 8:e69961
Kim JK, Kraemer GP, Yarish C (2015) Use of sugar kelp aquaculture in Long Island Sound and the Bronx River Estuary for nutrient extraction. Mar Ecol Prog Ser 531:155–166
Kittiwanich J, Yamamoto T, Kawaguchi O, Madinabeitia I (2016) Assessing responses of the Hiroshima Bay ecosystem to increasing or decreasing phosphorus and nitrogen inputs. Mar Pollut Bull 102:256–264
Lamb AL, Kim JK, Yarish C, Branco BF (2018) Identification of the bloom forming Ulva and macroalgal assemblage in Jamaica Bay, New York, USA. Rhodora 120:269–299
Latimer JS, Tedesco M, Swanson RL, Yarish C, Stacey SP, Garza C (eds) (2014) Long Island sound: prospects for the urban sea. Springer, New York, 558 p
Lee JE, Kang JW (2020) The interactive effects of elevated temperature and nutrients concentrations on the physiological responses of Ulva linza Linnaeus (Ulvales, Chlorophyta). J Appl Phycol 32:2459–2467
Li SX, Yu KF, Huo YZ, Zhang JH, Cai WuHL, Ce LYY, Shi DJ, He PM (2016) Effects of nitrogen and phosphorus enrichment on growth and photosynthetic assimilation of carbon in a green tide-forming species (Ulva prolifera) in the Yellow Sea. Hydrobiologia 776:161–171
Li S, Wang P, Zhang C, Zhou X, Yin Z, Hu TY, Hu D, Liu CC, Zhu LD (2020) Influence of polystyrene microplastics on the growth, photosynthetic efficiency and aggregation of freshwater microalgae Chlamydomonas reinhardtii. Sci Total Environ 714:136767
Lohman K, Priscu JC (1992) Physiological indicators of nutrients deficiency in Cladophora (Chlorophyta) in the Clark Fork of the the Columbia river, Montata. J Phycol 28:443–448
Lüning K (1990) Seaweeds: their environment, biogeography, and ecophysiology. John Wiley & Sons Inc., New York
Luo MB, Liu F, Xu ZL (2012) Growth and nutrients uptake capacity of two co-occurring species, Ulva prolifera and Ulva linza. Aquat Bot 100:18–24
Ma C, Qin S, Cui HL, Liu ZY, Zhuang LC, Wang Y, Zhong ZH (2021) Nitrogen enrichment mediates the effects of high temperature on the growth, photosynthesis, and biochemical constituents of Gracilaria blodgettii and Gracilaria lemaneiformis. Environ Sci Pollut Res 28:21256–21265
Martínez B, Arenas F, Rubal M, Burgués S, Esteban R, García-Plazaola I, Figueroa F, Pereira R, Saldaña L, Sousa-Pinto I (2012) Physical factors driving intertidal macroalgae distribution: physiological stress of a dominant fucoid at its southern limit. Oecologia 170:341–353
Martins NT (2016) Physiological responses of Ulva fasciata Delile (Ulvales, Chlorophyta): comparison of two populations from thermally distinct sites from Brazilian coast. MSc thesis, Universidade de São Paulo
Mawi S, Krishnan S, Din MF, Arumugam N, Chelliapan S (2020) Bioremediation potential of macroalgae Gracilaria edulis and Gracilaria changii co-cultured with shrimp wastewater in an outdoor water recirculation system. Environ Technol Innovat 17:100571
Nelson TA, Haberlin K, Nelson AV, Ribarich H, Hotchkiss R, Van Alstyne KL, Buckingham L, Simunds DJ, Fredrickson K (2008) Ecological and physiological controls of species composition in green macroalgal blooms. Ecol 89:1287–1298
Ober GT, Thornber CS (2017) Divergent responses in growth and nutritional quality of coastal macroalgae to the combination of increased pCO2 and nutrients. Mar Environ Res 131:69–79
Ott FD (1965) Synthetic media and techniques for the xenic cultivation of marine algae and flagellate. Virg J Sci 16:205–218
Park JS, Shin SK, Wu HL, Yarish C, Yoo HI, Kim JK (2021) Evaluation of nutrient bioextraction by seaweed and shellfish aquaculture in Korea. J World Aquacult Soc 52:1118–1134
Polo LK, Felix MR, Kreusch M, Pereira DT, Costa GB, Simioni C, de Paula MR, Latini A, Floh ES, Chow F (2015) Metabolic profile of the brown macroalga Sargassum cymosum (Phaeophyceae, Fucales) under laboratory UV radiation and salinity conditions. J Appl Phycol 27:887–899
Poloczanska ES, Brown CJ, Sydeman WJ, Kiessling W, Schoeman DS, Moore PJ, Brander K, Bruno JF, Buckley LB, Burrows MT, Duarte CM, Halpern BS, Holding J, Kappel CV, O’Connor MI, Pandolfi JM, Parmesan C, Schwing F, Thompson SA, Richardson AJ (2013) Global imprint of climate change on marine life. Nat Clim Change 3:919–925
Russell G, Bolton JJ (1975) Euryhaline ecotypes of Ectocarpus siliculosus (Dillw.) Lyngb. Estuar Coast Mar Sci 3:91–94
Reidenbach LB, Fernandez PA, Leal PP, Noisette F, McGraw CM, Revill AT, Hurd CL, Kübler JE (2017) Growth, ammonium metabolism, and photosynthetic properties of Ulva australis (Chlorophyta) under decreasing pH and ammonium enrichment. PLoS One 12:e0188389
Ribeiro ALN, Tesima KE, Souza J, Yokoya NS (2013) Effects of nitrogen and phosphorus availabilities on growth, pigment, and protein contents in Hypnea cervicornis J. Agardh (Gigartinales, Rhodophyta). J Appl Phycol 25:1151–1157
Saldarriaga-Hernandez S, Hernandez-Vargas G, Iqbal HMN, Barcelo D, Parra-Saldivar R (2020) Bioremediation potential of Sargassum sp. biomass to tackle pollution in coastal ecosystems: Circular economy approach. Sci Total Environ 715:136978
Salo T, Pedersen MF, Boström C (2014) Population specific salinity tolerance in eelgrass (Zostera marina). J Exp Mar Biol Ecol 461:425–429
Samanta P, Shin SK, Jang SJ, Kim JK (2019a) Comparative assessment of salinity tolerance based on physiological and biochemical performances in Ulva australis and Pyropia yezoensis. Algal Res 42:101590
Samanta P, Shin SK, Jang SJ, Song YC, Oh S, Kim JK (2019b) Stable carbon and nitrogen isotopic characterization and tracing nutrient sources of Ulva blooms around Jeju coastal areas. Environ Pollut 254:113033
Stengel D, Conde-Álvarez R, Connan S, Nitschke U, Arenas F, Abreu H, Barufi JB, Chow F, Robledo D, Malta E (2014) Short-term effects of CO2, nutrients and temperature on three marine macroalgae under solar radiation. Aquat Biol 22:159–176
Stocker TF, Qin D, Plattner G-K, Tignor MM, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (2014) Climate change 2013: The physical science basis. In: Contribution of Working Group I to the Fifth Assessment Report of IPCC the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 1535
Taylor R, Fletcher R, Raven J (2001) Preliminary studies on the growth of selected ‘green tide’algae in laboratory culture: effects of irradiance, temperature, salinity and nutrients on growth rate. Aquat Biol 22:159–176
Wang CY, Su RG, Guo LD, Yang B, Zhang Y, Zhang L, Xu H, Shi WJ, Wei LS (2019) Nutrient absorption by Ulva prolifera and the growth mechanism leading to green-tides. Estuar Coast Shelf Sci 227:106329
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Wernberg T, Bennett S, Babcock RC, de Bettignies T, Cure K, Depczynski M, Dufois F, Fromont J, Fulton CJ, Hovey RK, Harvey ES, Holmes TH, Kendrick GA, Radford B, Santana-Garcon J, Saunders BJ, Smale DA, Thomsen MS, Tuckett CA, Tuya F, Vanderklift MA, Wilson S (2016) Climate-driven regime shift of a temperate marine ecosystem. Science 353:169–172
Wiens JJ (2016) Climate-related local extinctions are already widespread among plant and animal species. PLoS Biol 14:e2001104
Wu HL, Huo YZ, Zhang JH, Liu YY, Zhang YT, He PM (2015) Bioremediation efficiency of the largest scale artificial Porphyra yezoensis cultivation in the open sea in China. Mar Pollut Bull 95:289–296
Wu HL, Kim JK, Huo YZ, Zhang JH, He PM (2017) Nutrient removal ability of seaweeds on Pyropia yezoensis aquaculture rafts in China’s radial sandbanks. Aquat Bot 137:72–79
Wu HL, Huo YZ, Yarish C, Kim JK He PM (2018a) Bioremediation and nutrients migration during blooms of Ulva the Yellow Sea, China. Phycologia 57:223-231
Wu HL, Shin SK, Jang SJ, Yarish C, Kim JK (2018b) Growth and nutrient bioextraction of Gracilaria chorda, G. vermiculophylla, Ulva prolifera, and U. compressa under hypo- and hyper-osmotic conditions. Algae 33:329–340
Xing QK, Han S, Park JS, Yarish C Kim JK (2023) Comparative transcriptome analysis reveals the molecular mechanism of heat-tolerance in Neopyropia yezoensis induced by Sargassum horneri extract. Front Mar Sci 10:1142483
Xu Q, Zhang H, Ju L, Chen M (2014) Interannual variability of Ulva prolifera blooms in the Yellow Sea. Int J Remote Sens 35:4099–4113
Ye NH, Zhang XW, Mao YZ, Liang CW, Xu D, Zou J, Zhuang ZM, Wang QY (2011) ‘Green tides’ are overwhelming the coastline of our blue planet: Taking the world’s largest example. Ecol Res 26:477–485
Yu Z, Zhu X, Jiang Y, Luo P, Hu C (2014) Bioremediation and fodder potentials of two Sargassum spp. in coastal waters of Shenzhen South China. Mar Pollut Bull 85:797–802
Zhang JH, Huo YZ, Yu KF, Chen QF, He Q, Han W, Chen LP, Cao JC, Shi DJ, He PM (2013a) Growth characteristics and reproductive capability of green tide algae in Rudong coast, China. J Appl Phycol 25:795–803
Zhang JH, Huo YZ, Zhang ZL, Yu KF, He Q, Zhang LH, Yang LL, Xu R, He PM (2013b) Variations of morphology and photosynthetic performances of Ulva prolifera during the whole green tide blooming process in the Yellow Sea. Mar Environ Res 92:35–42
Zou DH, Gao KS (2014) The photosynthetic and respiratory responses to temperature and nitrogen supply in the marine green macroalga Ulva conglobata (Chlorophyta). Phycologia 53:86–94
Funding
This study was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1A6A1A06015181) and by the Ministry of Science and ICT (2022R1A2C1011394), and funded by the Ministry of Oceans and Fisheries of Korea (Project No. 20190518).
Author information
Authors and Affiliations
Contributions
MB and JK designed the experiment.
MB and JP conducted the experiment.
MB and QX analyzed the data and wrote the first draft of manuscript.
JK provided fund for the experiment and supervised the experiment.
All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest associated with the material presented in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bao, M., Xing, Q., Park, JS. et al. Population specific responses to temperature and nutrients in the bloom forming Ulva prolifera. J Appl Phycol 36, 459–470 (2024). https://doi.org/10.1007/s10811-023-03143-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03143-4