Abstract
The responses of Ulva species to diurnal temperature difference remain poorly understood. In this present study, we cultured Ulva prolifera under different diurnal temperature treatments with 22°C for photoperiod and 22, 20, 18, 16, 14, and12°C for dark period, respectively (noted as 22-22, 22-20, 22-18, 22-16, 22-14, and 22-12°C treatments). The growth, pigment contents, photosynthesis, superoxide dismutase (SOD) activity, soluble proteins, and sugars were measured. The main results were shown as follows: (1) The growth of U. prolifera was enhanced by the moderate diurnal temperature difference, and the highest growth rate was observed at 22-18°C. (2) Compared with 22-22°C treatment, the thalli grown under 22-18°C condition showed lower chlorophyll a (Chla) content, respiration rate (Rd), the ratio of Rd, and gross photosynthetic rate (Rd/Pg) as well as the net photosynthetic rate (Pn), while the lowest Pn was observed at 22-12°C. (3) The maximum quantum yield (Fv/Fm) was enhanced by diurnal temperature difference, while the effective quantum yield (Fv′/Fm′) decreased with the decreased in temperature in the nighttime. (4) With the increase of the diurnal temperature difference gradient, the SOD activity decreased and then increased, with the lowest value observed at 22-18 °C, and the soluble protein content showed similar trend. Then we cultured this species at 22-22°C and 22-16°C both under 250 and 60 μmol m−2s−1 conditions in order to study the combined effects of diurnal temperature change and light intensities. It was found that under both two light levels, 22-16°C-grown thalli showed higher growth rate, while the SOD activity was lower than that grown under 22-22°C condition. Overall, the suitable range of diurnal temperature difference for the growth of U. prolifera was about 4–6°C and also was mediated by light intensity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Green tides, because of the excessive grown of green seaweed and dominated by Ulva species, are becoming a worldwide environmental problem and have caused serious impact on the environment, tourism, and economy (Guo et al. 2021; Smetacek and Zingone 2013). Until 2021, the size and biomass of green tide in Yellow Sea in China is unexpectedly massive (Song et al. 2022). It is well known that the suitable temperature, light, nutrition, and wind promote the formation and flotation of the green tides (Liu et al. 2013; Wu et al. 2022a). In fact, we should notice that the harvest of the Ulva at the suitable time can provide the valuable ecosystem service for their quick absorption of nitrogen and phosphorus, as well as their important role in the carbon neutrality (Wu et al. 2018; Gao et al. 2022). Compared with non-green tides Ulva strains, green tides strains show higher protein content (Fort et al. 2020) and they are rich in fatty acids (McCauley et al. 2016), then they can be used as feedstock in food, medicine, and biorefining industries (Calheiros et al. 2021), and Ulva also have aquaculture potential (Jaiswar et al. 2022). Additionally, Ulva can be used as a model organism in the green seaweed research for system biology (Blomme et al. 2023).
Due to the celebrity effects of Ulva species during green tides, there are too many studies about how Ulva respond to environmental changes, such as temperature, light intensity, salinity, and nutrient by using physiological and multi-omic methods (Cui et al. 2015; Feng et al. 2021; Huo et al. 2021; Wang et al. 2018; Zanolla et al. 2019; Zhao et al. 2023ab). Although there are species-specific acclimatization capacities of macroalge, Ulva can grow under a wide temperature range from 5 to 35°C (Cui et al. 2015; Wang et al. 2018), a large light levels range from 10 to 500 μmol m−2s−1 and have high tolerance to low nutrient concentration (Luo et al. 2012). The spore induction in U. lactuca was also affected by temperature and photoperiod (Jaiswar et al. 2022). In order to adapt the hypersalinity and excess light, Ulva fasciata can modify the gene expression of carotene biosynthesis-related protein (Hsu and Lee 2012). In U. prolifera, the carotenoid biosynthesis is modulated by R2R3-MYB transcription factor MYB44 (He et al. 2022). Additionally, different day-aged U. prolifera gametophytes showed varying growth rates and physiological characteristics (Zhao et al. 2023b). Among these environmental factors, the effects of temperature were mostly important (Zheng et al. 2022). When U. prolifera were exposed to 35°C for 3 h and 30°C for 12 h, the abscisic acid signal transduction pathway was upregulated (Fan et al. 2018; Zhao et al. 2023a), and the role of Ca2+-related channels was not to be ignored when they respond to high temperature (Fan et al. 2022). When the thalli grow under low temperature, vegetative growth dominated, while when they were cultivated under high temperature, they entered reproductive growth sooner (Feng et al. 2021). Actually, during the outbreak of the green tide, the gradient of the sea surface temperature (SST) showed regional and time difference (Keesing et al. 2011). Considering temperature dependence in the growth, photosynthesis, glucose, and amino acid metabolism of Ulva species (He et al. 2018; Zhao et al. 2023a), as well as the light and dark period difference in the regulation of the key antioxidant enzymes activity and content (Poor et al. 2018), whether the changes in temperature gradient affect the outbreak of green tide needs to be studied.
Previous studies showed that the growth and soluble carbohydrate content of Pyropia haitanensis were increased by diurnal temperature variation (Chen et al. 2019), as well as the fluctuation temperatures (Wu et al. 2022b). In fact, Ulva species showed special diurnal growth patterns with biomass accumulation observed during night period (Fort et al. 2019); lower temperature in nighttime enhanced the growth rate of U. prolifera in the daytime (Li et al. 2019). The latest research showed that the growth rate of U. prolifera was significantly improved by the suitable temperature difference between day and night (4°C), especially under eutrophication conditions (Chen et al. 2023). Additionally, a more rapid temperature increase in the nighttime than that in daytime was observed (Sillmann et al. 2013). The knowledge gap is that how the bloom forming Ulva species respond to diurnal temperature difference? Is there an optimal range of temperature difference between day and night for the growth of U. prolifera? So, in this present study, we set a certain diurnal temperature difference conditions based on the trend of SST changes in the Yellow Sea and the East China Sea, and cultured U. prolifera under these temperature conditions. The growth, respiration and photosynthesis, as well as the cellular component were determined and analyzed, with the aim to investigate how diurnal temperature difference impacts the growth of this species and find the suitable temperature ranges for the growth of this species.
Materials and methods
Materials and pre-treatment
U. prolifera thalli were provided by Xiangshan Xuwen Seaweed Development Co., Ltd. (Xiangshan, Ningbo, China) and were aerated and cultured in glass triangular bottles (500 mL) with artificial seawater (salinity 25) enriched with f/2 medium at 100 μmol m−2s−1 (12L:12D) and 22°C. A “germing cluster” method (Hiraoka and Oka 2008) was used to culture this species in laboratory for almost 5 years. Before the experiment, the “germing cluster” was cultured under above conditions, the seawater medium with f/2 medium was aerated vigorously (600 mL min−1) and was changed every other day. When the length of the thalli reached 3–5 cm, they were used in the following experiment. The changes of the length of thalli during the culture period are shown in Fig. S1.
Experimental setup
The suitable temperature for the growth of U. prolifera was about 15–25°C (Cui et al. 2015; Wang et al. 2018), the seawater temperature of Xiangshan bay was about 22°C when the U. prolifera grown quickly in the field, and this species was cultured in laboratory at 22°C for almost 5 years, we also did the pre-experiment and found that there were no significant difference between 22 and 18°C on the growth of this species, so the daytime temperature was set as 22°C, while the nighttime temperature was set as 22, 20, 18, 16, 14, and 12°C, respectively, so the treatments were noted as 22-22°C, 22-20°C, 22-18°C, 22-16°C, 2214°C, and 22-12°C. Other conditions were kept same as above.
U. prolifera thalli (3–5 cm length of the thalli; 0.625g FW L−1) were cultured under the different temperature treatments. A previous study showed that the growth of macroalgae was affected by culture density (Jiang et al. 2019), so during the experiment, the medium was changed every two days and the new biomass was removed at the same time in order to avoid the effect of algal density (Xu and Gao 2012; Zheng et al. 2019). Each treatment was set up in 4 replicates, and the other parameters, including pigment content, respiration and photosynthetic rate, chlorophyll fluorescence parameters, superoxide dismutase (SOD) activity, and cellular component, were determined when they acclimated to the different temperature treatments.
Additionally, during the adaptation of the Ulva spp., the light intensity of the sea surface was about 30–300 μmol m−1s−1 on cloudy day and more than 300 μmol m−1s−1 on sunny days (Wang et al. 2018). So, in order to study the combined effects of diurnal temperature difference and light intensity, we cultured this species under 22-22 °C and 22-16 °C under 60 (low light level) and 250 μmol m−2s−1 (high light level) for 3 days. The growth rate, Chla content, and SOD activity were measured.
Determination of relative growth rate
The fresh weights were measured every two days when the culture medium was renewed. The biomass accumulation rates (RGR) were calculated by using the following formula: RGR (% d−1) = ln (Wt1/Wt0)/(t1−t0)×100, where Wt0 and Wt1 are the fresh weight (g) of the thalli at t0 and t1 days, respectively. The data of the RGR was reported as the after they grown under different treatments for 5 days.
Extraction and determination of pigment content
Certain fresh algae (about 0.008–0.010 g for all the treatments) was weighed and placed in a test tube; then 3 mL methanol (100%) was added to extract at 4°C overnight in the dark condition. The absorbance of the extraction solution was measured by using UV spectrophotometer (METASH, Shanghai Yuanxi instrument Co., Ltd.); then according to Wellburn (1994), the chlorophyll a (Chla), chlorophyll b (Chlb), and carotenoid (Car) content were calculated. The units of Chla, Chlb, and Car are presented as mg g−1 Fw.
Determination of respiration and photosynthetic rate
At the mid-photoperiod, a Clark-type oxygen electrode (Hansatech, UK) was used to determine the net photosynthetic rates under different light intensity (P-I curves) of U. prolifera grown under different treatments. In details, about 0.01 g thalli (fresh weight) was transferred to the chamber containing fresh medium enriched with f/2 medium (2 mL). The temperature was controlled at 22°C and the light intensity was set as 0, 50, 80, 400, and 680 μmol m−2 s−1. The formula Pn = Pmax × tanh (α×I/Pmax) + Rd was used to fitted the P-I curves (Jasby and Platt, 1976), where Pmax is the maximum photosynthetic rate, α is the light energy utilization efficiency, and Rd is the dark respiration rate. Then, according to Henley (1993), the saturating (Ik; μmol m−2s−1) and compensation (Ic; μmol m−2s−1) light intensity for photosynthesis as well as the total photosynthetic rate (Pg) and the ratio between the total photosynthetic rate and dark respiration rate (Pg/Rd) were calculated based on the Pmax, Rd, and α.
Determination of chlorophyll fluorescence parameters
A PSI fluorometer (AquaPen-C, Photon System Instruments, Czech Republic) was used to determine the Fv/Fm, Fv′/Fm′, and the rapid light curves (RLC) for the electron transport rate (rETR). Fv/Fm was determined after the thalli acclimated to dark conditions for 15 min. Fv′/Fm′ was measured with the actinic light as 100 μmol m−2s−1 (growth light level). The RLCs were fitted as follows: rETR = I/(a × I2 + b × I + c), where I is the photon flux density of activity light (μmol m−2s−1) and a, b, and c are the adjustment parameters (Eilers and Peeters, 1988). Then the maximum relative electron transfer rate (rETRmax), the initial light saturation point (Ek), and the surface utilization efficiency (α) were calculated by using the formula: rETRmax = 1/[b + 2 (ac) 1/2]; Ek = rETRmax/α; α = 1/c.
Determination of the SOD activity, soluble protein, and soluble sugar content
About 0.1g fresh thalli were ground to homogenate with 2.0 mL phosphate buffer solution (PBS, 0.05 mol L−1, pH = 7.8) under the ice bath condition, the homogenate was centrifuged for 20 min at 4 °C (10,000g min−1). The supernatant was used for the SOD activity, soluble protein, and soluble sugar determination.
A nitrogen blue tetrazolium photoreduction method was used to determine the SOD activity (Giannopolitis and Ries 1977; Li et al. 2017). Soluble protein and sugar were determined by Coomassie brilliant blue G-250 method (Bradford 1976) and anthrone-sulfuric acid colorimetry (Li et al. 2017), respectively.
Data analysis
The statistical analysis in this experiment was performed using the software SPSS18.0. One-way ANOVA was used to analyze the significance of the difference between different day and night temperature gradients. Two-way ANOVA and Tukey’s post hoc test were used to analyze the interactive effects of diurnal temperature variation and light intensity. We set 0.05 as the significant level.
Results
Effects of diurnal temperature variation on the growth and pigment content
Compared with 22-22°C, the growth rate of U. prolifera was increased by the moderate diurnal temperature difference between day and nighttime, while when the nighttime temperature decreased to 14 and 12°C, the growth rate significantly decreased, with the highest growth rate observed at 22-18°C treatment (Fig. 1A). During the culture period, the length of the thalli also was affected by the diurnal temperature difference, with the longest thalli observed at 22-18°C (Fig. S1).
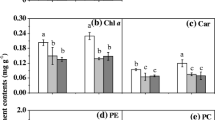
As for Chla content, the lowest value was observed at 22-18°C treatment, while there were no significant differences among other temperature treatments (Fig. 1B). Compared to 22-22°C treatment, the ratio of Chla and Chlb content was increased by the diurnal temperature difference, and the higher value was observed at 22-18°C, while the ratio of Chla and Car, as well as the ratio of Chlb and Car, was decreased by the diurnal temperature difference (Table 1).
Different letters indicate significant differences among different treatments at P<0.05
Effects of diurnal temperature variation on the photosynthesis, respiration, and chlorophyll fluorescence
The effects of diurnal temperature difference on the net photosynthetic rate (Pn) and rETR of U. prolifera are shown in Fig. 2. In details, thalli grown under 22-16°C and 22-14°C condition showed higher Pn, especially under higher light conditions when the PAR was larger than 200 μmol m−2 s−1, while the 22-12°C-grown thalli showed lowest value, but no significant differences among other three temperature conditions were observed (Fig. 2A). For all the temperature treatments, 22-12°C-grown thalli showed lowest rETRmax and Ik, while there were no significant differences among other temperature treatments (Fig. 2B, Table 2).
Compared with 22-22°C treatment, the lowest dark respiration rate was observed at 22-18°C treatment, and lower Rd/Pg value was observed at both 22-18°C and 22-16°C conditions (Fig. 3A, B). But 22-22°C-grown thalli showed lower Fv/Fm, and for all the temperature treatments, higher Fv′/Fm′ was observed at 22-18°C condition (Fig. 3C, D).
Effect of diurnal temperature variation on the SOD activity, soluble sugar, and protein content
Under 22-22°C condition, SOD activity of thalli was about 325 U g−1 Fw. With the decrease of the temperature in the night period, SOD activity decreased firstly then increased, with the lowest value (105 U g−1 Fw) was observed at 22-18°C treatment (Fig. 4).
The soluble protein content of U. prolifera under different temperature conditions showed similar trend as SOD activity, but there was no significant difference among 22-18, 22-16, and 22-14°C treatments (Fig. 5A). Additionally, there were no significant effects of diurnal temperature difference on the soluble sugar content except for 22-16°C treatment (Fig. 5B).
Combined effects of diurnal temperature difference and light level on the growth, Chla content, and SOD activity
For the growth rate, the effects of separate temperature changes (P = 0.000) and light intensity were significant (P = 0.003), while the interactive between these two factors were insignificant (P = 0.393). Compared with low light intensity, the growth was significantly increased from 25–32% d−1 to 61–72 d−1. Compared with 22-22°C, the growth of U. prolifera under low light intensity was enhanced by 28% by 22-16°C, but the increase was insignificant (P = 0.160), while under high light condition, the growth rate was significantly enhanced by 18% by 22-16°C (P= 0.016) (Fig. 6A). High light significantly decreased the Chla content under both temperature conditions, and the Chla content was enhanced by the diurnal temperature difference under low light intensity but not for high light treatment, while no significantly interactive effects between light and temperature were observed (P = 0.218) on the Chla content (Fig. 6B).
The relative growth rate (A) and SOD activity (C) of U. prolifera grown under different light and temperature conditions. The different lowercase letters indicate significant differences between 22-22°C and 22-16°C at low or high light, respectively, while the different capital letters indicate significant differences between low and high light under 22-22°C and 22-16°C, respectively, at P<0.05.
For SOD activity, the effects of separate temperature changes (P = 0.000), light intensity (P = 0.000), and the interactive between these two factors were significant (P = 0.005). Under low light intensity, the SOD activity was about 365 U g−1 Fw; then under high light intensity, the thalli grown under 22-16°C condition showed the lowest SOD activity (65 U g−1 Fw) (Fig. 6C).
Discussion
Both the growth and photosynthesis of macroalgae are affected by temperature, Ulva species had a large tolerance temperature range, not only for the biomass but also for the morphological characters (Cui et al. 2015; Gao et al. 2016; Feng et al. 2021; Huo et al. 2021; Wang et al. 2018; Wu et al. 2022a). Previous studies showed that the suitable temperature for the growth of U. prolifera was about 15–25°C (Cui et al. 2015); especially, the 20°C was best (Wang et al. 2018; Wu et al. 2022a), but another study reported that the highest growth rate of this species was observed at 15°C (Feng et al. 2021). In fact, the physiological responses of macroalgae to chronic and acute temperature stress were affected by their acclimation history of temperature (Page et al. 2021), length of the experiment period (Zanolla et al. 2019; Feng et al. 2021), culture density (Jiang et al. 2017), light levels (Huo et al. 2021; Jiang et al. 2020; Wu et al. 2022a), and the nutrient conditions (Bao et al. 2023; Jiang et al. 2022). Low temperature history can induce the over-compensatory growth of U. prolifera when they were transferred to optimal growth condition (Yu et al. 2022). The fluctuating temperatures increased from 20°C to 24°C then decreased to 20°C at the rate of 1°C per day enhanced the growth of Pyropia haitanensis (Wu et al. 2022a). These studies indicated the importance of the temperature change pattern during the study of impacts of temperature. So, in this experiment, the effects of diurnal temperature difference that simulate natural rhythms on the photosynthetic physiological response of U. prolifera were studied. Because this species was cultured at 22°C for almost 5 years by using the “germing cluster” method, 22°C was set as daytime temperature. Considering the suitable temperature range for the growth of this species was 15-25°C (Cui et al. 2015; Wang et al. 2018), both 22 and 18°C were within the suitable temperature range. Then combined the results of the pre-experiment—the thalli grown at 22°C and 18°C under constant temperature conditions showed similar growth rate (our unpublished data, or see Cui et al. 2015); the results of this study confirmed that a suitable temperature difference between photo-period and dark-period can promote the growth of this species, and this enhancement was affected by light intensity.
It is well known that the thermal photosynthetic plasticity can help Ulva survive and reproduce in marine coastal ecosystem (Zanolla et al. 2019). The results of this study also revealed a noticeable tolerance and adaptive capacity of U. prolifera to diurnal temperature difference, with the daily growth rate higher than 25% even when they grow under up to 10°C temperature difference between day and night condition.
Apart from the biomass, the length and numbers of the branches of Ulva was also affected by the temperature. In details, compared with 25°C, more branches were induced by the 20°C (Gao et al. 2016); however, in another study, more branches were observed in extreme temperature conditions (Wu et al. 2022a). In this study, we took “germing cluster” as materials which also was used in tank cultivation of Ulva (Hiraoka and Oka 2008). So, we did not pay more attention on the numbers of branches and only measured the length of the “germing cluster,” with the longest observed at 22-18°C (Fig. S1). This was consistent with the trend of biomass (fresh weight) under different temperature conditions.
For macroalgae, the enhanced growth by diurnal temperature variation also was observed in Pyropia haitanensis, and the nitrate reductase activity of P. haitanensis was also enhanced by diurnal temperature difference (Chen et al. 2019). Similarly, when the temperature was higher than 15°C, there were a positive correlation between the growth of U. prolifera and nitrate reductase activity (Feng et al. 2021). The activity of membrane transporters, enzyme activity, and the rate of diffusion of nutrients were also affected by temperature (Roleda and Hurd 2019). Additionally, for U. prolifera, high growth rate during the night was observed (Fort et al. 2019; Li et al. 2019), which was positively with nitrates accumulated during the night (Fort et al. 2019). In fact, the enhanced growth of U. prolifera was induced by the 4°C; temperature difference between day and night was affected by nutrient conditions (Chen et al. 2023). Additionally, the content of soluble protein was decreased then increased when the temperature in night period decreased from 22 to 14°C. This phenomenon could be explained by the temperature dependence of the relative enzymes; there is a possibility that low temperature decreased the synthesis and activity of the relative enzymes (Li et al. 2019; Eggert 2012). On the other hand, soluble proteins are important osmotic regulators, and play a protective role in the vital substances and biofilms of cells. So, with the decreased of the temperature in night period, the soluble protein content increased in order to keep the thalli from the extreme difference in temperature between day and night. This tendency was consistent with changes of dark respiration and SOD activity.
Low temperature reduced the dark respiration (Bao et al. 2023), leading lower photosynthate consumption during night for these suitable diurnal temperature range treatments. Indeed, the lower dark respiration rate also was observed under 22-18°C treatment. But we should notice that compared with the P. haitanensis grown under constant temperature condition (19°C), the dark respiration rate for that grown under 21°C and 17°C for day and night period, respectively, was enhanced (Chen et al. 2019). On the one hand, these seemingly contradictory results indicated the species-specific responses to diurnal temperature changes. On the other hand, the higher dark respiration rate can explain why the growth rates of U. prolifera grown under large diurnal temperature range, such as 22-14°C and 22-12°C treatments, were decreased. This was also observed at Wu et al. (2022a), although the way of the temperature changes is not exactly the same. Additionally, compare with 22-18°C treatment, thalli grown under 22-14°C and 22-12°C treatments showed higher SOD activity, indicating cold stress occurred in night, but there have the different explanation for 22-14°C and 22-12°C treatments. For 22-14°C-grown thalli, the photosynthesis, dark respiration and SOD activity were enhanced, but when the thalli grown under 22-12°C, the photosynthesis and dark respiration were inhibited, while the SOD activity and soluble protein content increased, indicating the cold stress occurred during the night period. Respiration includes the growth respiration and maintenance respiration (Amthor et al. 2019). Under large diurnal temperature range condition, trade-offs with growth and maintenance in energy budgets need to take place.
The most important finding of this study was that the growth of U. prolifera was enhanced by suitable diurnal temperature difference, with the highest growth rate observed at 22°C and 18°C for day and night periods, respectively, but the growth was inhibited by the large diurnal temperature difference. These findings indicated that the seasonal variations in day and night temperature differences can not be ignored in the study of the outbreak of green tide dominated by U. prolifera and shed the new light to study the adaptation of Ulva species to climate changes and the land-based farming of economic macroalgae in the future.
References
Amthor JS, Bar-Even A, Hanson AD, Millar AH, Stitt M, Sweetlove LJ, Tyerman SD (2019) Engineering strategies to boost crop productivity by cutting respiratory carbon loss. Plant Cell 31:297–314
Bao ML, Xing QK, Park JS, He PM, Zhang JH, Yarish C, Kim JK (2023) Temperature and high nutrients enhance hypo-salinity tolerance of the bloom forming green alga, Ulva prolifera. Harmful Algae 123:102402–102402
Blomme J, Wichard T, Jacobs TB, De Clerck O (2023) Ulva: an emerging green seaweed model for systems biology. J Phycol 59:433–440
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Calheiros AC, Sales LPM, Pereira Netto AD, Cavalcanti DN, Castelar B, Reis RP (2021) Commercial raw materials from algaculture and natural stocks of Ulva Spp. J Appl Phycol 33:1805–1818
Chen BB, Xia JR, Zou DH, Zhang X (2019) Responses to ocean acidification and diurnal temperature variation in a commercially farmed seaweed, Pyropia haitanensis (Rhodophyta). Eur J Phycol 54:184–192
Chen YL, Zheng MS, Jiang JN, Hu W, Xu NJ, Li YH (2023) Enhancement of growth in Ulva prolifera by diurnal temperature difference combined with nitrogen enrichment. Mar Environ Res 186:105905
Cui JJ, Zhang JH, Huo YZ, Zhou LJ, Wu Q, Chen LP, Yu KF, He PM (2015) Adaptability of free-floating green tide algae in the Yellow Sea to variable temperature and light intensity. Mar Pollut Bull 101:660–666
Fan MH, Sun X, Liao Z, Wang JX, Li YH, Xu NJ (2018) Comparative proteomic analysis of Ulva prolifera response to high temperature stress. Proteome Sci 16:17
Eggert A (2012) Seaweed responses to temperature. In: Wiencke C, Bischof K (eds) Seaweed Biology. Springer-Verlag, Berlin, Germany, pp 47–66
Fan MH, Tang XW, Yang ZX, Wang JX, Zhang XL, Yan XJ, Li P, Xu NJ, Liao Z (2022) Integration of the transcriptome and proteome provides insights into the mechanism calcium regulated of Ulva prolifera in response to high-temperature stress. Aquaculture 557:738344
Feng LN, Shi XY, Chen YH, Tang HJ, Wang LS (2021) Effects of temperature on the nitrate reductase activity and growth of Ulva prolifera. J Phycol 57:955–966
Fort A, Lebrault M, Allaire M, Esteves-Ferreira AA, McHale M, Lopez F, Farinas-Franco JM, Alseekh S, Fernie AR, Sulpice R (2019) Extensive variations in diurnal growth patterns and metabolism among Ulva spp. strains. Plant Physiol 180:109–123
Fort A, Mannion C, Farinas-Franco JM, Sulpice R (2020) Green tides select for fast expanding Ulva strains. Sci Total Environ 698:134337
Gao G, Gao L, Jiang MJ, Jian A, He LW (2022) The potential of seaweed cultivation to achieve carbon neutrality and mitigate deoxygenation and eutrophication. Environ Res Lett 17:014018
Gao G, Zhong ZH, Zhou XH, Xu JT (2016) Changes in morphological plasticity of Ulva prolifera under different environmental conditions: a laboratory experiment. Harmful Algae 59:51–58
Giannopolitis CN, Ries SK (1977) Superoxide dismutases, 1: occurrence in higher plants [Corn, oats, peas]. Plant Physiol 59:309–314
Guo XN, Zhu AN, Chen RS (2021) China’s algal bloom suffocates marine life. Science 373:751
He YL, Hu CY, Wang YH, Cui DD, Sun X, Li YH, Xu NJ (2018) The metabolic survival strategy of marine macroalga Ulva prolifera under temperature stress. J Appl Phycol 30:3611–3621
He Y, Li MR, Wang YH, Shen SD (2022) The R2R3-MYB transcription factor MYB44 modulates carotenoid biosynthesis in Ulva prolifera. Algal Res 62:102578
Hiraoka M, Oka N (2008) Tank cultivation of Ulva prolifera in deep seawater using a new “germling cluster” method. J Appl Phycol 20:97–102
Hsu Y-T, Lee T-M (2012) Modulation of gene expression of carotene biosynthesis-related protein by photosynthetic electron transport for the acclimation of intertidal macroalga Ulva fasciata to hypersalinity and excess light. Physiol Plant 144:225–237
Huo YZ, Kim JK, Yarish C, Augyte S, He PM (2021) Responses of the germination and growth of Ulva prolifera parthenogametes, the causative species of green tides, to gradients of temperature and light. Aquat Bot 170:103343
Jaiswar S, Mungalapara U, Kazi MA, Balar N (2022) Evidence from preliminary experiments revealed drifted Ulva biomass has seedling and aquaculture potential. Aquac Int 30:2833–2846
Jiang H, Gong JY, Lou WY, Zou DH (2019) Photosynthetic behaviors in response to intertidal zone and algal mat density in Ulva lactuca (Chlorophyta) along the coast of Nan’ao Island, Shantou, China. Environ Sci Pollut Res 26:13346–13353
Jiang JN, Yu YY, Zheng MS, Liu NN, Li YH, Xu NJ (2020) High light might alleviate inhibitory effects of high temperature on growth and physiological parameters of Ulva prolifera. Aquac Res 51:2062–2070
Jiang MJ, Gao L, Huang RP, Lin X, Gao G (2022) Differential responses of bloom-forming Ulva intestinalis and economically important Gracilariopsis lemaneiformis to marine heatwaves under changing nitrate conditions. Sci Total Environ 840:156591
Keesing JK, Liu D, Fearns P, Garcia R (2011) Inter- and intra-annual patterns of Ulva prolifera green tides in the Yellow Sea during 2007-2009, their origin and relationship to the expansion of coastal seaweed aquaculture in China. Mar Pollut Bull 62:1169–1182
Li YH, Wang D, Xu XT, Gao XX, Sun X, Xu NJ (2017) Physiological responses of a green algae (Ulva prolifera) exposed to simulated acid rain and decreased salinity. Photosynthetica 55:623–629
Li YH, Zheng MS, Lin JJ, Zhou SD, Sun TC, Xu NJ (2019) Darkness and low nighttime temperature modulate the growth and photosynthetic performance of Ulva prolifera under lower salinity. Mar Pollut Bull 146:85–91
Liu DY, Keesing JK, He PM, Wang ZL, Shi YJ, Wang YJ (2013) The world’s largest macroalgal bloom in the Yellow Sea, China: formation and implications. Estuar Coast Shelf Sci 129:2–10
Luo MB, Liu F, Xu ZL (2012) Growth and nutrient uptake capacity of two co-occurring species, Ulva prolifera and Ulva linza. Aquat Bot 100:18–24
McCauley JI, Meyer BJ, Winberg PC, Skropeta D (2016) Parameters affecting the analytical profile of fatty acids in the macroalgal genus Ulva. Food Chem 209:332–340
Page TM, Bergstrom E, Diaz-Pulido G (2021) Acclimation history of elevated temperature reduces the tolerance of coralline algae to additional acute thermal stress. Front Mar Sci 8:660196
Poor P, Ordog A, Czekus Z, Borbely P, Takacs Z, Kovacs J, Tari I (2018) Regulation of the key antioxidant enzymes by developmental processes and environmental stresses in the dark. Biol Plant 62:201–210
Sillmann J, Kharin VV, Zhang X, Zwiers FW, Bronaugh D (2013) Climate extremes indices in the CMIP5 multimodel ensemble: Part 1. Model evaluation in the present climate. J Geophys Res-Atmos 118:1716–1733
Smetacek V, Zingone A (2013) Green and golden seaweed tides on the rise. Nature 504:84–88
Roleda MY, Hurd CL (2019) Seaweed nutrient physiology: application of concepts to aquaculture and bioremediation. Phycologia 58:552–562
Song MJ, Kong FZ, Li YF, Zhao J, Yu RC, Zhou MJ, Jiang P, Yan T (2022) A massive green tide in the Yellow Sea in 2021: field investigation and analysis. Int J Environ Res Public Health 19:11753
Wang SY, Huo YZ, Zhang JH, Cui JJ, Wang Y, Yang LL, Zhou QY, Lu YW, Yu KF, He PM (2018) Variations of dominant free-floating Ulva species in the source area for the world’s largest macroalgal blooms, China: differences of ecological tolerance. Harmful Algae 74:58–66
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology 144:307–313
Wu HL, Chen J, Feng JC, Liu YH, Li XB, Chen R, Xu JT (2022a) Thermal fluctuations and nitrogen enrichment synergistically accelerate biomass yield of Pyropia haitanensis. Aquat Bot 179:103501
Wu HL, Liu YM, Beardall J, Zhong ZH, Gao G, Xu JT (2022b) Physiological acclimation of Ulva prolifera to seasonal environmental factors drives green tides in the Yellow Sea. Mar Environ Res 179:105695
Wu HL, Zhang JH, Yarish C, He PM, Kim JK (2018) Bioremediation and nutrient migration during blooms of Ulva in the Yellow Sea, China. Phycologia 57(2):223–231
Xu JT, Gao KS (2012) Focus issue on the plant physiology of global change: future CO2-induced ocean acidification mediates the physiological performance of a green tide alga. Plant Physiol 160:1762–1769
Yu YY, Lin JJ, Jiang JN, Hu SS, Kang C-K, Xu NJ, Li YH (2022) Environmental history affects the growth and photosynthesis of a green-tide macroalgae Ulva prolifera. Aquac Res 53:2509–2517
Zanolla M, Carmona R, Kawai H, Stengel DB, Altamirano M (2019) Role of thermal photosynthetic plasticity in the dispersal and settlement of two global green tide formers: Ulva pertusa and U. ohnoi. Marine Biology 166:123
Zhao H, Liu XH, Jiang T, Cai C, Gu K, Liu YL, He PM (2023a) Activated abscisic acid pathway and C4 pathway, inhibited cell cycle progression, responses of Ulva prolifera to short term high temperature elucidated by multi-omics. Mar Environ Res 183:105796
Zhao S, Xia ZY, Liu JL, Sun JY, Zhang JH, He PM (2023b) Morphology, growth, and photosynthesis of Ulva prolifera OF Müller (Chlorophyta, Ulvophyceae) gametophytes, the dominant green tide species in the Southern Yellow Sea. J Sea Res 193:102375
Zheng MS, Lin JJ, Zhou SD, Zhong JL, Li YH, Xu NJ (2019) Salinity mediates the effects of nitrogen enrichment on the growth, photosynthesis, and biochemical composition of Ulva prolifera. Environ Sci Pollut Res 26:19982–19990
Zheng LX, Wu MQ, Cui YT, Tian L, Yang PS, Zhao LJ, Xue MY, Liu JY (2022) What causes the great green tide disaster in the South Yellow Sea of China in 2021? Ecol Indic 140:108988
Funding
This study was supported by the Natural Science Foundation of Zhejiang Province (LY23D060003; LY19D060002) and the Key Program of Science and Technology Innovation in Ningbo (2021Z114, 2023Z118).
Author information
Authors and Affiliations
Contributions
LYH conceived and designed the experiments. QWD, YYY, ZMS and JJN contributed to carry out the experiments. QWD, YYY and ZMS analyzed data. QWD and LYH wrote the paper. QWD, YYY, JJN, ZWR and XNJ revised the manuscript and approved this version for submission. LYH, ZWR and XNJ provided project funding support.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests.
Additional information
Handling editor: ronan sulpice
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 448 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qie, W., Yu, Y., Zheng, M. et al. Growth and photosynthetic changes of Ulva prolifera in response to diurnal temperature variations. Aquacult Int 32, 3233–3247 (2024). https://doi.org/10.1007/s10499-023-01320-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01320-3