Abstract
A potential microalgal strain was isolated from dairy industrial effluent contaminated water and genetically identified as a close relative of Ascochloris sp. The current study demonstrates growth, biomass and lipid productivity of Ascochloris sp. ADW007 and simultaneous bioremediation of raw dairy waste water (RDW). Indoor microalgal cultivation studies were conducted in controlled conditions of light and temperature, while outdoor pilot-scale experiments were performed in errant conditions using semi-cylindrical barrel shaped open troughs. The rate of biomass productivity of ADW007 was improved with RDW as growth nutrient in indoor bench-scale (0.102 ± 0.003 g/L/d) and outdoor pilot-scale cultivations (0.207 ± 0.003 g/L/d) when compared with the algal growth in synthetic BG 11 medium (0.086 ± 0.004 g/L/d) and TAP medium (0.099 ± 0.003 g/L/d), respectively. Similarly, in outdoor conditions, the lipid content reached maximum to 34.98 ± 0.21% with volumetric and areal lipid productivities of 0.072 ± 0.001 g/L/d and 9.63 ± 0.08 g/m2/d, respectively. With this, the estimated annual algal oil production is nearly 20,495 ± 1953 gallons/acre/yr, if cultivated throughout the year. C18:0/C18:1 were the predominant fatty acids in lipid which indicates a great potential of ADW007 for biodiesel production and simultaneous bioremediation processes using RDW. Post-harvesting process includes hollow fiber filtration followed by activated carbon treatment and resulted in 95.1, 79.7 and 98.1% reduction in chemical oxygen demand, nitrate and total phosphate, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well established fact that microalgae-based biofuels, such as biodiesel, is having a great potential in replacing the conventional fossil fuels as evident from voluminous reviews on microalgae as sustainable renewable energy feed stock (Medipally et al. 2015; Singh and Sharma 2012). The high cost of nutrients and decreasing availability of fresh water are the major bottlenecks that currently hinder and pose a great challenge for commercialization of microalgae-based biodiesel production. Dairy industrial waste water generated from different processing streams is one such source of nutrients and water which when utilized for microalgae cultivation leads to bioremediation of waste water in addition to algae biomass. Recently, use of microalgae for treatment of dairy industrial waste water for simultaneous removal of high concentration of nitrogen and phosphorus is well studied (Hena et al. 2015; Lu et al. 2015; Qin et al. 2016). The improper discharge of dairy wastewater in the rivers and lakes without proper treatment not only causes environment pollution, contaminates ground water and water bodies, but also have a negative impact on human health. On the other hand, the nutrients present in such effluents could be effectively utilized for agricultural and aquaculture development along with the recently developing fields of alternative biorenewable energy production either by employing conventional anaerobic digestion systems or biological treatment methods (Rico et al. 2011). The development of rapid and cost-effective cultivation methods of potential microalgae using industrial waste water is still in nascent stage and must compete with the existing biological systems. Though waste water treatment usually involves additional cost but if the treatment of waste water itself produces valuable raw materials, cleans water there by reducing water pollution and complies with international standards, it takes the industry in the direction of profitability and sustainability (Hena et al. 2015).
In recent times, research toward development of integrated processes for microalgae cultivation and simultaneous treatment of industrial effluents is of high priority (Kothari et al. 2012; Woertz et al. 2009). The major targeted industries include municipal sewage, breweries, textile industries and dairy industries, etc. Among these, wastewater effluents from dairy industries are rich in ammoniacal nitrogen content, organic and inorganic phosphates and other valuable nutrients which support microalgae cultivation and growth. Studies for establishing the concept of enhanced growth of microalgae with the addition of wastewater to the culture medium are very limited, and in many cases supply of pure CO2, sterilization of RDW, addition of other carbon sources or pH adjustment are observed. Moreover, the integration of microalgal cultivation and bioremediation processes reduces the overall capital expenditure on post-treatment processes of wastewater for safe discharge. Huo et al. (2012) evaluated the feasibility of outdoor cultivation of Chlorella zongfingensis in dairy wastewater and achieved 97.5 and 51.7% removal of total nitrogen and total phosphorus in 5 days. Ding et al. (2014) studied the cultivation of microalgae in different dilutions of dairy wastewater (5, 10 and 20%) and achieved 99, 91 and 89% removal of ammoniacal nitrogen, total phosphorus and COD.
Microalgae as an algal-based biodiesel feedstock have great potential especially in developing countries such as India due to its limited arable land and fresh water availability for microalgae cultivation. Moreover, cultivation of microalgae with high biomass and lipid productivities in industrial waste water could avoid competitiveness for land and fresh water. However, development of rapid and cost-effective cultivation methods of potential microalgae using industrial waste streams is still in nascent stage and must compete with the existing biological systems. Although microalgae is projected to be the most prominent source for next generation biodiesel, but high culture medium costs and complicated microalgae production processes are seen as the major bottlenecks that currently hinder and pose a great challenge for commercialization of microalgae-based biofuels production. Among different microalgae strains available today, Chlorella and Scenedesmus sp are most predominantly used microalga for commercial applications due to their fast-growing nature and have the ability to utilize inexpensive raw materials for growth (Gouveia et al. 2016; Lu et al. 2015).
Evaluation of rate of algal biomass and lipid productivity are two important parameters that need to be considered during microalgal cultivation in order to test the feasibility of employing dairy wastewater in culture medium. Several studies have been reported on biomass and lipid productivity in different industrial wastewaters. Lu et al. (2015) reported that Chlorella sp. reached maximum biomass productivity of 260 and 110 mg/L/d in indoor bench-scale and outdoor pilot-scale cultivation in raw dairy wastewater. In the same study, they have also reported that C16/C18 were the most dominant fatty acids determined in outdoor cultures which showed production of high-quality biodiesel. Kothari et al. (2012) evaluated growth of Chlorella sp. in dairy influent and effluent where maximum biomass yields reached were 18.8 and 14.1 g/L, respectively. Moreover, they have reported that biodiesel production from influent was fourfold higher than the effluent. Guruvaiah et al. (2015) evaluated utilization of dairy wastewater and simultaneous removal of pollutants for high biomass and lipid production from Chloromonas playfairii and Desmodesmus opoliensis. More than 90% removal of COD, ammoniacal nitrogen and total phosphorus was reported with both these strains with maximum biomass production of 1.7 and 1.2 g/L and maximum lipid production of 15 and 12% after 15 days of cultivation. Woertz et al. (2009) reported integration of microalgae cultivation in dairy and municipal wastewater for simultaneous nutrient removal and lipid production using a polyculture of microalgae and diatoms. Maximum lipid productivity of 17 mg/L/d was obtained with batch-type outdoor cultivation system.
The study conducted here evaluates the potential of an isolated microalga Ascochloris sp. ADW007 for utilization of raw dairy wastewater in indoor as well as outdoor cultivation systems, simultaneous nutrient removal along with high biomass and lipid productivity. No studies have been reported on bioremediation of dairy waste water using Ascochloris sp. and its growth, biomass and lipid productivity. Here, we have attempted to evaluate the outdoor cultivation of microalga without external supplementation of CO2, no sterilization of the dairy wastewater or addition of bacterial inhibitors, pH adjustments or addition of other carbon sources or nutrients. A simple cost-effective batch-type outdoor cultivation system was developed that could be easily scaled up for mass production of microalgal biomass. Besides evaluation of lipid productivities, this study also involves generation of utilizable water after treatment of highly turbid, high pollutant dairy wastewater which meets the Indian Central Pollution Control Board water quality standards designated for Type E water. Overall the process developed here is simple, integrated, low-cost zero waste process generating value added products viz., high lipid containing (oleaginous) microalgal biomass for biodiesel production and utilizable water for irrigation.
Materials and methods
Medium and chemicals
Synthetic media (BG 11 and TAP) were used for preliminary enrichment of microalgal biomass (Gorman et al. 1965; Stanier et al. 1971). Analytical grade chemicals were used for microalgal growth in synthetic media, lipid extraction studies and other biochemical experiments. HPLC grade chemicals were used for quantitative and qualitative analysis of fatty acids. Fatty acid standards were purchased from Sigma-Aldrich, USA. Raw dairy wastewater (RDW) was obtained from Amul dairy, one of the largest dairy industries in India, located in Anand district, Gujarat, India. RDW was collected directly from the common storage tank, where all the wastewater streams generated from different dairy product processing units were pooled and readied for anaerobic digestion. In this study, RDW represents undiluted 100% raw dairy wastewater, unless specified separately. Physico-chemical characterization of the RDW includes pH, conductivity, total nitrogen (TN), total phosphorus (TP), organic and inorganic phosphates, total hardness, ammonia, nitrates, total dissolved solids (TDS), COD, sulfate, chloride and sodium adsorption ratio (SAR) measurements (Tchobanoglous et al. 2003).
Isolation and identification of microalgae
Microalgae samples were collected and isolated from nearby water bodies contaminated with dairy processing effluent treatment plants located in Anand district, Gujarat, India. Water samples were collected in sterile 50-ml sealed falcon tubes and were stored at 4 °C until isolation. Physico-chemical analysis of water samples were performed following the standard methods described in APHA (1998). Primary screening was done by suitable dilution of samples in sterile distilled water and spread plating on a sterile agar media supplemented with BG11 medium. The petriplates were then incubated for a period of 7–15 days in a temperature regulated growth room maintained at 25 ± 1 °C in the presence of light (3978 W/m2) by providing light/dark cycle of 8: 16 h. The pure microalgal cells were isolated and then cultured and grown in BG11 medium for biomass enrichment under similar conditions as described above. The purity and morphological characteristics of microalga were examined intermittently during algal growth using phase contrast microscopy (Carl Zeis, USA).
DNA was isolated from 7-day BG11 grown microalga culture using plant DNA isolation kit (Qiagen). The DNA fragment was PCR amplified using U515 and U1390 primers following the protocol described by described by Moro et al. (2009). Each 20 µL PCR consisted of 4 µL of 5× Phusion high fidelity Buffer (New England BioLabs, Inc.), 1.6 µL of dNTPs (2.5 µM each), 1 µL of forward and reverse primer (10 µM), 0.6 µL of DMSO, 10 ng of template DNA, 0.2 µL of DNA Polymerase (0.4 units) and 10.6 µL of double distilled water. PCR was performed with a thermal cycler (Bio-Rad, USA) using the following program: an initial denaturation at 98 °C for 1 min; 25 cycles at 98 °C for 10 s (denaturation), 56 °C for 30 s (annealing) and 72 °C for 20 s (extension); and a final extension at 72 °C for 5 min. The concentration of each PCR amplicon was estimated by agarose gel electrophoresis. DNA sequencing reaction of the PCR amplicon was carried out using BDT v3.1 cycle sequencing kit on ABI 3730xl Genetic Analyzer. Consensus sequence of 574 bp 28S rDNA was generated using aligner software. BLAST analysis was performed using open source NCBI genbank database, multiple sequence alignment using Clustal X, distance matrix using RDP database and phylogenetic tree was constructed using MEGA5.
Microalgae growth
Growth studies were performed in both indoor bench-scale and outdoor pilot-scale conditions. Rate of algal growth, lipid production and nutrient removal from raw dairy wastewater were evaluated. Indoor cultivations were performed in BG11, TAP and RDW as culture media. Prior to cultivation, RDW was filtered through a non-woven geotextile membrane (100GSM grade) to remove large aggregated particles and used directly without sterilization. Laboratory-scale experiments include 1–5 L round bottom glass flasks and bench-scale includes 25 L capacity photobioreactor. The growth containers were incubated for 5–17 days in a temperature regulated growth room maintained at 25 ± 1 °C in the presence of light intensity between 3366 and 3978 W/m2 and light/dark cycle of 8: 16 h. Samples were collected at regular intervals and microalga growth profile, wet/dry weight, and lipid content were measured. Besides, indoor growth studies were also performed in mixed growth media, where RDW was diluted with BG11 medium at different ratios. The tested dilutions include 20, 50, 80 and 100% of RDW, respectively.
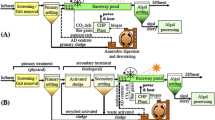
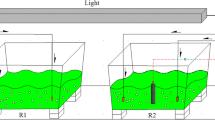
Outdoor cultivation studies were performed in small semi-cylindrical polypropylene troughs of size 0.9 m × 0.5 m. Maximum working culture volume of 56–60 L was achieved in each trough at 0.20 m depth. Scale-up cultivation was achieved at a total cultivation volume of 448–480 L in one batch using eight troughs placed in series adjacent to each other. Schematic representation of outdoor pilot-scale microalgae cultivation system is shown in Fig. 1a. 1 mm pore size perforated PVC tubes of 0.75 cm i.d. (inner diameter) were placed at the bottom of each trough and interconnected with valve junctions and regulators. At one terminal end of the tubes, an air compressor (capacity 0.35 hp) was connected for aeration in troughs.
10% (v/v) freshly grown algal culture (~ 2.6–3.1 OD per ml at 600 nm or 1.2–1.4 g/L dry biomass) was used as inoculum. All pilot-scale studies were performed in 100% RDW without any dilutions and external supplementation of nutrients. Temperature and light intensity were measured periodically during the cultivation period. Culture samples were withdrawn at regular intervals and evaluated for algal growth, biomass and lipid analysis and physico-chemical properties of RDW were studied pre- and post-microalgal treatment.
Post-harvesting process
Tangential flow hollow fiber filtration (TFF) was performed with a 0.2 µm Microza hollow fiber microfiltration cartridge (Pall filtration systems, USA) for enriching microalgal biomass concentration. Prior to TFF, the cultures were passed through 5-mm stainless steel (SS) filter and large aggregated particles were removed. The retentate, i.e., concentrated biomass obtained from TFF treatment was collected and then passed through 200 GSM non-woven geotextile membrane and then the wet algal biomass was dewatered using horizontal batch-type solar tunnel dryer (45–60 kg capacity of PV module with collector area 6.9 m2) until the moisture content reached ≤ 5% (w/w). The dried biomass was powdered and then stored at room temperature in air lock sealed containers until lipid extraction experiments. The algal-free treated water was passed through activated carbon filter (SUPRAcap™50, Pall filtration systems, USA). Physico-chemical properties of the treated water were studied at each step of post-processing.
Lipid and fatty acid analysis
Algal lipid was extracted following standard chloroform/methanol (1:1) extraction method (Axelsson and Gentili 2014). Further, to enhance the total lipid extract, the dried biomass was subjected to microwave pretreatment (2400 Hz) for 5 min with 30-s pulse interval prior to chemical treatment (Lee et al. 2010). The lipid fraction was separated, solvent was evaporated under vacuum, and fatty acids were extracted using standard ethanolic KOH method (Salimon et al. 2011). Qualitative and quantitative analysis of total free fatty acids were performed by HPLC (Luna C18 column, 250 × 4.6 mm 100 Å) using UV detector at 208 nm and referred against standard fatty acids (Sigma-Aldrich, USA).
Biomass measurement, growth rate and biomass productivity calculation
Microalgal culture samples grown in BG11, TAP and different dilutions of RDW were collected at regular growth intervals (24 h) and were subjected to centrifugation at 10,000 rpm for 15 min, and the pellet was dried at 60 °C to determine the microalgal biomass dry weight (g/L) (Ding et al. 2014). Simultaneously, optical density (absorbance) of the microalgal cultures was also measured at 600 nm using UV–visible spectrophotometer (Schimadzu, USA).
Growth rate of the cultures were calculated by Eq. 1
where x0 = initial biomass concentration (g/L), at time t0; x = final biomass concentration (g/L) and µ = specific growth rate of the culture (day−1).
The total lipid content was measured gravimetrically and stored at − 20 °C until further analysis. Lipid content was then calculated by Eq. 4
Process flow for microalgal cultivation from dairy waste water and post-harvesting process
An integrated process for microalgal cultivation using dairy waste water with simultaneous waste water bioremediation was demonstrated in the current investigation. The entire process flow with individual process steps are shown in Fig. 2. Step 1: collection of dairy wastewater from the common effluent treatment plant of the selected dairy industry Step 2: primary settling of raw dairy waste water and removal of scum. Step 3: scum-free waste water was now added to the open tub reactor system for microalgal cultivation. Step 4 and 5: cultivated microalgae was passed through TFF for harvesting. Step 6: flow through obtained after TFF was then passed through activated carbon filter to obtain clarified water. Step 7: the retentate enriched with algal slurry was concentrated by geotextile membrane. Step 8: algal cake obtained was dewatered completely by drying in solar tunnel dryer. Step 9: lipid extracted from the dried algal powder by chloroform/methanol extraction method. Step 10: lipid obtained from algae used for biodiesel production via transesterification. Step 11: utilization of lipid depleted algae for producing other value added products.
Pictorial view of each individual process of microalgal cultivation and post-harvesting is depicted in graphical abstract. The cost economics, mass balance and energy evaluation studies of the proposed process will further clearly demonstrate the techno-economic feasibility of the integrated process.
Analytical methods
Temperature, pH, electrical conductivity and TDS were monitored at regular intervals during microalgal growth using electrode probe based equipment (Eutech instruments Cyberscan series 600 Portable Meter). Light intensity for indoor and outdoor cultivation was measured using digital lux meter (TES-1332A, TES Electrical Electronic Corp. Taiwan). All the experiments related to microalgal growth, biomass and lipid contents, bioremediation and harvesting processes were performed for three times, and the aggregated mean values are presented.
Statistical analysis
All the statistical analysis was performed using GraphPad Prism version 6.07 and Mintab version 16. Statistical validation studies were performed using multiple Student’s t test, one-way and two-way analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test or the Wilcoxon rank sum test and/or Tukey’s multiple comparison tests. The results were expressed as mean ± standard error of the mean (SEM). p values < 0.05 were considered statistical significant.
Results and discussion
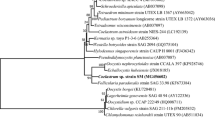
A potential microalgal strain designated as ADW007 was isolated from dairy effluent contaminated water bodies. Phase contrast microscopic image of the purified strain is shown in Fig. S1. Nucleotide blast analysis of ADW007 showed 81% sequence identity (Expect value 3e-92) with Ascochloris multinucleata UTEX 2013 26 and 28 s ribosomal RNA gene sequences (NCBI GenBank ID: AF395492.1 and AF277652.1) and phylogenetic analysis confirmed the close relativity to Ascochloris sp. (Figs. S2, S3). The gene sequence was submitted to NCBI with GenBank ID: KU725690.1. Studies on cultivation of fresh water green algae, especially Chlorella sp and Scenedesmus sp. in different wastewaters for bioremediation applications were studied extensively as evident from voluminous research publications (Gouveia et al. 2016; Gong et al. 2014).
Biomass growth and productivity
The growth rate of Ascochloris sp. ADW007 in indoor culture (laboratory and bench-scale) was highest in RDW followed by TAP and BG11 culture media (Fig. 3, Table 1). The biomass yield (g/L) in laboratory-scale studies for RDW, TAP and BG11 were 2.23 ± 0.15, 1.57 ± 0.03 and 1.05 ± 0.07, respectively, on 17th day. Similar trend was observed in bench-scale studies with biomass yields (g/L) of 1.73 ± 0.04, 1.68 ± 0.03 and 1.46 ± 0.07 in RDW, TAP and BG11, respectively. Detailed biomass and lipid productivities are given in Table 1. All the calculations were based on dry weight basis (dwb) after measuring the moisture content of the wet biomass.
Growth profile of ADW007 in different growth media. Raw dairy wastewater (RDW), synthetic Blue–Green (BG11) and tris–acetate phosphate (TAP) media at various strengths. (n = 3); Level of significance were analyzed with t test with Bonferroni corrections (*p < 0.05 and **p < 0.001 against RDW. Error bars represent ± SEM
Addition of RDW to the BG11 improved the growth rate of ADW007 in indoor laboratory-scale studies (Table 2, Fig. S4). Similar observation was also recorded in indoor bench-scale studies (Figs. 3, S5). Interestingly, increased % of RDW not only increased the microalgal growth rate but also significantly increased the biomass productivity. The biomass yields (g/L) on 17th day were 1.74 ± 0.005, 1.71 ± 0.003, 1.62 ± 0.006 and 1.54 ± 0.018 with 100, 80, 50 and 20% RDW addition, respectively (Table 2), while with BG11 the biomass production was reduced to 1.46 ± 0.007 g/L, which was nearly 19.1% lower compared to 100% RDW. However, when growth studies were performed in nutrient-rich synthetic TAP medium, the biomass yield was elevated to 1.68 ± 0.005 g/L on 17th day. Higher biomass productivities in RDW and TAP might be due to the presence of high amounts of ammoniacal nitrogen (0.1–0.3 g/L) in culture media. Supporting this observation, BG11 medium contained ammoniacal nitrogen concentration of 0.006 g/L in the form of ferric ammonium citrate, whereas RDW contained an estimated ammoniacal nitrogen concentration between 0.11 and 0.16 g/L. The data clearly indicate that ADW007 prefers ammoniacal nitrogen for growth. This might be one of the reasons for enhanced biomass productivities in RDW.
Outdoor cultivation studies were performed between the month of September and March during which the areal temperature and light intensities were in the range of 29–42 °C and 28,944–196,015 W/m2, respectively. A series of individual troughs were placed side-by-side occupying a total area of 3.6 m2 with maximum cultivation volume of 480 L (Fig. 1b). Even though there was undulation of illumination intensity and temperature in outdoor cultivation system, when compared to indoor cultivation, the rate of biomass productivity in outdoor cultivation was significantly higher. Compared to the biomass productivity in synthetic BG 11 medium (0.086 ± 0.004 g/L/d), the growth rate of microalga was boosted significantly with RDW in indoor bench-scale (0.102 ± 0.003 g/L/d) and outdoor pilot-scale cultivation (0.207 ± 0.003 g/L/d).
Unlike reported by Lu et al. (2015) on initial inhibitory effect of RDW on the growth of Chlorella sp, no such effect was seen with ADW007. This may possibly be due to symbiotic microalgal-microbial consortia dominated by Ascochloris sp during growth on RDW. Also, continuous aeration of 11 L/h was provided for 8 h/day into each open barrel troughs using an air pump of capacity 88L/h through perforated PVC tubes placed at the bottom of trough ensured effective mixing of culture. Based on the pump flow rate of 88 L/h air and at approximate CO2 and O2 concentrations of 0.04 and 20.95% in air, respectively, the open barrel trough was enriched with approximately 0.281 L of CO2 and 147.5 L of O2 per day of cultivation.
In contrast, in indoor cultivation, intermittent periodical physical shaking of conical flasks was provided. It is well known that CO2 is one of the key requisites for microalgal biomass production (Singh and Singh 2014). During high day light intensities (80,000–100,000 lx), a temporary shade was provided in open cultivation to reduce the light intensity to < 40,200 W/m2 in order to avoid cell damage by UV radiation. Gong et al. (2014) also reported that the optimum light intensity for obtaining maximum biomass productivity with Chlorella sp. was nearly 31,838 W/m2 under controlled indoor cultivation. However, the light intensity during indoor cultivation was around 3978 ± 158 W/m2 and attempts were made to increase the total illumination intensity by placing multiple tubular lights directly inside the microalgal cultivation tank (data not shown). However, no significant increase in biomass or lipid productivity was observed.
Post-harvesting processes
Different types of harvesting techniques for microalgae separation were reviewed recently (Barros et al. 2015). Although microalgal cells could be completely separated by centrifugation method, it is an energy intensive process. Other microalgal harvesting methods such as centrifugation, vacuum filtration and chemi-flocculation were also evaluated (data not shown). But, due to high energy requirement and post-harvesting process costs, these methods were not investigated in detail in scale-up studies and were limited to laboratory-scale small culture volume studies only. Since TFF is a well-known developed technology for microalgae harvesting and a detailed techno-economic feasibility study was reported (Stevens et al. 2013), here we have selected TFF system for microalgae harvesting (Fig. S6). A 30× fold concentration of microalgal cells was achieved after 0.7 m2 TFF at a flow rate of 67 L/h/m2. The total suspended solids were increased from 1.2 to 36.0%. No microalgal cells were detected in the flow through, and all the cells were retained with 0.2 µm filter membrane. Within 4 h, the initial feed volume of 30 L was reduced to 0.6 L (Table 3).
Danquah et al. (2009) also reported use of tangential flow filtration for dewatering of microalgal culture where 78× concentration fold was achieved. However, the initial feed concentration was only 0.06% (w/v) solids and a cassette-based THF system was used instead of hollow fiber filtration system for dewatering. Further dewatering of the concentrated retentate using a geotextile membrane resulted in thick wet biomass containing 90% moisture content (Fig. 4).
Lipid and fatty acid profile
The lipid contents from 17th day bench-scale grown culture of ADW007 in BG11, TAP and RDW under indoor cultivation conditions were 10.67 ± 0.12%, 29.25 ± 1.05% and 31.72 ± 0.77% (v/v), with a volumetric productivity of 0.009 ± 0.000, 0.029 ± 0.002 and 0.032 ± 0.001 g/L/d, respectively (Table 1). The lipid productivities obtained from this study were comparatively higher than Woertz et al. 2009, where maximum of 29% of lipid was produced using dairy wastewater in 6 d grown culture with a volumetric productivity of 0.017 g/L/d. Although the total lipid content from 7th day grown culture of ADW007 in RDW was only 24.99 ± 0.29%, the lipid productivity was significantly higher (0.059 ± 0.009 g/L/d), indicating that the rate of lipid production was decreased after 7 days. Besides, a threefold lower lipid was produced in BG11 medium compared to TAP and RDW. However, albeit less the lipid content of ADW007 grown in BG11 was slowly accumulated and reached a maximum lipid content of 22 ± 1.6% (v/v) after 28-day growth. On the other hand, in outdoor conditions, higher lipid yields were achieved in 7 days with a lipid concentration and volumetric and areal productivities of 34.98 ± 0.21%, 0.072 ± 0.001 g/L/d and 9.63 ± 0.08 g/m2/d, respectively. This would be equivalent to 20,495 ± 1953 gallons/acre/yr of algal oil, if cultivated throughout the year. Moreover, as compared to side by side, the areal productivities and estimated bio-oil yields from indoor cultivation were lower than the outdoor cultivation conditions as shown in Table 1. These clearly indicate scale-up outdoor cultivation has significantly improved the total bio-oil production utilizing raw dairy wastewater as sole source of growth nutrient for liquid biofuels applications. The predominant fatty acids determined from lipid extracts of BG11 grown cells were linolenic acid (C18:3) followed by linoleic (C18:2) and oleic acid (C18:1). Although similar fatty acids were identified from lipid extracts of TAP and RDW grown cells, the total peak area of stearic acid (C18:0) and eicosahexanoic acid (C22:6) was higher in lipid extracts of RDW grown cells (Fig. S7). The percentage distribution of fatty acids measured from the chromatogram is shown in Table 4. > 50% of total lipids comprised of long-chain saturated and unsaturated fatty acids. Nearly, threefold higher levels of saturated fatty acids were observed with lipid extracts of TAP and RDW grown cells. Moreover, RDW induced production of very long-chain poly unsaturated fatty acids, and it was evident from the lipid profile data where twofold higher levels of C22:6 fatty acid were observed in RDW lipid extracts.
Nutrient removal
Over 95.1% of COD was reduced from the raw dairy wastewater after 7 days of microalgal cultivation both in indoor and in outdoor cultivation (Fig. 5). Maximum reduction in COD, total nitrogen and total phosphorus was 965 ± 16, 19.27 ± 1.2 and 14.72 ± 0.9 mg/L/d in the treated water of 100% RDW observed (Table 5). For 20, 50 and 80% RDW dilution experiment, initial concentrations of total ammoniacal nitrogen were 35 ± 2, 46 ± 3 and 58 ± 12 mg/L and were reduced < 5 mg/L in 7 days (data not shown). These were similar to results reported by Woertz et al. (2009) but were with mixed algal and diatom cultures. The initial fluoride content in dairy wastewater was 4.83 ± 0.8 mg/L, and nearly 92% removal was seen in 7 days, indicating ADW007 could effectively remove fluorine from fluoride contaminated waters. Besides, the physico-chemical characteristics of activated carbon treated water were within the limits of the Indian water quality standards of Central Pollution Control Board (CPCB) Type E water designated for irrigation usage (Table 5). Physico-chemical characterization of raw dairy waste water and its comparison with microalgal treated water are listed in Table 5. There is no significant difference on the effect of growth (in terms of length of culm and leaves) and visual color appearance of garden grasses after sprinkling every day with the treated water for 30 days (data not shown).
Conclusion
The current study provided proof-of-concept for an integrated process comprising of a newly isolated microalgal strain belonging to Ascochloris sp. ADW007 in dairy waste water along with nutrient removal, high biomass and lipid productivity in a simple outdoor cultivation system. COD reduction and fluoride removal in dairy waste water reached > 90% after ADW007 treatment for period of 7 days. Pilot-scale cultivation studies were performed in outdoor conditions without external supplementation of additional nutrients and CO2 in low-cost polypropylene semi-cylindrical barrels. With a biomass and lipid yields of 1.44 ± 0.03 g/L and 34.98 ± 0.21% in 7 days, using 100% RDW makes ADW007 as a potential third-generation biofuel feedstock for scale-up studies. Overall, this study gives a glimpse of an integrated approach on waste-to-biofuel and utilizable water that mainly avoids environmental concerns and provides a possible solution for availability of clean water and alternative renewable feedstocks for liquid fuels to meet the demand of developing countries such as India which is rapidly increasing its industrialization process.
References
American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF) (1998) Standard methods for the examination of water and wastewater, 20th edn. United Book Press Inc, Baltimore
Axelsson M, Gentili F (2014) A single-step method for rapid extraction of total lipids from green microalgae. PLoS ONE 9:e89643
Barros AI, Goncalves AL, Simoes M, Pires JCM (2015) Harvesting techniques applied to microalga: a review. Renew Sustain Energy Rev 41:1489–1500
Danquah MK, Ang L, Uduman N, Moheimani N, Forde GM (2009) Dewatering of microalgal culture for biodiesel production: exploring polymer flocculation and tangential flow filtration. J Chem Technol Biotechnol 84:1078–1083
Ding JF, Zhao FM, Cao YF, Xing L, Liu W, Mei S, Li SJ (2014) Cultivation of microalgae in dairy wastewater without sterilization. Int J Phytoremediation 17:222–227
Gong Q, Feng Y, Kang L, Luo M, Yang J (2014) Effects of light and pH on cell density of Chlorella vulgaris. Energy Proc 61:2012–2015. https://doi.org/10.1016/j.egypro.2014.12.064
Gorman DS, Levine RP (1965) Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci USA 54:1665–1669
Gouveia L, Graça S, Sousa C, Ambrosano L, Ribeiro B, Botrel EP, Neto PC, Ferreira AF, Silva CM (2016) Microalgae biomass production using wastewater: treatment and costs Scale-up considerations. Algal Res 16:167–176
Guruvaiah M, Narra M, Shah E, James J, Kurchania A (2015) Utilization of dairy wastewater for pollutants removal and high lipid biomass production by a newly isolated microalgal strains Chloromonas playfairii and Desmodesmus opoliensis. Int J Appl Sci Biotechnol 3:699–707
Hena S, Fatimah S, Tabassum S (2015) Cultivation of algae consortium in a dairy farm wastewater for biodiesel production. Water Resour Ind 10:1–14
Huo SH, Wang ZM, Zhu SN, Zhou WZ, Dong RJ, Yuan ZH (2012) Cultivation of Chlorella zofingiensis in bench-scale outdoor ponds by regulation of pH using dairy wastewater in winter, South China. Bioresour Technol 121:76–82
Kothari R, Pathak VV, Kumar V, Kumar V, Singh DP (2012) Experimental study for growth potential of unicellular alga Chlorella pyrenoidosa on dairy wastewater: an integrated approach for treatment and biofuel production. Bioresour Technol 116:466–470
Lee JY, Yoo C, Jun SY, Ahn CY, Oh HM (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresour Technol 101:S75–S77
Lu W, Wang Z, Wang X, Yuan Z (2015) Cultivation of Chlorella sp. using raw dairy wastewater for nutrient removal and biodiesel production: characteristics comparison of indoor bench-scale and outdoor pilot-scale cultures. Bioresour Technol 192:382–388
Medipally SR, Yusoff Md F, Banerjee S, Shariff M (2015) Microalgae as sustainable renewable energy feedstock for biofuel production. Biomed Res Int 2015:519513
Moro CV, Crouzet O, Rasconi S, Thouvenot A, Coffe G, Batisson I, Bohatier J (2009) New design strategy for development of specific primer sets for PCR-based detection of Chlorophyceae and Bacillariophyceae in environmental samples. Appl Environ Microbiol 75:5729–5733
Qin L, Wang Z, Sun Y, Shu Q, Feng P, Zhu L, Xu J, Yuan Z (2016) Microalgae consortia cultivation in dairy wastewater to improve the potential of nutrient removal and biodiesel feedstock production. Environ Sci Pollut Res Int 23:8379–8387
Rico C, Rico JL, Tejero I, Munoz N, Gomez B (2011) Anaerobic digestion of the liquid fraction of dairy manure in pilot plant for biogas production: residual methane yield of digestate. Waste Manag 31:2167–2173
Salimon J, Abdullah BM, Salih N (2011) Hydrolysis optimization and characterization study of preparing fatty acids from Jatropha curcas seed oil. Chem Cent J 5:67
Singh RN, Sharma S (2012) Development of suitable photobioreactor for algae production—a review. Renew Sustain Energy Rev 16:2347–2353
Singh SP, Singh P (2014) Effect of CO2 concentration on algal growth: a review. Renew Sustain Energy Rev 38:172–179
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue–green algae (Order Chroococcales). Bacteriol Rev 35:171–205
Stevens DM, Stone ML, Peterson ES, Newby DT (2013) Cross-flow filtration of multiple algal strains and mixed populations using embedded membranes. Idaho National Laboratory Idaho Falls, Idaho, p 83415
Tchobanoglous G, Burton FL, Stensel HD (2003) Waste water engineering, treatment and reuse, 4th edn. Metcalf and Eddy, Inc., McGraw Hill Companies, Inc., New York
Woertz I, Feffer A, Lundquist T, Nelson Y (2009) Algae grown on dairy and municipal wastewater for simultaneous nutrient removal and lipid production for biofuel feedstock. J Environ Eng 135:1115–1122
Acknowledgements
The authors are thankful to the Director, Sardar Patel Renewable Energy Research Institute, Gujarat, India, for supporting this research. The research work is financially supported by Indian Council of Agricultural Research (ICAR), under All India Coordinated Research Project (AICRP)—EAAI program, Government of India with Grant No. VVN/RES/DRET-LBT/2014/1 and Department of Science and Technology (DST), Government of India, GUJCOST Minor Research Project with Grant No. GUJCOST/MRP/2014-15/2016.
Author information
Authors and Affiliations
Contributions
AKK is a Principal Scientist and he has designed and supervised the research work. SS and GD are Research Fellows, while ES, BSP and AP are Scientific Technical Assistants at Sardar Patel Renewable Energy Research Institute. SG is a Post-Doctoral Fellow at University of Missouri and JMD is an Associate Professor in the Department of Statistics, Sardar Patel University. SS, ES, BSP and AP have performed the laboratory experiments and compiled the research data. GD, SG and JMD performed statistical analysis. AKK and SS have written the manuscript. All authors have read and approved the final manuscript. This work has not been submitted in any other journal/article or either published earlier in any journal elsewhere. All the authors have agreed to publish this work and do not have any conflict in publishing this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Editorial responsibility: M. Abbaspour.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, A.K., Sharma, S., Shah, E. et al. Cultivation of Ascochloris sp. ADW007-enriched microalga in raw dairy wastewater for enhanced biomass and lipid productivity. Int. J. Environ. Sci. Technol. 16, 943–954 (2019). https://doi.org/10.1007/s13762-018-1712-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1712-0