Abstract
Microalgae are a unique renewable resource utilized since ages, serving as a reservoir for the production of various metabolites. In this study, dairy waste water (DWW) is used as the nutrient media for the cultivation of microalgae. This study focuses on the phycoremediation process of converting rich nutrients in the effluent into biomass and removing contaminants using microalgae. The specific growth rate reached the maximum of 0.55 day−1 in Desmococcus olivaceous, followed by 0.39 day−1 for Scenedesmus dimorphus, 0.23 day−1 in DCS (consortia composing all three strains in equal ratio), and lastly 0.22 day−1 in Chlorella vulgaris. The biomass productivity was 1.44 g L−1 day−1, 1.06 g L−1 day−1, 0.88 g L−1 day−1, and 0.65 g L−1 day−1 in D. olivaceous, S. dimorphus, C. vulgaris, and DCS, respectively. The COD and BOD removal percentage was 82.85% and 45.40% in D. olivaceous, 81.98% and 44.25% in C. vulgaris, 80.73% and 53.45% in S. dimorphus, and 80.10% and 43.10% in DCS, respectively. These results emphasize the promising role of algae in dairy effluent treatment, highlighting the effluent as a suitable medium for microalgae cultivation. It verifies the circular bio-economy concept where the treated wastewater is converted into value-added products.
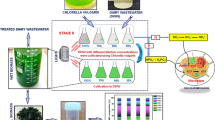
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
India, one of the major industrialized countries with diversified areas, emerged as an indigenous industrial hub surpassing global anticipation and emerging as one of the world’s booming economies. But on the other hand, rising environmental pollution decreases the quality of life, causing global ecological crises and climatic fluctuations (Renuka et al., 2015). A few major sources of pollution are large volumes of effluents, both in solid and liquid forms, generated by breweries, sugar mills, distilleries, dairy industries, food-processing industries, tanneries, and paper-pulp industries (Enamala et al., 2018; Ghashghaie et al., 2022). These threats have posed thoughtful challenges for the scientific community in maintaining the sustainability of our planet for the current and future generations.
Untreated wastewater from industries and agricultural runoff has excess nutrients like nitrogen and phosphorous that lead to eutrophication, oxygen exhaustion, and loss of important flora and fauna, and sometimes to the total degradation of water bodies that can be estimated using SWAT (the Soil and Water Assessment Tool) software (Ostad-Ali-As, 2022; Renuka et al., 2015). Therefore, there is an immediate need to detect inexpensive, eco-friendly techniques that require nominal infrastructure and inputs that can be utilized by the common person or poorly educated population. Though many physical and chemical methods are developed for wastewater treatment, they have their limitations due to the toxicity of the end products released (Abatenh et al., 2017; Arora & Khosla, 2021; Azubuike et al., 2016).
Bioremediation is an integrated approach which includes degradation, depletion, adsorption, or detoxification of hazardous wastes from the surrounding by the action of microorganisms. Bioremediation employs microbes like bacteria, fungi, actinomycetes, and earthworms to transform toxic chemicals to release CO2/CH4 and water (Choi et al., 2018). In phycoremediation, researchers employed plants (including algae and lower plants) and associated microflora to remove contaminants including nutrients and heavy metals. Transferring this technology for industrial wastewater treatment is a promising option due to the following merits of microflora: (i) CO2 sequestration potency (Enamala et al., 2018); (ii) biotransformation, bioaccumulation, or bioaugmentation of the toxic chemical substances in the environment; (iii) easy adaptability to the natural environment and climatic fluctuations; and (iv) eco-friendly and cheap alternative to other methods ( Sirisha et al., 2017; Swetha et al., 2016).
In India, milk is one of the most important commodities needed for everyday life. In 1970, the Indian Government initiated a new operation flood called the White Revolution after witnessing the success of the Green Revolution that increased crop productivity in wheat and rice. The main objectives of the operation were (i) to feed the growing population, (ii) to increase the market share value to the producer, (iii) to improve productivity for the dairy farmers, (iv) to generate income for small farmers and landless people, and (v) to move cattle farms from cities to rural areas. These strategies rapidly increased the country’s milk production and promoted India as one of the largest milk producers in the world (Feil et al., 2020). Operation flood was a rural development program started by the National Dairy Development Board of India (NDDB) in three phases during the period 1970–1996, and later, this operation transformed into the White Revolution by AMUL (Anand Milk Union Limited), a Gujarat-based cooperative societies lead to the success of the Operation Flood Programme (Amul - Milk, 2021).
Currently, India produces 147 million tonnes of milk annually, which is double the quantity produced by the USA. The excess milk production makes India the largest milk exporter with annual milk production worth Rs. 2422.85 crores (295.469 million USD) (Moitra, 2021).
Control operations are involved in all production stages, including sanitary conditions (cleaning), storage, transportation, processing, and packaging to maintain the good quality of produced milk. It is one of the most polluting industries generating about 0.2–10 L of effluent per liter of processed milk, which is 2.5 times more than the volume of milk production (Liu et al., 2016). In 2020, the FAO estimates that 928 million tonnes of milk were produced, generating approximately 2320 million tonnes of wastewater (FAO, 2021). Dairy effluent from all the above processes contains soluble organics, suspended solids, chloride, sulfates, trace organics, oil, and grease (Chokshi et al., 2016; Maurya et al., 2016). These components increase the biological oxygen demand (BOD) and chemical oxygen demand (COD) (Kothari et al., 2012; Ummalyma & Sukumaran, 2014). Due to the fermentation of milk sugar to lactic acid, dairy waste becomes rapidly acidic and contributes to immediate high oxygen demand.
Besides milk constituents, the wastewater has detergents, sanitizers, and caustic soda used for washing purposes (Chokshi et al., 2016; Kothari et al., 2012). The essential nutrients (N and P) in dairy effluent are suitable for bacterial growth, favoring the anaerobic decomposition of pollutants (Barrera Bernal et al., 2008).
Deploying microalgae for remediation is the most cost-effective technology to remove nutrients and increase biomass productivity for by-product development (Do et al., 2019; Kumar et al., 2019; Ummalyma & Sukumaran, 2014).
The emerging applications of biotechnology in efforts to reduce environmental degradation and promote long-term sustainability have broadened the scope of algal bioremediation. Moreover, the treated water can be used for irrigation purposes, with enriched nutrient content that can promote plant growth and development (Dominic et al., 2009). Microalgal remediation is a simple, economical, and environmentally friendly approach in treating effluent while simultaneously enhancing the agronomic traits of plants. The novelty involved in the study is to reduce the cost of media by suggesting an alternative cheap and effective commercial-grade fertilizer mix with nitrogen, phosphate, and potassium, thereby reducing the cost of effluent treatment. The media cost of microalgal culturing is not feasible for large-scale production. Replacing commercial-grade fertilizers will reduce the production cost and space occupied by conventional unit operations. Also, the treated residual water and biomass are recommended for irrigation to produce plants with higher yield and significant biomass, indirectly making the industry a place of zero-waste discharge. This sustainable method of remediation is also capable of remediating hazardous substances disposed off in the environment (Ostad-Ali-Askari, 2022).
The Indian Government has strict rules and regulations for effluent discharge to protect the environment (Prevention and Control of Pollution Act 1974; The Environment Protection Act 1986). The main objective of this study is to focus on addressing the reformation of water quality to meet the minimum standards set by the government for the discharge of effluents from the dairy industry by implementing appropriate industry transferrable technology cost-effectively, benefitting both the industry and society.
Materials and methods
Collection of dairy effluent
The dairy waste water (DWW), collected at the inlet of ETP (effluent treatment plant) of a dairy industry located in Tamil Nadu, was filtered using a 100 mesh strainer to remove solid suspended particles and stored in the cold room at 4 °C until further investigation (Chinnasamy et al., 2010).
Selection of species and maintenance of culture
Based on previous studies, Desmococcus olivaceous (NRMCF0124), Scenedesmus dimorphus (NRMCF0157), and Chlorella vulgaris (NRMCF0128) (Kumar et al., 2019; Umamaheswari & Shanthakumar, 2016; Zhang et al., 2018) were obtained for the study from the National Repository for Microalgae and Cyanobacteria [NRMC], Bharathidasan University, Tiruchirappalli, Tamil Nadu, India.
A morphological study of the selected microalgae strains was conducted under a trinocular light microscope LM-52–1803 to check cross-contamination. SEM was done to analyze the structural changes in the algal cells after the absorption of contaminants from untreated raw effluent. Later, 10 ml of each microalgal strain was inoculated into 180 ml of Chu10 medium in a 250-ml Erlenmeyer flask and kept in an incubator shaker (100 rpm, 25 °C, 2500 lx).
Bioreactor designing and setup
Scaling up to large volumes (culture: effluent volume was maintained in a 4:1 ratio) was done using sterilized tap water and added commercial fertilizers with an equal proportion of nitrogen, phosphorus, and potassium (Purity Originals Npk 19:19:19). Plastic bubble top PET cans with 10 L capacities and an upper lid with an opening for aeration tubes were used for scaling up to pilot scale. The selected cultures adapted to fertilizers were slowly scaled up to 4 L with an optical density of more than 0.2 and growth not exceeding 11–15 days of sub-culture (exponential phase) for better results. Aeration was provided using an electricity-powered air pump for proper mixing. LED lights of 60 µmol m−2 s−1 light intensity (white LED lights) were fitted to maintain 16:8 h light: dark period. After scaling to a 4 L culture volume, 1 L of effluent was added to the culture. After 24 h, 1 L culture was removed and replaced with 1 L raw effluent to mimic the effluent treatment process in industries with a semi-continuous inlet and outlet. The same process was repeated every 24 h for the next 5 days to replace the entire culture volume with effluent.
Algal growth conditions and biomass estimation
Microalgae growth parameters were monitored turbidimetrically at 680 nm and the OD obtained was used to calculate the doubling time and specific growth rate.
Algal dry cell weight (DCW) was calculated using the gravimetric method by harvesting a microalgal culture of 15 ml in a pre-weighed centrifuge tube, centrifuged at 5000 rpm for 5 min. The pellet was dried for 24 h in a hot air oven at 60 °C. The dry weight of biomass was calculated daily and calculated using the standard formula (Chen et al., 2018; Saranya & Shanthakumar, 2019) as given below:
Biomass yield (mg/L) = The final weight of the Eppendorf tube − Initial weight of empty Eppendorf.
Specific growth rate and doubling time
Algal growth was analyzed from the optical density reading measured at 680 nm every 24 h for calculating the doubling time and specific growth rate (Chokshi et al., 2016) using a UV–VIS spectrophotometer (Agilent—Cary 3500 UV–VIS spectrophotometer). Based on the OD reading, the doubling time and specific growth rate were calculated using the following formula (Eqs. 1 and 2):
(OD2 and OD1 are optical density readings of day 2 and day 1; t2 and t1 are the time of observation of reading) (Balaji et al., 2014b; Barrera Bernal et al., 2008).
Chlorophyll A estimation
Chlorophyll A content of untreated control effluent and treated effluent were estimated by centrifugation at 5000 rpm for 5 min, and the pellets were washed 2–3 times with distilled water, then re-suspended in 2 ml acetone and boiled till the pellets turned colorless (Saranya & Shanthakumar, 2019). After complete cell disruption, the solution was again centrifuged at 13,000 rpm for 5 min, and the supernatant was taken for measuring the optical density at 565 nm and 540 nm using a spectrophotometer (Spectro-quant@Pharo 300) (Balaji et al., 2014a). The chlorophyll A (Chl A) content was determined using the formula (Eq. 3):
Physicochemical parameter analysis
On the 5th and 10th days, physicochemical parameters were analyzed. Temperature and pH were recorded daily using the centigrade thermometer and electrode pH meter. Water quality is assessed before and after adding dairy effluent for the following parameters: sulfide, chloride, fluoride, nitrite, nitrate, ammoniacal nitrogen, alkalinity, and hardness were determined by following the APHA protocol (APHA, 2012). Dissolved oxygen content was recorded every 24 h using a DO meter to rapidly confirm the oxygen level increase on the decrease of BOD and COD. All the sample analysis was done following the standard the APHA (1998) protocol and Indian standard IS: 3025–1964, 10 days after the addition of effluent.
Nutrient removal efficiency of microalgae
Nitrite and nitrate estimation was done by direct nesslerization to induce the reduction of ammonia by the addition of reducing agent Devarda’s alloy before and after treatment (5th and 10th days). Similarly, ammoniacal nitrogen in treated samples was estimated using Nessler’s reagent, and the color developed was photometrically measured at 410 nm. Nitrates were estimated by coupling with diazotized sulfanilic acid and N-(1napththyl)-ethylene dihydrochloride to form reddish purple azo dye at pH 2.0 to 2.5 (Sahrawat & Prasad, 1975). Olsen’s method was used to estimate phosphorus in the effluent before and after treatment (Wolf & Baker, 1985). The reduction/removal percentage of BOD, COD, and TDS was calculated as follows (Eq. 4):
where Ci is the initial concentration (mg/L) and Cf is the final concentration (mg/L) (Asadi et al., 2019; Saranya & Shanthakumar, 2019).
Statistical analysis
All study experiments were performed in triplicates (n = 3), and the data was represented as mean ± standard error. One-way ANOVA was conducted to analyze the significant mean values of important parameters like BOD, COD, TDS, nitrogen, and phosphate using Tukey’s HSD (honestly significant difference) test for the four microalgal cultures (Barrera Bernal et al., 2008; Khemka & Saraf, 2015).
Results and discussion
Characterization of dairy effluent
We characterized the dairy effluent before and after treatment for various parameters like pH, TDS, BOD, COD, alkalinity, hardness, and turbidity as mentioned in Table.1. The nutrient profiling determined for DWW collected for the study was found to be similar to the characteristics of DWW reported earlier (de Queiroz et al., 2020; Malla et al., 2015).
Morphological analysis of algal cells
Figure 1 shows the cells of the microalgal species D. olivaceous, C. vulgaris, and S. dimporphus at 10 µm scale. In the SEM analysis, the cells looked swollen due to the accumulation of dissolved solids from the DWW on the 10th day of the experiment. The SEM images of the microalgae before and after treatment are shown in Fig. 2. The cells show prominent structural alteration in their surface due to exposure to pollutants from the effluent. Due to the absorption of pollutants, the cells of C. vulgaris are completely damaged with a granular, uneven, and porous surface.
Scaling up to bioreactor setup
The total cost of commercial-grade fertilizers is Rs. 215/- for 450 g. About 100 mg/L was observed to be necessary for the rapid growth of algal cells. Frequently used algal culture (chu10) media costs around 6872/- for 100 g and about 123 mg/L is required for effective growth. Commercial-grade fertilizers reduce the media cost to Rs. 8404.79/- for every 1000 L media. Substitution of the media with fertilizers is approximately 175 times cheaper and involves much less unit operation and infrastructure for the remediation.
Algal growth estimation
After the setup of the microalgal culture in 10 L plastic cans, the culture conditions were maintained at constant temperature (25 °C) and light intensity (2500 lx). The optical density at 680 nm was taken every 24 h to estimate the cell growth after adding the effluent.
The dry biomass estimated was 1.44 g L−1, 0.88 g L−1, 1.06 g L−1, and 0.65 g L−1 in D. olivaceous, S. dimorphus, C. vulgaris, and the DCS consortium after treatment with DWW (Fig. 3a). The dry biomass showed higher results when compared with the control. A higher biomass was obtained with D. olivaceous and S. dimorphus than the other two species.
Specific growth rate and doubling time
The specific growth rate reached the maximum of 0.55 day−1 for D. olivaceous, 0.22 day−1 for C.vulgaris, 0.39 day−1 for S. dimorphus, and 0.23 day−1 for the DCS mixed culture on the 5th day of cultivation. The growth slowed after the 5th day due to the entry of cells into the late stationary phase and nutrient depletion. In continuous mode, the growth rate was maintained for longer when the effluent is added daily. The algal cells survived until the 10th day of the experiment, even when the addition of effluent was stopped on the 5th day for the acclimatization process. A growth rate of 0.24 day−1 for D. olivaceous, 0.17 day−1 for C.vulgaris, 0.09 day−1 for S. dimorphus, and 0.20 day−1 for the DCS mixed culture was obtained, respectively. D. olivaceous had a peak growth rate on the 5th day of about 0.55 day−1 and maintained growth till the 10th day with a growth rate of 0.24 day−1. However, C. vulgaris could not survive after the 5th day, and the growth rate reached as low as 0.09 day−1 on the 10th day (Fig. 3b). The doubling time of the algal cells is the exact inverse of the specific growth rate with C. vulgaris and S. dimorphus having a maximum doubling time of 4.16 td and 7.93 td, followed by 2.83 td and 3.39 td in D. olivaceous and the DCS consortia on the 10th day of experiment (Fig. 3c).
Chlorophyll A estimation
The chlorophyll A content in D. olivaceous, S. dimorphus, C. vulgaris, and the DCS consortium was determined as 6.66 µg/mL, 2.22 µg/mL, 6.23 µg/mL, and 5.13 µg/mL respectively. Chlorophyll A content was 77% for S. dimorphus, 76% for DCS consortia, 57% for D. olivaceous, and 48% for C. vulgaris. It was difficult to grow Chlorella sp., under dark conditions (Fig. 3d). The other species with low chlorophyll content could adapt to the dark.
Physicochemical characteristics
The color of the effluent treated with D. olivaceous, S. dimorphus, C. vulgaris, and DCS consortium changed on day 2 to mild greyish white, on day 5 to greenish-yellow, and on day 10, it completely turned to green. The increase in algal biomass and their photosynthetic activity changed the color and odor of the dairy effluent. The physicochemical characteristics of dairy effluent before and after treatment are represented in Table 2. The odor changed to an acceptable level from an offensive smell.
The pH of the untreated effluent was found to be more acidic due to the accumulation of lactic acid produced by the decomposition of lactose under aerobic conditions (Benemann et al., 1987). In our study, the pH of the dairy effluent wastewater increased from 5.68 to 7.52 with D. olivaceous and C. vulgaris, whereas pH increased to 7.50 and 7.58 in S. dimorphus and the DCS consortia (Fig. 4a). An increase in pH is due to the uptake of CO2 and bicarbonate from the wastewater by the photosynthesis of microalgal species.
Fluoride concentration became significantly reduced from 4.0 to 0.2 mg/L (95%) in the S. dimorphus treated sample, followed by 62.50% in both D. olivaceous and DCS consortia, and 70% in C. vulgaris. Similarly, the chloride and sulfate concentrations reduced by 81.58% and 59.12% in D. olivaceous, 82.57% and 84.91% in S. dimorphus, 82.24% and 80.50% in C. vulgaris, and 83.22% and 83.02% in the DCS consortia.
Dissolved oxygen levels increased gradually from day 1 of effluent treatment because of the increased cell growth of the microalgae. After day 8, a few species gradually had a small reduction in DO level due to nutrient depletion and a fall in cell growth. The DO level increased from 3.73 to 6.67 in 8 days for D. olivaceous treated with effluent, whereas for S. dimorphus, C. vulgaris, and the DCS consortia, the level went up from 2.83 to 6.20 in 8 days, 3.50 to 6.57 in 7 days, and 1.77 to 5.23 in 7 days, respectively, after treatment on 10th day (Fig. 4b). The DO level was found to be inversely proportional to BOD and COD values. The higher the reduction percentage of BOD and COD, the higher the dissolved oxygen level assuring better water quality to support aquatic life. The photosynthetic activity of the green species increases dissolved oxygen content as the microbial biomass and growth rate increases gradually from day 1 to day 8 as reported by Umamaheswari and Shanthakumar (2016).
Nutrient removal efficiency
A high inoculation culture density is important in increasing algal growth and nutrient removal efficiency. Nitrate, nitrite, and ammoniacal nitrogen are the free inorganic forms of nitrogen in excess in agro-based wastewater and enhance microalgae growth. On the first day, immediately after the addition of the effluent, the initial values of most of the parameters like nitrate, nitrite, ammoniacal nitrogen, and phosphorous were recorded to have higher values than the permissible limits fixed by the government bodies. But microalgae cultures could utilize the available nutrients in the DWW for their growth and increase in biomass by simultaneously reducing the organic load of nutrients that pollute the environment they are discharged (Kothari et al., 2013; Wang et al., 2010).
The successful adaptation of dairy wastewater as a suitable growth medium in microalgal cultures was a good example of phycoremediation supported by the consumption of excess nitrogen and phosphorous from the DWW. As mentioned earlier, the physicochemical properties analyzed on the 10th day substantially reduced all the parameters. Previous studies describe the algal property of using nitrite and ammonia only when there is low availability of nitrates (Richards & Mullins, 2013). Nutrient removal depends directly on the uptake of nutrients and biomass harvest and indirectly depends on two mechanisms called ammonia–nitrogen volatilization and orthophosphate precipitation (Nurdogan & Oswald, 1995). The present analysis reports a reduction of nitrates from 32.6 to 10 mg/L (76.53%) in D. olivaceous, 9.8 mg/L (77%) in S. dimorphus, 14.3 mg/L (66.43%) in C. vulgaris, and 13.2 mg/L (69.01%) in DCS consortia-treated samples. Nitrite reduction was found to be reduced to 79.41% in S. dimorphus, 76.47% in DCS consortia, 73.53% in D. olivaceous, and 64.71% in C. vulgaris, respectively. Ammoniacal nitrogen was reduced to its maximum by C. vulgaris (93.57%), followed by S. dimorphus (92%), D. olivaceous (90.01%), and DCS consortia (88.80%), respectively.
Nutrients from animal manure accumulated in the soil, but they get washed off, leading to a nutrient loss for plants and eutrophication of the water reducing the water quality.
The high percentage of phosphorous in raw effluent may be due to overusing detergents in our daily life. Phosphate increases the biological activity of microbes and decreases the DO content of wastewater, thereby decreasing the water quality (Nandini et al., 2013). In our study, the phosphorous content was reduced by 70.77% in D. olivaceous, 66.65% in S. dimorphus, 79.58% in C. vulgaris, and 82.04% in the DCS consortia after the 10th day of treatment. The DCS consortia-treated effluent found a high reduction percentage (Khemka & Saraf, 2015). The reduction of nitrites, nitrates, ammoniacal nitrogen, and phosphorus is recorded in Table 2, showing the 5th- and 10th-day reduction. A similar phosphate reduction of 83% by Scenedesmus was observed in fermented swine effluent. In another case study, high phosphate reduction was recorded in effluent treatment using S. quadricauda (Kim et al. (2007). In our study, D. olivaceous and S. dimorphus exhibited significant removal of organic nitrogen sources, whereas the DCS consortia reduced the maximum organic nitrogen.
The reduction percentage of COD was lower than the BOD because of the dominance of microalgae. The major indicators of organic pollutant load are the reduction of BOD and COD. Biological activity by microalgae is the main reason for the decrease in the effluent after treatment. Kotteswari et al. (2012) documented a 47.34% reduction in BOD and a 24.69% COD reduction in dairy effluent when treated with Spirulina platensis. The removal efficiency of Scenedesmus sp. was reported to be maximum in domestic effluents by Zhang et al. (2018). BOD and COD reduced from 3.34 to 1.23 mg/L and 9.1 to 4.93 mg/L in Chlorella pyrenoidosa and from 3.34 to 1.84 mg/L and 9.1 to 5.2 mg/L in Scenedesmus abundans after treatment (Nandini et al., 2013).
In our study, the maximum reduction of BOD was found to be in S. dimorphus (53.45%) followed by D. olivaceous (45.40%), C. vulgaris (44.25%), and the DCS consortia (43.10%), respectively, on the 10th day of treatment. Similarly, COD reduction was recorded to be maximum in D. olivaceous (82.85%), followed by C. vulgaris (81.98%), S. dimorphus (80.73%), and finally the DCS consortia (80.10%) (Fig. 5a-b).
In this study, the total dissolved solids (TDS) were reduced to 53.93% by D. olivaceous, which is much lower when compared to the influent. In reality, the TDS range of the dairy effluent exceeded the limits prescribed by CPCB (1995). In our study, TDS was reduced to 75.82%, 72.89%, 70.70%, and 69.05% by the microalgal species D. olivaceous, S. dimorphus, C. vulgaris, and the DCS culture. The overall removal efficiency of pollutant removal is depicted in Fig. 6 for BOD, COD, and TDS in percentage. Similar results have been reported by Kotteswari et al. (2012), where S. platensis reduced TDS by 74.37%. Murugesan and Dhamotharan (2007) reported a 36.19% and 19.16% reduction of TDS in oil refinery and petroleum effluents, respectively (Kotteswari et al., 2012).
Statistical analysis
Tukey’s HSD between the different physicochemical variables among the four microalgae in treating dairy wastewater showed a statistically significant correlation (p < 0.001; n = 3) for the removal of the following parameters like nitrate (C. vulgaris), ammoniacal nitrogen (D. olivaceous, C. vulgaris), and TDS (all 4 species). Univariate analysis of variance (ANOVA) showed a significant effect (p < 0.05) for sulfide removal in D. olivaceous. The majority of the other physicochemical parameters including nitrite (all species excluding C. vulgaris), nitrate (all species), ammoniacal nitrogen (S. dimorphus, DCS consortium), fluoride (all species), phosphorous (all species), chloride (all species), and sulfide (all species excluding D. olivaceous) showed significant correlation with p < 0.01. The pH of D. olivaceous, C. vulgaris, and S. dimorphus did not show any significant increase, whereas the changes in pH of the DCS consortium were substantial correlations (p < 0.01) (Table 3).
Conclusion
This study showed that microalgae are an effective alternative to conventional wastewater treatment. The substantial reduction of the nitrogen and phosphorus load on the 10th day in D. olivaceous and S. dimorphus confirms the elimination of the major agents of eutrophication, including TN, TP, TDS, BOD, and COD. In this study, the reduction percentage of BOD (53.45%), COD (82.25%), and TDS (75.82%) was maximally obtained by D. olivaceous and S. dimorphus. The effective use of commercial-grade fertilizers for scaling up microalgal cultures makes the technology economically adaptable to the industrial scale. In the future, successful technology transfer to industries is required, leading to large-scale microalgal biomass production. This opens up an avenue for producing other value-added products like biofuel, biofertilizer, and livestock production, thereby making wealth from waste leading to zero-waste management. Therefore, phycoremediation has dual benefits for industries and society, providing a greener environment with additional inexpensive and safe by-products.
Availability of data and materials
We have incorporated all the data associated with this manuscript.
References
Abatenh, E., Gizaw, B., Tsegaye, Z., & Wassie, M. (2017). The role of microorganisms in bioremediation-A review. Open Journal of Environmental Biology, 2(1), 038–046.
Amul - Milk. (2021). The inspiration behind a revolution. Retrieved August 21, 2021, from https://www.amuldairy.com/history.php/
APHA. (1998). Standard methods for the examination of water and wastewater. 20th edn. APHA, American Water Works Association, and Water Pollution Control Federation, Washington, DC, USA.
APHA. (2012). Standard methods for the examination of water and wastewater. Washington DC American Public Health Association.
Arora, V., & Khosla, B. (2021). Conventional and contemporary techniques for removal of heavy metals from soil. In Biodegradation technology of organic and inorganic pollutants. IntechOpen.
Asadi, P., Rad, H. A., & Qaderi, F. (2019). Comparison of Chlorella vulgaris and Chlorella sorokiniana pa.91 in post treatment of dairy wastewater treatment plant effluents. Environmental Science and Pollution Research, 26(28), 29473–29489. https://doi.org/10.1007/s11356-019-06051-8
Azubuike, C. C., Chikere, C. B., & Okpokwasili, G. C. (2016). Bioremediation techniques–Classification based on site of application: Principles, advantages, limitations and prospects. World Journal of Microbiology and Biotechnology, 32(11), 1–18.
Balaji, S., Kalaivani, T., & Rajasekaran, C. (2014a). Biosorption of zinc and nickel and its effect on growth of different Spirulina strains. Clean - Soil, Air, Water, 42(4), 507–512. https://doi.org/10.1002/clen.201200340
Balaji, S., Kalaivani, T., Rajasekaran, C., Shalini, M., Siva, R., Singh, R. K., & Akthar, M. A. (2014b). Arthrospira (Spirulina) species as bioadsorbents for lead, chromium, and cadmium - A comparative study. Clean - Soil, Air, Water, 42(12), 1790–1797. https://doi.org/10.1002/clen.201300478
Barrera Bernal, C., Vázquez, G., Barceló Quintal, I., & Laure Bussy, A. (2008). Microalgal dynamics in batch reactors for municipal wastewater treatment containing dairy sewage water. Water, Air, and Soil Pollution, 190(1–4), 259–270. https://doi.org/10.1007/s11270-007-9598-3
Benemann, J. R., Tillett, D. M., & Weissman, J. C. (1987). Microalgae biotechnology. Trends in Biotechnology, 5(2), 47–53. https://doi.org/10.1016/0167-7799(87)90037-0
Chokshi, K., Pancha, I., Ghosh, A., & Mishra, S. (2016). Microalgal biomass generation by phycoremediation of dairy industry wastewater: An integrated approach towards sustainable biofuel production. Bioresource Technology, 221, 455–460. https://doi.org/10.1016/j.biortech.2016.09.070
Central Pollution Control Board, CPCB. (1995). Classification of inland surface waters (CPCB Standards). Water Quality Parivesh, 1(4), 6.
Chen, H., Wang, J., Zheng, Y., Zhan, J., He, C., & Wang, Q. (2018). Algal biofuel production coupled bioremediation of biomass power plant wastes based on Chlorella sp. C2 cultivation. Applied Energy. https://doi.org/10.1016/j.apenergy.2017.11.058
Chinnasamy, S., Bhatnagar, A., Hunt, R. W., & Das, K. C. (2010). Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresource Technology, 101(9), 3097–3105. https://doi.org/10.1016/j.biortech.2009.12.026
Choi, Y. K., Jang, H. M., & Kan, E. (2018). Microalgal biomass and lipid production on dairy effluent using a novel microalga, Chlorella sp. isolated from dairy wastewater. Biotechnology and Bioprocess Engineering, 23(3), 333–340. https://doi.org/10.1007/s12257-018-0094-y
de Queiroz, R. D. C. S., Maranduba, H. L., Hafner, M. B., Rodrigues, L. B., & de Almeida Neto, J. A. (2020). Life cycle thinking applied to phytoremediation of dairy wastewater using aquatic macrophytes for treatment and biomass production. Journal of Cleaner Production, 267, 122006. https://doi.org/10.1016/j.jclepro.2020.122006
Do, J. M., Jo, S. W., Kim, I. S., Na, H., Lee, J. H., Kim, H. S., & Yoon, H. S. (2019). A feasibility study of wastewater treatment using domestic microalgae and analysis of biomass for potential applications. Water, 11(11), 2294.
Dominic, V. J., Murali, S., & Nisha, M. C. (2009). Phycoremediation efficiency of three micro algae Chlorella vulgaris, Synechocystis salina and Gloeocapsa gelatinosa. SB Academic Review, 16(1&2), 138–146.
Enamala, M. K., Enamala, S., Chavali, M., Donepudi, J., Yadavalli, R., Kolapalli, B., Aradhyula, T. V., Velpuri, J., & Kuppam, C. (2018). Production of biofuels from microalgae - A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renewable and Sustainable Energy Reviews, 94, 49–68. https://doi.org/10.1016/j.rser.2018.05.012
FAO. (2021). Dairy market review. Retrieved November 28, 2022, from https://www.fao.org/3/cc1189en/cc1189en.pdf
Feil, A. A., Schreiber, D., Haetinger, C., Haberkamp, Â. M., Kist, J. I., Rempel, C., Maehler, A. E., Gomes, M. C., & da Silva, G. R. (2020). Sustainability in the dairy industry: A systematic literature review. Environmental Science and Pollution Research, 27(27), 33527–33542. https://doi.org/10.1007/s11356-020-09316-9
Ghashghaie, M., Eslami, H., & Ostad-Ali-Askari, K. (2022). Applications of time series analysis to investigate components of Madiyan-rood river water quality. Applied Water Science, 12(8), 1–14.
Khemka, A., & Saraf, M. (2015). Phycoremediation of dairy wastewater coupled with biomass production using Leptolyngbya sp. Journal of Environmental Science and Water Resources, 4(3), 104–111.
Kim, M. K., Park, J. W., Park, C. S., Kim, S. J., Jeune, K. H., Chang, M. U., & Acreman, J. (2007). Enhanced production of Scenedesmus spp. (green microalgae) using a new medium containing fermented swine wastewater. Bioresource Technology, 98(11), 2220–2228. https://doi.org/10.1016/j.biortech.2006.08.031
Kothari, R., Pathak, V. V., Kumar, V., & Singh, D. P. (2012). Experimental study for growth potential of unicellular alga Chlorella pyrenoidosa on dairy waste water: An integrated approach for treatment and biofuel production. Bioresource Technology, 116, 466–470. https://doi.org/10.1016/j.biortech.2012.03.121
Kothari, R., Prasad, R., Kumar, V., & Singh, D. P. (2013). Production of biodiesel from microalgae Chlamydomonas polypyrenoideum grown on dairy industry wastewater. Bioresource Technology, 144, 499–503. https://doi.org/10.1016/j.biortech.2013.06.116
Kotteswari, M., Murugesan, S., & Ranjith Kumar, R. (2012). Phycoremediation of dairy effluent by using the microalgae Nostoc sp. International Journal of Environmental Research and Development, 2(1), 35–43. ISSN 2249-3131.
Kumar, P. K., Krishna, S. V., Naidu, S. S., Verma, K., Bhagawan, D., & Himabindu, V. (2019). Biomass production from microalgae Chlorella grown in sewage, kitchen wastewater using industrial CO2 emissions: Comparative study. Carbon Resources Conversion, 2(2), 126–133. https://doi.org/10.1016/j.crcon.2019.06.002
Liu, C., Subashchandrabose, S., Ming, H., Xiao, B., Naidu, R., & Megharaj, M. (2016). Phycoremediation of dairy and winery wastewater using Diplosphaera sp. MM1. Journal of Applied Phycology, 28(6), 3331–3341. https://doi.org/10.1007/s10811-016-0894-4
Malla, F. A., Khan, S. A., Rashmi, Sharma, G. K., Gupta, N., & Abraham, G. (2015). Phycoremediation potential of Chlorella minutissima on primary and tertiary treated wastewater for nutrient removal and biodiesel production. Ecological Engineering, 75, 343–349. https://doi.org/10.1016/j.ecoleng.2014.11.038
Maurya, R., Paliwal, C., Ghosh, T., Pancha, I., Chokshi, K., Mitra, M., Ghosh, A., & Mishra, S. (2016). Applications of de-oiled microalgal biomass towards development of sustainable biorefinery. Bioresource Technology, 214, 787–796.
Moitra, M. (2021). Top 4 milk exporters in India 2022. Retrieved January 13, 2023. https://blog.exportsconnect.com/milk-exporters-india/
Murugesan, S. R., & Dhamotharan, J. K. (2007). Phycoremediation of oil refinery effulent using cyanobacterium. Ecology, Environment and Conservation, 13(4), 703–708.
Nandini, N., Kumar, M., Sivasakthivel, S., & Vijay Kumar, M. (2013). Efficacy of microalgae on the removal of pollutants from wastewater. International Journal of Emerging Technologies in Computational and Applied Sciences, 1(3), 82–86.
Nurdogan, Y., & Oswald, W. J. (1995). Enhanced nutrient removal in high-rate ponds. Water Science and Technology, 31(12), 33–43. https://doi.org/10.1016/0273-1223(95)00490-E
Ostad-Ali-As, K. (2022). Investigation of meteorological variables on runoff archetypal using SWAT: Basic concepts and fundamentals. Applied Water Science, 12(8), 1–18.
Ostad-Ali-Askari, K. (2022). Management of risks substances and sustainable development. Applied Water Science, 12(4), 1–23.
Renuka, N., Sood, A., Prasanna, R., & Ahluwalia, A. S. (2015). Phycoremediation of wastewaters: A synergistic approach using microalgae for bioremediation and biomass generation. International Journal of Environmental Science and Technology, 12(4), 1443–1460. https://doi.org/10.1007/s13762-014-0700-2
Richards, R. G., & Mullins, B. J. (2013). Using microalgae for combined lipid production and heavy metal removal from leachate. Ecological Modelling, 249, 59–67. https://doi.org/10.1016/J.ECOLMODEL.2012.07.004
Sahrawat, K. L., & Prasad, R. (1975). A rapid method for determination of nitrate, nitrite, and ammoniacal nitrogen in soils. Plant and Soil, 42(1), 305–308. https://doi.org/10.1007/BF02186992
Saranya, D., & Shanthakumar, S. (2019). Green microalgae for combined sewage and tannery effluent treatment: Performance and lipid accumulation potential. Journal of Environmental Management, 241, 167–178. https://doi.org/10.1016/j.jenvman.2019.04.031
Sirisha, K., Suganya, B., Sivasubramanian, V., Bs, V., Swaminathan, D., Babu, A. C., & Meyyappan, N. (2017). Studies on the effect of pulsed magnetic field on the productivity of algae grown in dye industry effluent. Journal of Applied Biotechnology & Bioengineering, 3(5), 409–413.
Swetha, C., Sirisha, K., Swaminathan, D., & Sivasubramanian, V. (2016). Study on the treatment of dairy effluent using Chlorella vulgaris and production of biofuel (algal treatment of dairy effluent). BioTechnology An Indian Journal, 21(1), 12–17.
Umamaheswari, J., & Shanthakumar, S. (2016). Efficacy of microalgae for industrial wastewater treatment: A review on operating conditions, treatment efficiency and biomass productivity. Reviews in Environmental Science and Biotechnology, 15(2), 265–284. https://doi.org/10.1007/s11157-016-9397-7
Ummalyma, S. B., & Sukumaran, R. K. (2014). Cultivation of microalgae in dairy effluent for oil production and removal of organic pollution load. Bioresource Technology, 165, 295–301.
Wang, L., Li, Y., Chen, P., Min, M., Chen, Y., Zhu, J., & Ruan, R. R. (2010). Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresource Technology, 101(8), 2623–2628. https://doi.org/10.1016/j.biortech.2009.10.062
Wolf, A. M., & Baker, D. E. (1985). Comparisons of soil test phosphorus by Olsen, Bray P1, Mehlich I and Mehlich III methods. Communications in Soil Science and Plant Analysis, 16(5), 467–484. https://doi.org/10.1080/00103628509367620
Zhang, L., Cheng, J., Pei, H., Pan, J., Jiang, L., Hou, Q., & Han, F. (2018). Cultivation of microalgae using anaerobically digested effluent from kitchen waste as a nutrient source for biodiesel production. Renewable Energy, 115, 276–287.
Acknowledgements
The authors thank the VIT management and the Dean SBST for providing the required facilities, infrastructure, support, and encouragement. A special acknowledgment to Bharathidasan University, Tiruchirappalli for providing us with the microalgal strains and helping with SEM analysis. The authors also acknowledge Mr. Arunram, Mr. Sasi Bhushan, Mr. Nagaraj, Mr. Manoj, and Ms. Chandra for their extended support in performing the experiments. The authors extend their gratitute to Prof. Michael Pillay, Erudite Scientific Editing & Science Writing Solutions, South Africa, for English correction and proofreading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Phycoremediation technology is robust due to minimum automation, maintenance, and skilled human resources.
• Algae are eligible candidates for bioremediation due to the following factors: (a) oxygenation of the environment, (b) CO2 sequestration ability—a solution for the threat of global warming, and (c) removal of excess nutrients that lead to eutrophication.
• Promote and ensure the integration of algae-feed-fertilizer production with livestock raising in the nitrogen recycling systems lead industries towards zero-waste management.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nachiappan, K., Chandrasekaran, R. Reformation of dairy effluent—a phycoremediation approach. Environ Monit Assess 195, 405 (2023). https://doi.org/10.1007/s10661-023-10995-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-10995-3