Abstract
Laminaria farlowii, golden kombu, is of interest as a novel species for aquaculture in Southern California, USA. Thermal biology and climate change resilience are important to the species' usefulness as a crop for the future. Wild populations of L. farlowii live primarily below the seasonal thermocline but individuals have been successfully cultivated in near surface conditions of light intensity and temperature. We examined the thermal biology of female gametophytes and juvenile sporophytes of L. farlowii. We grew the female gametophytes and juvenile sporophytes across gradients of temperature (9–20 °C) and light intensity (20–80 µmol photons m−2.s−1), finding growth rates were saturated by all light intensities tested and sensitive to temperature. Optimal growth temperatures were 16 °C for the female gametophytes and 15 °C for juvenile sporophytes. In a separate experiment, larger adult sporophytes were exposed to thermal regimes differing in mean temperature (10–20 °C) and thermal variability, under a photoperiod chosen to induce sorus formation. Growth rates were not significantly different from 14–18 °C but sori were formed only at a mean temperature of 15 °C. These results indicate that nursery and cultivation methods developed for a similar kelp, Saccharina latissima, are suitable for L. farlowii, and that the species can grow in the predominant coastal temperatures of Southern California.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temperate and polar oceanic ecosystems are rapidly changing environments with those changes reflected in dramatic fluctuations in distribution and abundance of kelps in coastal areas (Smale et al. 2013; Krumhansl et al. 2016; Hamilton et al. 2020). Warming is manifest in increasing average seawater temperatures and in thermal variability associated with increased frequency and duration of short- (≥ 5 days), or medium- (weeks or months), term periods of elevated seawater temperatures termed marine heatwaves (Oliver et al. 2018; Holbrook et al. 2019). Warming trends are causing geographic range shifts and often contractions of range with more rapid poleward advances of range limits at the trailing edge (Martínez et al. 2018). Marine heat waves have been linked to habitat loss and drastic changes to marine community structure (Jentsch et al. 2007; Wernberg et al. 2013; Cavanaugh et al. 2019; Filbee-Dexter et al. 2020). Persistent events like El Nino Southern Oscillation (ENSO), or the Northeast Pacific “Blob” caused dieback of kelp forests in California and Baja California, Mexico (Ladah et al. 1999; Edwards and Estes 2006; Rogers-Bennett and Catton 2019), especially when coupled with additional stressors (e.g., overgrazing by sea urchins).
Prior research on the thermal physiology of kelps shows that their broad-scale distributions are consistent with optimal performance for growth and survivorship at temperatures ≤ 20 °C and declining performance at higher temperatures (Fain and Murray 1982; Davison 1987; Gerard and Du Bois 1988; Egan et al. 1989; Gerard 1997; Park et al. 2017; Liesner et al. 2020; Gauci et al. 2022; Schimpf et al. 2022; Visch et al. 2023). More recently, recognition that small and microscopic gametophyte stages of kelps, which may be crucial for population persistence (Edwards 2022), has emphasized their responses to key variables, like temperature and light intensity. A central question is: are microscopic stages of kelps especially vulnerable to increased sea surface temperatures causing decreased growth and high mortality rates? (Lind & Konar 2017; Korabik et al. 2023), or do they escape thermal damage in microhabitat refugia, or have greater tolerance than sporophytes by virtue of more efficient selection on haploid genotypes (Ladah et al. 1999; Mohring et al. 2014; Becheler et al. 2022). The emerging evidence suggests the relative thermal tolerance among ploidy stages is both species- and population-specific (Ladah & Zertuche-González 2007; Schimpf et al. 2022; Strasser et al. 2022; Veenhof et al. 2022).
While ocean warming threatens persistence of kelp forests, demand for sustainable cultivation of seaweeds is rising as wild fisheries decline globally and the human population continues to rise. Macrocystis pyrifera, the giant kelp, is the primary wild harvested forage used for abalone aquaculture in California. Giant kelp is an abundant, fast-growing species with a broad geographic distribution (Schiel and Foster 2006). However, a severe dieback of giant kelp was documented during the “Blob” marine heat wave of 2015/2016 (Cavanaugh et al. 2019). That event required increased travel to collect kelp for abalone forage for aquaculture in Southern California at that time (Kübler et al. 2021). Recent climate change and associated marine heat waves illustrate the need for diversification of forage for abalone culture, both in terms of species and of production methods of that forage.
Beyond abalone forage, sustainable cultivation of seaweeds can contribute significantly to food security with production currently valued at 11 billion US$ (FAO 2020). Seaweed aquaculture is gaining interest in the USA and has the potential to be a key contributor to the American Blue Economy with extensive research focus on open-ocean cultivation systems, selective breeding, and improved hatchery protocols (Augyte et al. 2021; US Department of Energy [DoE, ARPA-E] 2021; Kite-Powell et al. 2022). An adaptive solution to benefit the seaweed aquaculture industry in California in the face of climate change is to replace wild harvest with cultivation of native species expected to be thermally resilient (Kübler et al. 2021). Six key species traits have been identified as important for open-ocean cultivation; (1) selecting a native species, (2) large in size with a simple morphology, (3) high growth rates, (4) high photosynthesis: respiration ratio, (5) thermal tolerance to warm temperatures, and (6) the capacity to store a large internal reserve of nitrogen (Kübler et al. 2021).
The kelp Laminaria farlowii Setchell, golden kombu, is a viable candidate for kelp aquaculture in California (Kübler et al. 2021). It is distinguished by longitudinally oriented corrugations on a prostrate blade and may reach up to 5 m in length (Druehl and Clarkston 2016). Its habitat distribution is the subtidal with a range from Bahia del Rosario, Baja California, Mexico to Central California with an isolated collection in Vancouver Island, Canada (www.Algaebase.org, accessed 31 Jul 2019; Lonhart et al. 2019), suggesting that it may be adapted to warmer water. The average annual sea surface temperature over the typical range of the species is 10–20 °C (NOAA OSPO). It often dominates the deep-water algal assemblages (typical depths of 40–60 m) and its large blades form horizontal structure providing shelter and protection for juvenile kelp perch and kelp bass (Lissner & Dorsey 1986; Anderson 1994). Additionally, white abalone, Haliotis sorenseni, have shown to be significantly more abundant in areas with high cover of L. farlowii (Lafferty et al. 2004).
Like other Laminaria species, L. farlowii has a heteromorphic haplodiplontic life-cycle with alternations between microscopic gametophytes and macroscopic sporophytes. The different developmental stages of this intricate life-cycle exhibit distinct thermal response patterns and survival thresholds (Augyte et al. 2019; Martins et al. 2020). Depending on species, kelp gametophytes have a broad tolerance to temperature and irradiance while some processes can be more sensitive to evnironemtnal fluctuations including gametogenesis and early sporophyte development when compared to gametophyte vegetative growth (Veenhof et al. 2022). Previous studies indicate that kelp gametophytes exhibit a higher thermal tolerance allowing them to tolerate elevated seawater temperatures compared to sporophytes—an evolutionary trait that enables the species to persist in adverse conditions for brief periods of time at the edges of their distribution (Bolton & Lüning 1982; Martins et al. 2020; Veenhof et al. 2022).
Similar to sugar kelp, (Saccharina sp.), which is extensively cultivated in temperate waters globally, Laminaria farlowii shares likenesses in morphology and reproduction, making it a promising candidate for cultivation on lines in Southern California. Wild populations of L. farlowii live primarily below the seasonal thermocline but individuals have been successfully cultivated in near surface conditions of light intensity and temperature (Kübler et al. 2021). Pilot scale cultivation of L. farlowii is underway in a near-shore farm in Ensenada, MX but no commercial production has yet been reported (J. Zertuche-González, pers comm.). Unlike most kelps in cultivation, L. farlowii is a warm temperate, southern species that has been hypothesized, due to its geographic distribution to have greater resilience to seawater warming. Currently, the ecophysiological tolerance of this species is undocumented, leaving cultivation efforts subject to unknown levels of risk due to climate change.
We investigated the thermal tolerance of L. farlowii female gametophytes and sporophytes with the goal of developing methods to optimize cultivation. Results of this work will facilitate ongoing cultivation efforts with our industry partner, The Cultured Abalone, LLC and others. Here, we present data comparing the relative tolerances of gametophytes and sporophytes of L. farlowii to temperature and light intensity. Our experiment affords a test to distinguish among alternative hypotheses whether gametophytes of L. farlowii are more, or less, sensitive than very young sporophytes to temperature and light and to estimate the functional relationships of growth to each of these independent variables. These data can inform which stage of the life cycle is more vulnerable to thermal variation as well as conditions for maximizing growth in nursery settings.
Materials and methods
Gametophyte cultures
Laminaria farlowii blades with reproductive sorus tissue were collected by SCUBA offshore of Goleta, CA in April 2019, placed in Ziplock bags with seawater and shipped in coolers with ice packs overnight to the Seaweed Marine Biotechnology Laboratory, at the University of Connecticut, Stamford, CT for spore release (Fig. 1). Sorus tissue was prepared the night before meiospore release following Redmond et al. (2014). Meiospores were released the next morning into sterile seawater after quantification of spore solution (5000—40,000 spores mL−1 in a dilution series) and were pipetted onto glass slides placed at the bottom of deep-welled (250 mL) Petri dishes with Provasoli’s enriched seawater medium (PES) (Provasoli 1968). The dishes were then placed into a walk-in incubator at 10° C and 12:12 light:dark photoperiod at 35 µmol photons m−2 s−1 and allowed to develop for one month. Gametophytes were grown under red light to prolong the vegetative stage. Individual gametophytes were then isolated and grown in 20 mL scintillation vials with sterilized seawater enriched with half-strength PES plus germanium dioxide (GeO2) to prevent diatom growth, which was replaced approximately every 4–6 months. They were grown at 12 °C and photon flux density of 20 µm photons m−2 s−1 on a 12:12 light:dark cycle under red light.
Prior to the start of the experiment, female gametophytes were ground for 30 s in a blender and transferred into individual 125 mL flasks with sterile enriched seawater changed fortnightly and grown for one month as described above to provide sufficent biomass for the experiment. Female gametophyte cultures were combined into one batch to standardize biomass allocated to each experimental treatment. Fresh weight biomass at the start of the experiment was measured by scooping gametophyte tissue with a microscoop of known weight and measuring the difference between the total weight with and without gametophyte tissue. The total starting wet weight of gametophytes in each dish ranged from 11.6 to 13.8 mg (mean 12.58 ± 0.49 mg SD).
Experiment 1: Female gametophyte growth
The effects of light intensity and temperature on juvenile female L. farlowii gametophytes were measured on a temperature gradient table with a temperatures ranging from 8–20 °C and with light levels ranging from 18–65 µmol photons m−2 s−1 with overhead adjustable fluorescent lights (Yarish et al. 1979; Augyte et al. 2019). In total, the table held 48 deep culture petri dishes (9.5 cm diameter, 7.5 cm height) each containing 250 mL of half-strength PES plus GeO2. Dishes were spaced and insulated by 1″ thick styrofoam on top of the gradient table (Fig. 2). Each dish’s position on the table had a unique light and temperature combination. Light intensities were resolved to ± 1 µmol photon m−2 s−1, and temperatures were resolved to ± 0.1 °C. Each dish was aerated through a Pasteur pipette placed in a hole in the lid. Gametophytes were incubated under experimental conditions on the gradient table for 14 days when the experiment was terminated.
The end biomass of each dish was harvested by filtering tissue through a 20 µm Nitex nylon mesh, transfering to individual, pre-weighed centrifuge tubes with a Pasteur pipet and spinning for 30 s at 21,000 RCF. The water was removed from each centrifuge tube by pipetting followed by wicking any remaining moisture on the tube wall with a Kimwipe. End biomass was calculated as the difference in mass between weight of the empty tube and the tube with gametophytes.
Growth rates of gametophytes was estimated as growth rate in percent per day using the formula,
where b1 and b2 are fresh weight biomass at times t1 (initial – day 0) and t2 (final – day 14), respectively.
Light, temperature, salinity were measured periodically throughout the experiment. Light intensity as µmol photon m−2 s−1were recorded using an Apogee quantum meter (Model MQ-200) at the top of each dish the day after the start of the experiment and each week thereafter. Temperature of the media in each dish was measured on the same schedule. Spot temperature checks on two dishes at the edge of the table were performed every weekday (Control Company/Traceable digital thermometer). Every 2–3 days, salinity checks were performed using a handheld refractometer (Vee Gee STX-3), and salinity was maintained at 30 ppt by pipetting in filtered DI water to dishes as necessary.
Experiment 2: Juvenile sporophyte growth
A separate experiment measured the effects of light intensity and temperature on juvenile L. farlowii sporophytes on the gradient table. Very small sporophytes of L. farlowii growing in the flow-through seawater system at the Cultured Abalone Farm in Goleta, CA, USA were collected and shipped overnight to the lab at the University of Connecticut. The average biomass of a sporophyte at the start of the experiment was 243 ± 33 mg (mean ± s.e.). The sporophytes were immediately transferred to dishes maintained at 12 °C under 40 µm photons m−2 s−1 for 6 days until the beginning of the experiment.
For this experiment, temperature ranged from 8.6–19.7 °C and light ranged from 11–99 µm photons m−2 s−1. Forty-seven sporophyes were used in the experiment. Dishes were filled with 250 mL of PES plus GeO2. Light intensity and temperature measured was measured weekly and temperature was checked for two dishes at the ends of the gradient every weekday. Salinity was checked every 2–3 days and maintained at 30 ppt. Starting and end wet weights were measured after dabbing the sporophytes until no more visible moisture was seen on a Kimwipe. The experiment ran for 14 days and biomass of each sporophyte was measured at the start and end, and growth estimated using formula (1).
Experiment 3. Adult sporophyte sorus induction
In a separate experiment involving adult sporophytes at the Cultured Abalone Farm, larger sporophytes were grown in a range of temperatures to estimate growth and determine the thermal optimum for sorus induction. We compared six thermal regimes with different average temperatures (10, 13, 14, 15, 18, 20 °C). Temperatures in the tanks were manipulated using a combination of aquarium heaters and ice probe chillers. Each tank held an ibutton temperature logger which recorded the temperature every 60 s. Tanks were illuminated by fluoroescent light fixtures on two sides at 100 µmol photons m−2 s−1 measured at the center of the tank. Seawater in the tanks was aerated and enriched with PES. A photoperiod of 12:12 light:dark was chosen as the maximum daylength for possible sorus formation. Five fronds were tagged with labeled flagging tape and assigned to each tank. Each frond was cut 5 cm above the meristem to induce sorus formation in the distal segment. Weekly measurments included biomass (g) and length of the meristematic and distal segments. Photographs were taken and distal segments were visually assessed for sorus formation weekly.
Data analysis
Estimates of growth rates among the array of sampling units across different combinations of continuously varying light intensities and temperatures on the gradient table represents a multiple regression experimental design without replication. We analyzed the growth data using a Bayesian hierarchical model of multiple regression to characterize the functional relationships between growth and light, temperature and their interaction. Many previous studies of productivity of temperate macroalgae as a function of temperature (e.g., Davison 1987; Kübler and Davison 1993; Kübler and Davison 1993) have shown a hump-shaped thermal performance curve. Thus, in addition to including light and its interaction with temperature, our regression models included a second-order polynomial (i.e., quadratic) term for temperature in addition to the linear effect:
Regression analyses were modeled as standardized regressions by normalizing the data as standard deviates before analyzing as functions of the standard deviates of light and temperature to improve the efficiency of Markov Chain Monte Carlo (MCMC) sampling (Kruschke 2015). Data (i.e., yij’s) were modeled as t-distributed with mean, µij, standard deviation, σ, and normality parameter, ν. The t-distribution is a convenient and robust distribution for continuous data that are not normally distributed owing to heavy-tailed distributions. All model parameters were modeled with vague prior distributions. The vague prior for the variance of the t-distribution was modeled as a uniform distribution between 10–10 and 100 on the standardized scale. The prior of the normality parameter, ν, was modeled as an exponential distribution with mean equal to 30 that provides equal opportunity to values greater, or less, than 30 where most of the variation in the shape of the distribution occurs as it converges towards the normal distribution when ν = 30 (Kruschke 2015). Regression coefficients were modeled as normally distributed. Prior distributions of coefficients used values of mean equal to 0 and variance equal to 4 on the standardized scale to make them vague. Conventional regression coefficients were obtained by converting back from standardized coefficients.
Posterior distributions for each random variable were generated from Markov Chain Monte Carlo (MCMC) samples of four chains run in parallel. Each chain consisted of 500 adaptation steps to tune parameters plus 1000 burn-in steps, followed by 187,500 steps that were thinned every 50 to yield 3,750 saved steps (i.e., 15,000 saved steps across the four chains). Diagnostics of MCMC chains, including trace plots, the Gelman-Rubin statistic, and autocorrelation were examined to confirm convergence of values among chains for each parameter. The effective sample sizes (ESS) for all regression coefficients were > 3556 and > 3747 for gametophyte and sporophyte analyses, respectively.
Regression coefficients for light, its interaction with temperature and, both, linear and quadratic effects of temperature were examined by comparing the modes and 95% credible intervals of their respective posterior distributions against the value of zero. Outcomes in which zero was outside of the 95% credible interval were considered strong effects estimated by the mode (i.e., most probable value). Bayesian models were constructed using jags for MCMC sampling in R (version 3.5.2) with the DBDA2E programs (Kruschke 2015).
Results
Experiments 1 & 2
Growth rates of L. farlowii were sensitive to temperature. As expected, both female gametophytes and sporophytes showed typical thermal performance curves characterized by positive regression coefficients with respect to the linear effect of temperature on growth, but negative coefficients with respect to the quadratic component, thereby yielding a convex curve with the peak near the middle (Figs. 3 and 4). Female gametophytes were more sensitive to temperature having regression coefficients that were 2X and 1.5X those of small sporophytes with respect to both linear and quadratic components, respectively (Tables 1 and 2).
Growth rates of female gametophytes and small sporophytes were saturated by all light intensities tested. Thus, we observed neither an effect of light, nor an interaction between light and temperature on growth rates of either phase (Tables 1 and 2). Conventional regression coefficients for light and the interaction of light and temperature were on the order of 10–3 – 10–4 with 95% credible intervals that included zero in the posterior distributions of these coefficients in both female gametophytes and sporophytes (i.e., growth was independent of light intensity).
Results reveal that optimal growth temperatures were 16 °C for female gametophytes with a max growth rate of 9.7% and 15 °C for sporophytes with a max growth rate of 3.5%.
Experiment 3
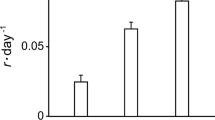
Distal segments of larger sporophytes, being disconnected from their meristems did not increase in size, though some increased in biomass. Biomass growth rates were not significantly different from 14–18 °C. Biomass growth rates were negative at 10 and 20 °C, losing biomass in contrast to 14–18 °C (Fig. 5).
Sorus formation was only observed in the two tanks with mean temperatures ~ 15 °C. (Fig. 5).
Discussion
The findings of this study indicate that temperature has a notable impact on the growth rates of L. farlowii juvenile gametophytes and sporophytes. For domestication of L. farlowii it is recommended to maintain adult blades to induce spore formation at 15 °C in short day photoperiods and grow out female gametophytes at 16 °C under low light ≤ 80 µmol photons m−2 s−1.
We found no effect of light intensity (18–65 µmol photons m−2 s−1 for gametophytes and 11–99 µmol photons m−2 s−1 for juvenile sporophytes) and no significant interaction of light inensity and temperature of the range of temperatures and light intensities tested. The observation that there was no increase in growth rate with increasing light intensity over 18 and 11 µmol photons m−2 s−1 indicates that growth rates of both microstages were efficient at using light for growth. That is in keeping with microscopic stages of this species developing in low light conditions at 40-60 m depth in plankton-rich Pacific coastal waters. In hatchery conditions, optimal growth rates could be achieved at optimal temperature for the life history stage and 30 µmol photons m−2 s−1.
High temperatures have been shown to inhibit growth of kelp gametophytes by changing pigment content, PSII reaction center densities, and activity of related enzymes of photosynthesis, and by increasing reactive oxygen species formation (Zhang et al. 2013). Interestingly, L. farlowii female gametophytes exhibited growth rates of 9% even at 18 °C, suggesting tolerance of elevated temperature. Martins et al. (2017) also showed that in L. digitata gametophyte optimal growth ranges from 10–18 °C and that range is broader than for sporophytes. Studies of other kelp species have found that gametophytes show greater thermal tolerance than sporophytes (Ladah et al. 1999; Mohring et al. 2014; Becheler et al. 2022).
Our findings suggests that this species may possess a higher temperature tolerance and could be a suitable candidate for climate change resilient cultivation. However, the work presented here only addreses the microscopic stages of L. farlowii and thus applies to hatchery work. For hatchery cultivation, we would recommend growing gametophytes at 16 °C and decreasing to 15° while small sporophytes are becoming established on seed string. Additional research needs to be conducted for temperature tolerance in open-water cultivation systems. For example, long-term cold thermal priming of Laminaria digitata gametophytes has produced thermally resilient sporophyte offspring to temperature extremes and has been proposed as an additional strategy to enhance productivity for both aquaculture and restoration efforts (Gauci et al. 2022). Other techniques such as direct selection of gametophytes able to withstand higher temperatures may further be used for breeding high temperature resistant varieties and hybrids (Zhang et al. 2013).
In this study, juvenile sporophytes grew more slowly than gametophytes and showed a broader thermal optimum, especially into lower temperatures. We measured growth rates of 3% day−1 at 11 °C to about 3.5% day−1 at 15 °C. Similarly, Lüning and Neushul (1978) found that nine distinct kelp species in central California exhibit a limited thermal tolerance range during embryonic development. Specifically, temperatures below 12 °C and above 17 °C hindered the growth of some juvenile kelp sporophytes. They also showed that a temperature of 12 °C favored the onset of fertility in the L. farlowii female gametophytes tested.
These results apply to L. farlowii in unialgal cultivation. In its wild habitat, studies show that the smaller, understory kelps L. farlowii and Pterygophora california compete with the giant kelp, Macrocystis pyrifera, for space, light and nutrients and the strength of competition depends on ambient environment and the top-down burden of herbivory (Liu and Gaines 2022). Increasing ocean temperatures gives a competitive advantage to M. pyrifera over P. california and may increase M. pyrifera’s dominance along the central California coast (Howard 2014). Furthermore, it has been observed that a higher density of M. pyrifera leads to the competitive exclusion of L. farlowii (Tegner et al. 1997). Therefore, the fate of wild populations of L. farlowii as the ocean warms and becomes more variable, will depend on complex community interactions in the kelp forest.
Early life-history stages of species with distribution ranges restricted to higher latitudes appear to be more vulnerable to ocean warming than kelp species with wider ranges (Lind and Konar 2017). Laboratory experiments indicate that kelp gametophytes sourced from cold waters such as the Arctic have upper survival temperatures up to 20 °C compared to warm temperate species that can tolerate up to 30 °C (tom Dieck 1993) and even up to 31 °C for male gametophytes of Eckloniopsis radicosa (Komazawa et al. 2015). Kelp species with warm temperate distributions typically exhibit a greater thermal tolerance enabling them to survive experimental temperatures exceeding the maximum encountered by the source population and undergo gametogenesis across a broad range of temperatures (10–25 °C) (Veenhof et al. 2022 and references therein). While kelp gametophytes develop and persist at higher laboratory temperatures than they experience in situ, developmental failure starts appearing in the egg production and sporophyte development stages (Hollarsmith et al. 2020). The results of our research indicate that increasing surface seawater temperatures may significantly affect growth rates of the early-life stages of L. farlowii. Extreme temperature events such as marine heat waves may thus contribute to latitudinal range shift for the species. Comparing that thermal tolerance to the annual sea surface temperature in Santa Barbara, California from 2007 to 2021, we predict that L. farlowii could be actively growing near the surface 75% of the time and survive brief marine heat waves of a few °C above typical seasonal averages (Kübler et al. 2021).
When choosing species for cultivation in open-ocean farms, it is crucial to take into account economic factors in the context of the evolving climate of the California coastal system. Prioritizing species that possess resilient traits to climate change and examining the physiological diversity within those species will support the growth and advancement of the industry (Kübler et al. 2021). In this regard, species displaying desirable compositional traits emerge as highly suitable candidates for farming. L. farlowii has a relatively high dry mass to wet mass ratio of 13% (Miller et al. 2012) and high vegetative phenolic levels, secondary metabolites whose functions may include protecting plants from pathogens and damage by UV radiation, as well as deterring feeding from herbivores (Van Alstyne et al. 1999). Overall, L. farlowii is a good novel candidate for farming in California because of its temperature tolerance and compositional characteristics.
Data availability
On publication, data files will be available through CSUN Scholarworks, an open data repository. The datasets generated during and/or analyzed during the current study are also available from the corresponding author on reasonable request.
References
Anderson TW (1994) Role of macroalgal structure in the distribution and abundance of a temperate reef fish. Mar Ecol Prog Ser 113:279–290
Augyte S, Yarish C, Neefus CD (2019) Thermal and light impacts on the early growth stages of the kelp Saccharina angustissima (Laminariales, Phaeophyceae). Algae 34:153–162
Augyte Simona, Kim JK, Yarish C (2021) Seaweed aquaculture—From historic trends to current innovation. J World Aquacult Soc 52:1004–1008
Becheler R, Haverbeck D, Clerc C, Montecinos G, Valero M, Mansilla A, Faugeron S (2022) Variation in thermal tolerance of the giant kelp’s gametophytes: Suitability of habitat, population quality or local adaptation? Front Mar Sci 9:802536
Bolton JJ, Lüning K (1982) Optimal growth and maximal survival temperatures of Atlantic Laminaria species (Phaeophyta) in culture. Mar Biol 66:89–94
Cavanaugh KC, Reed DC, Bell TW, Castorani MCN, Beas-Luna R (2019) Spatial variability in the resistance and resilience of giant kelp in southern and Baja California to a multiyear heatwave. Front Mar Sci 6:00413
Davison IR (1987) Adaptation of photosynthesis in Laminaria saccharina (Phaeophyta) to changes in growth temperature. J Phycol 23:273–283
Dieck IT (1993) Temperature tolerance and survival in darkness of kelp gametophytes (Laminariales, Phaeophyta) - Ecological and biogeographical implications. Mar Ecol Prog Ser 100:253–264
Druehl L, Clarkston BE (2016) Pacific seaweeds: updated and expanded edition. Harbour Publishing
Edwards MS (2022) It’s the little things: The role of microscopic life stages in maintaining kelp populations. Front Mar Sci 9:871204
Edwards MS, Estes JA (2006) Catastrophe, recovery and range limitation in NE Pacific kelp forests: A large-scale perspective. Mar Ecol Prog Ser 320:79–87
Egan B, Vlasto A, Y C, (1989) Seasonal acclimation to temperature and light in Laminaria longicruris de la Pyl. (Phaeophyta). J Exp Mar Biol Ecol 129:1–16
Fain SR, Murray SN (1982) Effects of light and temperature on net photosynthesis and dark respiration of gametophytes and embryonic sporophytes of Macrocystis pyrifera. J Phycol 18:92–98
Food and Agriculture Organization (FAO) of the United Nations (2020) The state of world fisheries and aquaculture 2020. Retrieved from https://www.fao.org/3/ca9229en/CA9229EN.pdf
Filbee-Dexter K, Wernberg T, Grace SP, Thormar J, Fredriksen S, Narvaez CN, Feehan CJ, Norderhaug KM (2020) Marine heatwaves and the collapse of marginal North Atlantic kelp forests. Sci Rep 10:13388
Gauci C, Bartsch I, Martins N, Liesner D (2022) Cold thermal priming of Laminaria digitata (Laminariales, Phaeophyceae) gametophytes enhances gametogenesis and thermal performance of sporophytes. Front Mar Sci 9:862923
Gerard VA, Du Bois KR (1988) Temperature ecotypes near the southern boundary of the kelpL aminaria saccharina. Marine Biology 97:575–580
Hamilton SL, Bell TW, Watson JR, Grorud-Colvert KA, Menge BA (2020) Remote sensing: generation of long-term kelp bed data sets for evaluation of impacts of climatic variation. Ecology 101:e03031
Holbrook NJ, Scannell HA, Sen Gupta A, Benthuysen JA, Feng M, Oliver ECJ, Alexander LV, Burrows MT, Donat MG, Hobday AJ, Moore PJ, Perkins-Kirkpatrick SE, Smale DA, Straub SC, Wernberg T (2019) A global assessment of marine heatwaves and their drivers. Nat Commun 10:2624
Hollarsmith JA, Buschmann AH, Camus C, Grosholz ED (2020) Varying reproductive success under ocean warming and acidification across giant kelp (Macrocystis pyrifera) populations. J Exp Mar Biol Ecol 522:151247
Howard AC (2014) Effects of temperature on sexual competition of kelps: Implications for range shifts in foundation species. Master’s thesis, San Jose State University
Jentsch A, Kreyling J, Beierkuhnlein C (2007) A new generation of climate-change experiments: events, not trends. Front Ecol Environ 5:365–374
Kite-Powell HL, Ask E, Augyte S, Bailey D, Decker J, Goudey CA, Grebe G, Li Y, Lindell S, Manganelli D, Marty-Rivera M, Ng C, Roberson L, Stekoll M, Umanzor S, Yarish C (2022) Estimating production cost for large-scale seaweed farms. Appl Phycol 3:435–445
Komazawa I, Sakanishi Y, Tanaka J (2015) Temperature requirements for growth and maturation of the warm temperate kelp Eckloniopsis radicosa (Laminariales, Phaeophyta). Phycol Res 63:64–71
Korabik AR, Winquist T, Grosholz E, Hollarsmith JA (2023) Examining the reproductive success of bull kelp (Nereocystis luetkeana) in climate change conditions. J Phycol. https://doi.org/10.1111/jpy.13368
Krumhansl KA, Okamoto DK, Rassweiler A, Novak M, Bolton JJ et al (2016) Global patterns of kelp forest change over the past half-century. Proc Natl Acad Sci 113:13785–13790
Kruschke J (2015) Doing Bayesian Data Analysis, 2nd edn. Academic Press, New York
Kübler JE, Davison IR (1993) High-temperature tolerance of photosynthesis in the red alga Chondrus crispus. Mar Biol 117:327–335
Kübler JE, Dudgeon SR, Bush D (2021) Climate change challenges and opportunities for seaweed aquaculture in California, the United States. J World Aquacult Soc 52:1069–1080
Ladah LB, Zertuche-González JA (2007) Survival of microscopic stages of a perennial kelp (Macrocystis pyrifera) from the center and the southern extreme of its range in the Northern Hemisphere after exposure to simulated El Niño stress. Mar Biol 152:677–686
Ladah LB, Zertuche-González JA, Hernandez-Carmona G (1999) Giant kelp (Macrocystis pyrifera, Phaeophyceae) recruitment near its southern limit in Baja California after mass disappearance during ENSO 1997–1998. J Phycol 35:1106–1112
Lafferty KD, Behrens MD, Davis GE, Haaker PL, Kushner DJ, Richards DV, Taniguchi IK, Tegner MJ (2004) Habitat of endangered white abalone, Haliotis sorenseni. Biol Cons 116:191–194
Liesner D, Shama LNS, Diehl N, Valentin K, Bartsch I (2020) Thermal Plasticity of the Kelp Laminaria digitata (Phaeophyceae) Across Life Cycle Stages Reveals the Importance of Cold Seasons for Marine Forests. Frontiers in Marine Science 7. https://doi.org/10.3389/fmars.2020.00456
Lind AC, Konar B (2017) Effects of abiotic stressors on kelp early life-history stages. Algae 32:223–233
Lissner AL, Dorsey JH (1986) Deep-Water biological assemblages of a hard-bottom bank-ridge complex of the Southern California continental borderland. Bull South Calif Acad Sci 85:87–101
Liu OR, Gaines SD (2022) Environmental context dependency in species interactions. Proc Natl Acad Sci U S m 119:e2118539119
Lonhart SI, Jeppesen R, Beas-Luna R, Crooks JA, Lorda J (2019) Shifts in the distribution and abundance of coastal marine species along the eastern Pacific Ocean during marine heatwaves from 2013 to 2018. Mar Biodivers Rec 12, https://doi.org/10.1186/s41200-019-0171-8
Lüning K, Neushul M (1978) Light and temperature demands for growth and reproduction of laminarian gametophytes in southern and central California. Mar Biol 45:297–309
Martínez B, Radford B, Thomsen MS, Connell SD, Carreño F, Bradshaw CJA, Fordham DA, Russell BD, Gurgel CFD, Wernberg T (2018) Distribution models predict large contractions of habitat-forming seaweeds in response to ocean warming. Divers Distrib 24:1350–1366
Martins N, Pearson GA, Bernard J, Serrão EA, Bartsch I (2020) Thermal traits for reproduction and recruitment differ between Arctic and Atlantic kelp Laminaria digitata. PLoS One 15:e0235388
Martins N, Tanttu H, Pearson GA, Serrão EA, Bartsch I (2017) Interactions of daylength, temperature and nutrients affect thresholds for life stage transitions in the kelp Laminaria digitata (Phaeophyceae). Botanica Marina 60(2):109–121. https://doi.org/10.1515/bot-2016-0094
Miller RJ, Harrer S, Reed DC (2012) Addition of species abundance and performance predicts community primary production of macroalgae. Oecologia 168:797–806
Mohring MB, Wernberg T, Wright JT, Connell SD, Russell BD (2014) Biogeographic variation in temperature drives performance of kelp gametophytes during warming. Marine Ecology Progress Series 513:85–96
Oliver ECJ, Donat MG, Burrows MT, Moore PJ, Smale DA, Alexander LV, Benthuysen JA, Feng M, Sen Gupta A, Hobday AJ, Holbrook NJ, Perkins-Kirkpatrick SE, Scannell HA, Straub SC, Wernberg T (2018) Longer and more frequent marine heatwaves over the past century. Nature Communications 9(1):1–12. https://doi.org/10.1038/s41467-018-03732-9
Park J, Kim JK, Kong JA, Depuydt S, Brown MT, Han T (2017) Implications of rising temperatures for gametophyte performance of two kelp species from Arctic waters. Bot Mar 60:39–48
Provasoli L (1968) Media and prospects for the cultivation of marine algae. In: Watanabe H, Hattori A (eds) Culture and collection of algae. Proceedings U.S.-Japan Conference. Japanese Society of Plant Physiology, Hakone, pp 63–75
Redmond S, Green L, Yarish C, Kim J, Neefus C (2014) New England Seaweed Culture Handbook-Nursery Systems. Connecticut Sea Grant CTSG-14-01; R/A 38. p 93. Retrieved from https://digitalcommons.uconn.edu/seagrant_weedcult/1/
Schiel DR, Foster MS (2006) The population biology of large brown seaweeds: Ecological consequences of multiphase life histories in dynamic coastal environments. Ann Rev Ecol Evol Syst 37:343–372
Schimpf NM, Liesner D, Franke K, Roleda MY, Bartsch I (2022) Microscopic stages of North Atlantic Laminaria digitata (Phaeophyceae) exhibit trait-dependent thermal adaptation along latitudes. Front Mar Sci 9:870792
Smale DA, Burrows MT, Moore P, O’Connor N, Hawkins SJ (2013) Threats and knowledge gaps for ecosystem services provided by kelp forests: A northeast Atlantic perspective. Ecol Evol 3:4016–4038
Strasser FE, Barreto LM, Kaidi S, Sabour B, Serrão EA, Pearson GA, Martins N (2022) Population level variation in reproductive development and output in the golden kelp Laminaria ochroleuca under marine heat wave scenarios. Front Mar Sci 9:943511
Tegner MJ, Dayton PK, Edwards PB, Riser KL (1997) Large-scale, low-frequency oceanographic effects on kelp forest succession: A tale of two cohorts. Mar Ecol Prog Ser 146:117–134
US Department of Energy (DoE), ARPA-E (2021) Macroalgae research inspiring novel energy resources (MARINER). Retrieved from https://arpa-e.energy.gov/technologies/programs/mariner
Van Alstyne KL, McCarthy JJ, Hustead CL, Duggins DO (1999) Geographic variation in polyphenolic levels of northeastern Pacific kelps and rockweeds. Mar Biol 133:371–379
Veenhof RJ, Champion C, Dworjanyn SA, Wernberg T, Minne AJP, Layton C, Bolton JJ, Reed DC, Coleman MA (2022) Kelp gametophytes in changing oceans. Oceanogr Mar Biol 60:335–371
Visch W, Larsson AI, Åberg P, Toth GB (2023) Adherence of kelp (Saccharina latissima) gametophytes on ropes with different binder treatments and flow regimes. J Appl Phycol 35:195–200
Wernberg T, Smale DA, Tuya F, Thomsen MS, Langlois TJ, De Bettignies T, Bennett S, Rousseaux CS (2013) An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat Clim Chang 3:78–82
Yarish C, Lee KW, Edwards P (1979) An improved apparatus for the culture of algae under varying regimes of temperature and light intensity. Botanica Mar 22:395–397
Zhang L, Cui C, Li X, Zhang Z, Luo S, Liang G, Liu Y, Yang G (2013) Effect of temperature on the development of Saccharina japonica gametophytes. J Appl Phycol 25:261–267
Funding
This work was supported by California Sea Grant #R/AQ-148,NA18OAR417 to J.E. Kubler, S. Augyte and D. Bush
Author information
Authors and Affiliations
Contributions
SA, JK – conceptualization, funding acquisition, writing – original draft of the manuscript.
SD, JK – data analysis.
CY, MMR, CN, SD – writing, review and editing of the manuscript.
MMR, CN, JK – data collection.
CY- supervision of research, PI UConn.
Corresponding authors
Ethics declarations
Competing interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Augyte, S., Dudgeon, S.R., Yarish, C. et al. Thermal characteristics of early life stages of Laminaria farlowii, a deep-water kelp from Southern and Central California. J Appl Phycol 35, 2543–2553 (2023). https://doi.org/10.1007/s10811-023-03064-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03064-2