Abstract
The kelp Lessonia corrugata (Ochrophyta, Laminariales) is being developed for integrated multi-trophic aquaculture (IMTA) trials in the vicinity of salmon cages in Tasmania, Australia. Gametophytes are vegetally maintained before seeding on hatchery twine; however, the optimal temperature and light conditions for growth and sexual development are unknown. We measured vegetative size of female and male gametophytes and sexual development of females over a range of temperatures and irradiances using a temperature gradient table and neutral density light filters. Over a 4-week experiment, gametophytes were exposed to a combination of thermal (5.7–24.9 °C) and irradiance (10–100 μmol photons m−2 s−1) gradients, to assess biological performance. At the temperature extremes (hottest = 24.9 °C, coldest = 5.7 °C), we observed the critical thermal limits for this species and the results reveal a narrow optimal temperature range for growth and sexual development between 15.7 and 17.9 °C, with irradiances between 40 and 100 μmol photons m−2 s−1 resulting in fertile female gametophytes. Lessonia corrugata inhabits a small geographic range, found only around Tasmania, south of the Australian mainland, hence oceanic changes such as ongoing increases in sea surface temperatures (SSTs), and altered irradiance regimes may limit recruitment of the early microscopic life stages in the future. Our findings provide optimised culture conditions for aquaculture and information to predict the future geographic range of L. corrugata under ocean global change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brown macroalgae of the order Laminariales (true kelps) are important in temperate marine ecosystems as they form dense forests providing food, habitat and key ecosystem services for a great diversity of organisms (Bennett and Wernberg 2014; Bennett et al. 2016). They also take up nutrients including nitrogen and phosphorus, and as such, kelps are increasingly being used in integrated multi-trophic aquaculture (IMTA) systems where seaweeds are grown in conjunction with finfish and shellfish to take up excess nutrients produced by the fed species (Buschmann et al. 2001; Chopin et al. 2001; Hurd et al. 2014; Buck et al. 2017; Roleda and Hurd 2019). However, the development of IMTA and kelp aquaculture may be hindered by environmental changes in sea surface temperature (SST), nutrients (primarily nitrogen) and irradiance, which currently limit seaweed recruitment and biogeographic distribution of kelp forests in coastal marine waters around the world (Deysher and Dean 1986; Steneck et al. 2002; Mabin et al. 2019; Smale 2020).
Kelp forests currently experience multiple anthropogenic stressors including ocean warming and altered underwater irradiance regimes which may be exacerbated in the future (Smale et al. 2013; Krumhansl et al. 2016; Smale 2020). Global SSTs have increased ~ 0.11 °C per decade since the industrial revolution and the east coast of Tasmania, Australia, is predicted to experience warming ~ 3.8 times the global average (2–3 °C) making this one of the fastest warming regions in the southern hemisphere—a global ‘hotspot’ (Ridgeway 2007; Johnson et al. 2011; IPCC 2014). Increased SSTs have the potential to impact seaweeds by impairing respiration, photosynthesis, basic cellular maintenance and juvenile recruitment (Wernberg et al. 2010). As the kelp canopy is destabilised by increased SST, higher irradiances penetrate the understorey algal community, including juvenile kelps and gametophytes (Wernberg et al. 2010). Furthermore, changes to underwater irradiance regimes caused by increased precipitation, storm events and pollution may affect kelp survival by reducing the irradiance available (Tait 2019; Blain and Shears 2020). Understanding how temperature and irradiance that are likely to interact and influence the microscopic life stage of kelps is crucial for determining productivity of the order Laminariales both in natural populations and in an aquaculture setting into the future.
The kelp life cycle is a heteromorphic alteration of generations (Hurd et al. 2014). Mature diploid sporophylls (2n) produce and release zoospores (n) which settle and germinate into dioecious haploid gametophytes (n). Gametophytes mature into female and males before reproduction is induced (Lüning and Dring 1975; Lüning and Neushul 1978; Gerard 1990). During reproduction, eggs produced in the female oogonium are fertilised by sperm produced in the male antheridium, driving the formation of the zygote and re-starting the sporophyte (2n) stage (Maier et al. 1987; Müller et al. 1979). The development of the gametophyte and fertilisation of the egg is, however, affected by environmental factors, particularly temperature and irradiance (Matson and Edwards 2007; Cie and Edwards 2008; Mohring et al. 2013; Bringloe et al. 2018). Environmental extremes in either sides of their optimal range can reduce gametophyte growth and sexual development, which may hinder population recruitment (Augyte et al. 2019).
Temperature has a significant regulatory effect on the growth and sexual development of the microscopic life stages of kelp through changes to zoospore germination success, morphology and development of gametophytes, as well as growth of juvenile sporophytes (Lüning and Neushul 1978; Nelson 2005; Oppliger et al. 2012; Murúa et al. 2013). Lower and upper critical temperatures vary depending on species and biogeographic location; only within the optimal temperature window can gametophytes germinate, become sexually mature and fertilise into zygotes (tom Dieck 1993). This optimal temperature window highlights the importance of physiological plasticity and local adaptation in kelps as recruitment varies in respect to seasonality and latitudinal distribution (Lee and Brinkhuis 1988; Martínez 1999; Oppliger et al. 2012; Murúa et al. 2013; Mohring et al. 2014).
Organisms respond to their environments according to their tolerances and sensitivities, and these responses can be described through performance curves that represent the degree of physiological plasticity of species and populations (Fernández et al. 2020). The performance curves depict the tolerance range and critical zones where organisms can enter into stress and be lethal (Gaitán-Espitia et al. 2013). Although temperature is recognised as the main environmental factor shaping these performance curves (and thus plasticity), for kelps, these curves can additionally be shaped by the interactions with other factors such as irradiance, nutrients and salinity (Gaitán-Espitia et al. 2014b; Fernández et al. 2020).
The order Laminariales contains 113 species in 33 genera globally (Bolton 2010), one of which is Lessonia corrugata A.H.S. Lucas, an endemic kelp found around Tasmania, a temperate island at the southern tip of Australia (Scott 2017). Lessonia corrugata commonly grows at 1–4 m depth but has been recorded to 18 m (Scott 2017). It is being grown around finfish farms in Tasmania as part of a trial IMTA system. Gametophyte cultures are initiated and maintained in a laboratory before being sprayed onto twine and spun around rope suspended from buoys adjacent to the fish farms (Barrington et al. 2009; Edwards and Watson 2011; Flavin et al. 2013). However, despite being an ecosystem dominant in Tasmania, L. corrugata is poorly studied and the temperature and irradiance conditions necessary for optimal gametophyte growth are unknown. Therefore, the aim of this study was to identify the thermal performance range (minimum, maximum and optimal temperatures) for gametophyte growth and sexual development under a range of irradiances (10, 40, 70 and 100 μmol photons m−2 s−1). The results of this study will help to future proof the seaweed aquaculture industry of Tasmania under future oceanic change by providing optimal culture conditions for gametophyte germplasm preservation (i.e. zoospores or haploid gametophytes stored in a lab, also known as a ‘seed bank’) (Edwards 2000; Wade et al. 2020) and provide information that will enable predictions of the future biogeographic range of L. corrugata as the oceans continue to warm.
Methods
Seaweed collection and zoospore release
Lessonia corrugata sori were collected using snorkel from Crayfish Point (43° 01′ S, 147° 33′ E), South-Eastern Tasmania on the 28th of May 2018 (late Autumn; L. corrugata is found to have reproductive sori year-round; J.C. Sanderson, pers. obs.). Specimens were found growing sub-tidally on rock substratum between 2 and 6 m and once removed from the water were promptly placed into a dark cool box and transported to the laboratory (20 min away).
For zoospore release from kelp tissue, we followed the protocols described by Edwards and Watson (2011) and Flavin et al. (2013). In the laboratory, sorus tissue containing fertile sporangia were cut and isolated from the non-reproductive tissue and cleaned using tweezers to remove any obvious epiphytes before being placed in an iodine bath for 30 s (5 mL L−1 Betadine and filtered seawater 0.22 μm) (Flavin et al. 2013). Once removed from the iodine bath, sori were rinsed and soaked in sterilized seawater for 5 min then wiped with paper towel. This process was repeated three times each in fresh filtered seawater (0.22 μM), to ensure clean sori. Sorus tissue was then placed between sheets of clean, damp paper towel and left in a cool room (12 °C) overnight for slow, gentle desiccation. The following day, 1-L beakers were filled with filtered seawater (15 °C) in a laminar flow hood into which sorus tissue was immersed. Sori were stirred occasionally and left for 2 h for zoospore release. The initial zoospore density was estimated at 160,000 zoospores per millilitre by counting of zoospores using a haemocytometer under a light microscope (Nikon ECLIPSE Ts2). The following experiment was initiated the afternoon of the zoospore release.
Experimental design
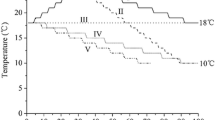
An aluminium temperature gradient table was used for the temperature × irradiance experiment (Edwards and van Baalen 1970). The temperature gradient along the table was created by a heating unit set at 28 °C at one end and a cooling unit set at − 8 °C at the other end of the aluminium block. The temperature table had a total of 72 wells (12 along the temperature gradient with six replicate wells per temperature), which fit 70-mL polypropylene specimen containers (Techno Plas). The table was set up to create 12 ecologically relevant temperature treatments, 5.7, 8.3, 11.0, 13.2, 15.3, 16.9, 18.5, 19.9, 21.1, 22.4, 23.6, and 24.9 °C, which were monitored using a spike probe thermometer (Testo 826-T4—IR and Probe Thermometer) over a 24-h period (SD ± 0.3 °C) prior to the experiment.
A LED panel light (Lumex NovaBlade) illuminated the base of the wells in the table from below. Neutral density filters were placed at the base of each well creating four irradiance treatments, 10, 40, 70, and 100 μmol photons m−2 s−1. Irradiance in each well was measured using a LI-COR Light Meter (LI-250A) with a flat quantum sensor attached. Due to logistical restraints of the 12 × 6 well temperature gradient table and in order to have three replicates for each factorial treatment, the irradiance treatments alternated with each temperature treatment (Fig. 1). We grouped 10 μmol photons m−2 s−1 and 70 μmol photons m−2 s−1 treatments as well as the 40 μmol photons m−2 s−1 and 100 μmol photons m−2 s−1 irradiance treatments and alternated them with each temperature treatment (Fig. 1). The light:dark cycle was 12:12 which was chosen to approximate the seasonal conditions on collection.
Schematic showing temperature and irradiance combinations across the temperature gradient table (not to scale). Black = 10 μmol photons m−2 s−1, dark grey = 40 μmol photons m−2 s−1, light grey = 70 μmol photons m−2 s−1 and white = 100 μmol photons m−2 s−1. Each irradiance had three replicates. Temperatures ranged between 24.9 and 5.7 °C. The temperature increment between treatments was ~ 1.75 °C. Once in position, replicates were kept in that position because of the difficulty of moving light filters
For the temperature × irradiance experiment, aliquots of the zoospore solution (438 μL) were added to each of the 72 containers and filled to 10 mL with F/2 enriched filtered seawater to achieve a density of ca 7000 zoospores per millilitre (~ 36 spores per mm2 once settled) (Tatsumi and Wright 2016). F/2 culture medium was used as it is known to support good gametophyte development (Nelson 2005; Ratcliff et al. 2017). The experiment was run for 4 weeks, and the culture medium in each of the 72 culture containers was exchanged with fresh F/2 enriched seawater every week following methods outlined in other gametophyte studies (tom Dieck 1987; Wiencke 1990; Nelson 2005; Murúa et al. 2013; Müller et al. 2019).
Measurement of gametophyte surface area and sexual development
To determine the combined effects of temperature and irradiance on gametophyte size (a measure of growth) and sexual development, three photographs were taken using an inverted light microscope at × 100 magnification (bright field; Leica Microsystems-Labovert) in each replicate container at the end of the experiment (day 30) (Fig. 2). Photographs were randomly taken along a pre-determined radial line at the base of each container. For each of the three photographs, five male and five female gametophytes were randomly selected and their surface area (mm2) measured in the software Fiji (Schindelin et al. 2012). These values were then averaged for each photo, then each container, to give three values per temperature × irradiance treatment (i.e. one value of surface area for each of the three replicate containers). The total number of oogonia on female gametophytes per mm2 (i.e. area of microscope field of view at × 100 magnification) was counted for each photo to determine sexual development of female gametophytes (Fig. 3). Again, oogonia count was averaged for each photo, then each container, to give one value per replicate container (i.e. three values per temperature × irradiance treatment). For all the traits analysed, the mean and variance of each replicate (i.e. each container) were included in the model fitting step of the performance curves.
Data analysis
The non-linear relationship between gametophyte size and the experimental factors (temperature and irradiance) was analysed following the method described in Fernández et al. (2020). Briefly, a continuous reaction norm or performance curve was constructed for female and male gametophyte surface area as well as for the number of oogonia using a model-fitting approach (Gaitán-Espitia et al. 2014a). Here, several non-linear functions (e.g. Gaussian, exponential modified Gaussian, Quadratic Lorentzian, Weibull) were tested using the lsfit function implemented in the easynls R package v.5.0 (Arnhold 2017) and the GraphPad Prism software (v.7.03). Thermal performance curve (TPC) parameters of the curve (maximal measurement—μmax, thermal optimum—Topt, critical thermal maximum and minimum at which gametophyte growth decreases—CTmin and CTmax) were numerically derived from the best-fitted models considering the ymax and the intersection points of the resulting curve with the temperature axis (μ = 0). The best fit models were assessed using the Akaike Information Criterion (AIC) (Angilletta 2006). Parameters of the curves were compared among treatments through the confidence intervals (CI) computed from the likelihood profile and using AIC and the extra sum-of-square F test. Finally, in order to better visualise the overall biological performance of L. corrugata gametophytes across the temperature ×irradiance environmental seascape, we used surface plots implemented in the R plotly package v. 4.9.2.1 (Sievert 2020). Comparison of functions used to describe the performance curves for size of female and male gametophytes retrieved the lowest Akaike’s information criterion (AIC) for the exponential modified Gaussian function (best-fitted model). For sexual development, the AIC retrieved the Gaussian function as the best-fitted model. These functions were used to fit the data for all the treatments in the corresponding datasets.
Results
Gametophyte size
The size of female and male gametophytes at a range of temperatures followed the typical bell shape curve described for ectothermic organisms (Angilletta Jr et al. 2002). However, these curves were influenced by irradiance showing a clear interaction between both factors. At the lowest irradiance (10 μmol photons m−2 s−1) gametophytes exhibited no-to-slow growth nor sexual development across the thermal gradient which revealed a sub-optimal irradiance response independent of temperature (Fig. 2a). The Topt, CTmax and μmax of the TPCs for both female and male gametophyte sizes were the highest under 70 μmol photons m−2 s−1; however, there was no statistical difference in the CTmin between all irradiances (Table 1). The graphs clearly indicate that the overall pattern of the TPCs is driven by temperature, and that variability around the curve is driven by irradiance and sexual dimorphism of the gametophyte. In cultures kept at 22.4 °C and above, zoospores did not survive, and no gametophytes were recorded in these cultures.
The TPCs for female gametophytes were similar to each other at the higher irradiances (40–100 μmol photons m−2 s−1; Fig. 2a). However, female gametophytes cultured at 70 μmol photons m−2 s−1 showed higher Topt (16.52 °C) and CTmax (22.30 °C) compared with their counterparts at the other two irradiances (40 μmol photons m−2 s−1: Topt = 15.82 °C, CTmax = 21.31 °C and 100 μmol photons m−2 s−1: Topt = 15.69 °C, CTmax = 21.12 °C; F18,48 = 11.26; P = 0.001; Table 1).
The TPCs for male gametophyte size showed similar Topt (16.17–16.54 °C) under each of the higher irradiances (40–100 μmol photons m−2 s−1; Table 1; Fig. 2b). Significant differences in thermal performance were detected for μmax (i.e. maximum size) and CTmax. Male gametophytes at 100 μmol photons m−2 s−1 showed the lowest thermal performance (μmax = 6.95 mm2 and CTmax = 21.1 °C) compared with those at 40 μmol photons m−2 s−1 (μmax = 9.01 mm2 and CTmax = 21.23 °C) and 70 μmol photons m−2 s−1 (μmax = 10.9 mm2 and CTmax = 22.1 °C) (F18,48 = 7.16, P < 0.001).
The modelled surface plots for L. corrugata gametophytes across the temperature × irradiance environmental seascape revealed the narrow optimal range for female and male gametophyte size (Fig. 3 a and b). The differences in parameters of the TPCs (Table 1) were significant within sex (females and males compared separately), but not between the sexes (i.e. comparison between female and male TPCs, P > 0.05) (Figs. 4, 5 and 6). The difference between sexes was non-significant despite the visual differences in the shape of the TPCs between female and male gametophytes with the different irradiance levels (Fig. 2 a and b).
Temperature-size response curves of a female and b male L. corrugata gametophytes incubated under four irradiances (orange square = 10 μmol photons m−2 s−1, blue triangle = 40 μmol photons m−2 s−1, green circle = 70 μmol photons m−2 s−1, purple star = 100 μmol photons m−2 s−1). Each point represents the average surface area of gametophytes in a replicate container i.e. n = 3 at each temperature treatment (5.9–24.9 °C)
Temperature response curve of number of oogonia per mm2 of female L. corrugata gametophytes incubated under four irradiances (orange square = 10 μmol photons m−2 s−1, blue triangle = 40 μmol photons m−2 s−1, green circle = 70 μmol photons m−2 s−1, purple star = 100 μmol photons m−2 s−1). Each point represents the average count of oogonia per mm2 in a replicate container i.e. n = 3 at each temperature treatment (5.9–24.9 °C)
Female gametophyte sexual development
Sexual development, reflected in female gametophyte oogonia count (per mm2), was strongly influenced by both temperature and irradiance (Fig. 4). The Topt was between 16.2 and 17.9 °C at 40, 70 and 100 μmol photons m−2 s−1. No oogonia were observed under 10 μmol photons m−2 s−1 at any of the experimental temperatures. In terms of the TPCs for each irradiance, the best-fitted model for oogonia count followed the Gaussian function evidencing different shapes among treatments (F9.6 = 11.36; P < 0.001).
Discussion
We found that gametophyte development for L. corrugata, a kelp with a narrow geographic range that is endemic to the island of Tasmania, Australia, has a narrow range of thermal tolerance and high sensitivity to changes in temperature and irradiance. This finding supports the idea of local thermal adaptation in species with a narrow biogeographic range (Bennett et al. 2019) and has also been documented in endemic brown seaweeds from other temperate and polar regions, for example, Durvillaea poha in New Zealand (Thomsen et al. 2019) and Laminaria solidungula in the Arctic (Roleda 2016). Compared with other kelp species that also grow in Tasmania but have very broad geographic distribution, Ecklonia radiata (Topt range of ~ 18 to 23 °C) (Mohring et al. 2014) and Undaria pinnatifida (a non-indigenous kelp in Tasmania, which has successfully invaded coastal areas around the world due to its wide temperature tolerance; CTmax of 27–28 °C and up to 33 °C under short term exposure) (Henkel and Hofmann 2008; Morita et al. 2003), L. corrugata has a narrow optimal thermal range (15.7–17.9 °C) for gametophyte growth and sexual development.
No differences were observed for the TPC traits between male and female gametophytes of L. corrugata. This contrasts with Undaria pinnatifida male gametophytes, which had both a higher temperature and irradiance tolerance than female gametophytes (Sato et al. 2020) and Ecklonia radiata males which had a higher temperature tolerance (Mabin et al. 2019). At temperatures > 21.1 °C, zoospores were unable to survive with a 100% mortality rate observed after 1 week. In the only prior study of L. corrugata gametophytes, an upper survival temperature of 23 °C was recorded (tom Dieck 1993) but that experiment used gametophytes previously grown vegetally in long-term laboratory culture under red light, whereas our study followed gametogenesis directly from freshly collected zoospores. The differences between our results which recorded zoospore mortality > 21.1 °C and the upper survival temperature of 23 °C found by tom Dieck (1993) suggest that L. corrugata zoospores are more susceptible to degradation and senescence under sub-optimal environmental conditions compared with gametophytes of L. corrugata.
The mortality of zoospores > 21.1 °C and small optimal temperature range for L. corrugata gametophyte size suggest that the species may be negatively impacted by the predicted 2–3 °C increase in SSTs by the end of the century (Allen et al. 2018). Many seaweed species in mainland Australia have experienced southward migration to lower latitudes as a result of increased SSTs (Wernberg et al. 2011), but L. corrugata is at its geographic limit in Tasmania as there is no suitable habitat further south. Our results suggest it is unlikely that L. corrugata will successfully recruit under these predicted temperatures, and a bottleneck for survival may occur with a local extinction of the species and subsequent range constriction (Wernberg et al. 2010; Harley et al. 2012). A mechanism by which L. corrugata may survive future water warming is vertical migration to cool, deeper waters as SSTs increase (Jorda et al. 2020). However, our results suggest that L. corrugata requires moderate irradiances for size and sexual development (discussed below), and migrating to cooler temperatures at depths could reduce the density of the population due to light limitation (Jorda et al. 2020). Additionally, although L. corrugata zoospores at low temperatures (< 8.3 °C) underwent gametogenesis, gametophytes remained small (< 1.64 mm2), did not become sexually mature and had a very slow growth rate (~ 2.8 μm day−1). These results suggest that L. corrugata gametophytes can survive in temperatures lower than those currently experienced in Tasmanian waters. As our experiment was run at the start of winter and seasonal changes would be expected to alter the thermal plasticity of kelp gametophytes, seasonal studies may elucidate annual responses of L. corrugata gametophytes to thermal ranges. Furthermore, L. corrugata has a broad genetic variability (Durrant et al. 2015), and this study provides a basis for future population studies around Tasmania. It would additionally be beneficial for future research to study the thermal range of the mature L. corrugata sporophyte to further understand the species biogeography and response to increase in SSTs.
Our results revealed a trade-off between maximal size (μmax) and optimal temperature (Topt) for sexual development but not for gametophyte size. Sexual development of cultures at 70 μmol photons m−2 s−1 exhibited higher Topt and CTmax than those at 40 and 100 μmol photons m−2 s−1; however, the greatest number of oogonia per mm2 was found under 40 μmol photons m−2 s−1. This trade-off between μmax and Topt for sexual development may have implications for L. corrugata under future ocean predictions. For example, the greatest number of oogonia formed under mid-low irradiance conditions (i.e. 40 μmol photons m−2 s−1) but was at the cost of a lower Topt. This temperature and irradiance interaction may be beneficial for L. corrugata gametophytes in terms of vertical migration to depth and competition under the canopy (Bennett and Wernberg 2014; Bennett et al. 2015); however, a higher Topt would be most beneficial under future SSTs (Harley et al. 2012). Moreover, mid-high irradiance (70 μmol photons m−2 s−1) supported a great number of oogonia yet with a higher Topt which would be beneficial under future SSTs. However, the CTmin at this irradiance was also higher than the other treatments which suggests that irradiance had a significant regulatory effect on the sexual development of L. corrugata. This finding indicates that moderate irradiances are optimal as the higher Topt at these irradiances provides higher chances of survival and fertilisation in changing environments (de Bettignies et al. 2018). On the other hand, no trade-offs were detected for gametophyte size and the optimal irradiance was between 70 and 100 μmol photons m−2 s−1. However, size of gametophytes under 70 μmol photons m−2 s−1 evidenced broader thermal breath than their counterparts at 100 μmol photons m−2 s−1, suggesting higher thermal plasticity and performance.
Conclusions
Our results suggest that growth and sexual development of L. corrugata gametophytes are highly sensitive to fluxes in temperature and irradiance. Understanding how this habitat-forming species will respond to increases in SSTs and alterations to irradiance regimes will help conserve the biodiversity of the temperate reefs that L. corrugata supports and aid in management decisions regarding the species’ aquaculture potential. Future experiments should analyse the thermal range of L. corrugata sporophytes and sporangia development as microscopic reproduction may have a lower thermal performance window than adult sporophyte size and survival (Ling et al. 2008; Andrews et al. 2014). Warming events may act as a strong selective force on thermal tolerant genotypes of L. corrugata, and further research is required to better understand the thermal responses of adult sporophytes. This future research will enhance our ability to predict the species vulnerability to climate change and marine heatwaves (Bennett et al. 2019). Finally, our study highlights the importance of including multiple environmental factors which co-vary seasonally in determining potential drivers of change in kelp forests, and our results may be used to assist in predicting distributional changes in L. corrugata biogeography as well as establishing and optimising kelp aquaculture practices.
Data availability
Contact corresponding author.
References
Allen MR, Dube OP, Solecki W, Aragón-Durand F, Cramer W, Kainuma M, Kala J, Mahowald N, Mulugetta Y, Perez R, Wairiu M, Zickfeld K (2018) Framing and context. In: Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. https://www.ipcc.ch/site/assets/uploads/sites/2/2019/05/SR15_Chapter1_Low_Res.pdf
Andrews S, Bennett S, Wernberg T (2014) Reproductive seasonality and early life temperature sensitivity reflect vulnerability of a seaweed undergoing range reduction. Mar Ecol Prog Ser 495:119–129
Angilletta MJ (2006) Estimating and comparing thermal performance curves. J Therm Biol 31:541–545
Angilletta MJ Jr, Niewiarowski PH, Navas CA (2002) The evolution of thermal physiology in ectotherms. J Therm Biol 27:249–268
Arnhold E (2017) easynls: easy nonlinear model. vol R package version. https://cran.r-project.org/web/packages/easynls/easynls.pdf
Augyte S, Yarish C, Neefus CD (2019) Thermal and light impacts on the early growth stages of the kelp Saccharina angustissima (Laminariales, Phaeophyceae). Algae 34:153–162
Barrington K, Chopin T, Robinson S (2009) Integrated multi-trophic aquaculture (IMTA) in marine temperate waters. Integrated mariculture: a global review. FAO Fisheries and Aquaculture Technical Paper., vol 529. FAO, Rome
Bennett S, Wernberg T (2014) Canopy facilitates seaweed recruitment on subtidal temperate reefs. J Ecol 102:1462–1470
Bennett S, Wernberg T, de Bettignies T, Kendrick G, Anderson R, Bolton J, Rodgers K, Shears N, Leclerc J, Lévêque L, Davoult D, Christie H (2015) Canopy interactions and physical stress gradients in subtidal communities. Ecol Lett 18:677–686
Bennett S, Wernberg T, Connell SD, Hobday AJ, Johnson CR, Poloczanska ES (2016) The 'Great Southern Reef': social, ecological and economic value of Australia's neglected kelp forests. Mar Freshw Res 67:47–56
Bennett S, Duarte CM, Marbà N, Wernberg T (2019) Integrating within-species variation in thermal physiology into climate change ecology. Phil Trans Roy Soc B 364:0550
Blain C, Shears N (2020) Differential response of forest-forming seaweeds to elevated turbidity may facilitate ecosystem shifts on temperate reefs. Mar Ecol Prog Ser 641:63–77
Bolton J (2010) The biogeography of kelps (Laminariales, Phaeophyceae): a global analysis with new insights from recent advances in molecular phylogenetics. Helgol Mar Res 64:263–279
Bringloe TT, Bartlett CAB, Bergeron ES, Cripps KSA, Daigle NJ, Gallagher PO, Gallant AD, Giberson ROJ, Greenough SJ, Lamb JM, Leonard TW, Mackay JA, McKenzie AD, Persaud SM, Sheng T, Stack Mills AME, Moore TE, Saunders GW (2018) Detecting Alaria esculenta and Laminaria digitata (Laminariales, Phaeophyceae) gametophytes in red algae, with consideration of distribution patterns in the intertidal zone. Phycologia 57:1–8
Buck BH, Nevejan N, Wille M, Chambers MD, Chopin T (2017) Offshore and multi-use aquaculture with extractive species: seaweeds and bivalves. In: Buck B, Langan R (eds) Aquaculture perspective of multi-use sites in the open ocean: the untapped potential for marine resources in the Anthropocene. Springer, Cham, pp 23–69
Buschmann AH, Troell M, Kautsky N (2001) Integrated algal farming: a review. Cah Biol Mar 42:83–90
Chopin T, Buschmann AH, Halling C, Troell M, Kautsky N, Neori A, Kraemer GP, Zertuche-González JA, Yarish C, Neefus C (2001) Integrating seaweeds into marine aquaculture systems: a key toward sustainability. J Phycol 37:975–986
Cie DK, Edwards MS (2008) The effects of high irradiance on the settlement competency and viability of kelp zoospores. J Phycol 44:495–500
de Bettignies T, Wernberg T, Gurgel C (2018) Exploring the influence of temperature on aspects of the reproductive phenology of temperate seaweeds. Front Mar Sci 5:218
Deysher LE, Dean TA (1986) In situ recruitment of sporophytes of the giant kelp, Macrocystis pyrifera (L.) C.A. Agardh: Effects of physical factors. J Exp Mar Biol Ecol 103:41–63
Durrant HMS, Barrett NS, Edgar GJ, Coleman MA, Burridge CP (2015) Shallow phylogeographic histories of key species in a biodiversity hotspot. Phycologia 54:556–565
Edwards MS (2000) The role of alternate life-history stages of a marine macroalga: a seed bank analogue? Ecology 81:2404–2415
Edwards P, van Baalen C (1970) An apparatus for the culture of benthic marine algae under varying regimes of temperature and light-intensity. Bot Mar 13:42
Edwards and Watson (2011) Aquaculture explained. Cultivating Laminaria digitata. BIM Bord Iascaigh Mhara, Irish Sea Fisheries Board, Dublin, Ireland
Fernández PA, Gaitán-Espitia JD, Leal PP, Schmid M, Revill AT, Hurd CL (2020) Nitrogen sufficiency enhances thermal tolerance in habitat-forming kelp: implications for acclimation under thermal stress. Sci Rep 10:3186
Flavin K, Flavin N, Flahive B (2013) Kelp farming manual: a guide to the processes, techniques, and equipment for farming kelp in New England waters. Ocean Approved, New England
Gaitán-Espitia J, Belén Arias M, Lardies M, Nespolo R (2013) Variation in thermal sensitivity and thermal tolerances in an invasive species across a climatic gradient: lessons from the land snail Cornu aspersum. PLoS One 8:e70662
Gaitán-Espitia JD, Bacigalupe LD, Opitz T, Lagos NA, Timmermann T, Lardies MA (2014a) Geographic variation in thermal physiological performance of the intertidal crab Petrolisthes violaceus along a latitudinal gradient. J Exp Biol 217:4379–4386
Gaitán-Espitia JD, Hancock JR, Padilla-Gamiño JL, Rivest EB, Blanchette CA, Reed DC, Hofmann GE (2014b) Interactive effects of elevated temperature and pCO2 on early-life-history stages of the giant kelp Macrocystis pyrifera. J Exp Mar Biol Ecol 457:51–58
Gerard VA (1990) Ecotypic differentiation in the kelp Laminaria saccharina: phase-specific adaptation in a complex life cycle. Mar Biol 107:519–528
Harley CDG, Anderson KM, Demes KW, Jorve JP, Kordas RL, Coyle TA, Graham MH (2012) Effects of climate change on global seaweed communities. J Phycol 48:1064–1078
Henkel SK, Hofmann GE (2008) Thermal ecophysiology of gametophytes cultured from invasive Undaria pinnatifida (Harvey) Suringar in coastal California harbors. J Exp Mar Biol Ecol 367:164–173
Hurd CL, Harrison PJ, Bischof K, Lobban CS (2014) Seaweed ecology and physiology, 2nd edn. Cambridge University Press, UK
IPCC (2014) Climate change 2014: synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, Switzerland
Johnson CR, Banks SC, Barrett NS, Cazassus F, Dunstan PK, Edgar GJ, Frusher SD, Gardner C, Haddon M, Helidoniotis F, Hill KL, Holbrook NJ, Hosie GW, Last PR, Ling SD, Melbourne-Thomas J, Miller K, Pecl GT, Richardson AJ, Ridgway KR, Rintoul SR, Ritz DA, Ross DJ, Sanderson JC, Shepherd SA, Slotwinski A, Swadling KM, Taw N (2011) Climate change cascades: shifts in oceanography, species' ranges and subtidal marine community dynamics in eastern Tasmania. J Exp Mar Biol Ecol 400:17–32
Jorda G, Marbà N, Bennett S, Santana-Garcon J, Agusti S, Duarte CM (2020) Ocean warming compresses the three-dimensional habitat of marine life. Nat Ecol Evol 4:109–114
Krumhansl KA, Okamoto DK, Rassweiler A, Novak M, Bolton JJ, Cavanaugh KC, Connell SD, Johnson CR, Konar B, Ling SD, Micheli F, Norderhaug KM, Pérez-Matus A, Sousa-Pinto I, Reed DC, Salomon AK, Shears NT, Wernberg T, Anderson RJ, Barrett NS, Buschmann AH, Carr MH, Caselle JE, Derrien-Courtel S, Edgar GJ, Edwards M, Estes JA, Goodwin C, Kenner MC, Kushner DJ, Moy FE, Nunn J, Steneck RS, Vásquez J, Watson J, Witman JD, Byrnes JEK (2016) Global patterns of kelp forest change over the past half-century. Proc Natl Acad Sci U S A 113:13785–13790
Lee JA, Brinkhuis BH (1988) Seasonal light and temperature interaction effects on development of Laminaria saccharina (Phaeophyta) gametophytes and juvenile sporophytes. J Phycol 24:181–191
Ling SD, Johnson CR, Frusher S, King CK (2008) Reproductive potential of a marine ecosystem engineer at the edge of a newly expanded range. Glob Change Biol 14:907–915
Lüning K, Dring MJ (1975) Reproduction, growth and photosynthesis of gametophytes of Laminaria saccharina grown in blue and red light. Mar Biol 29:195–200
Lüning K, Neushul M (1978) Light and temperature demands for growth and reproduction of laminarian gametophytes in southern and central California. Mar Biol 45:297–309
Mabin CJT, Johnson CR, Wright JT (2019) Family-level variation in early life-cycle traits of kelp. J Phycol 55:380–392
Maier I, Müller DG, Gassmann G, Boland W, Jaenicke L (1987) Sexual pheromones and related egg secretions in Laminariales (Phaeophyta). Z Naturforsch C 42:948–954
Martínez EA (1999) Latitudinal differences in thermal tolerance among microscopic sporophytes of the kelp Lessonia nigrescens (Phaeophyta: Laminariales). Pac Sci 53:74–81
Matson PG, Edwards MS (2007) Effects of ocean temperature on the southern range limits of two understory kelps, Pterygophora californica and Eisenia arborea, at multiple life-stages. Mar Biol 151:1941–1949
Mohring MB, Wernberg T, Kendrick GA, Rule MJ (2013) Reproductive synchrony in a habitat-forming kelp and its relationship with environmental conditions. Mar Biol 160:119–126
Mohring MB, Wernberg T, Wright JT, Connell SD, Russell BD (2014) Biogeographic variation in temperature drives performance of kelp gametophytes during warming. Mar Ecol Prog Ser 513:85–96
Morita T, Kurashima A, Maegawa M (2003) Temperature requirements for the growth and maturation of the gametophytes of Undaria pinnatifida and U. undarioides (Laminariales, Phaeophyceae). Phycol Res 51:154–160
Müller DG, Gassmann G, Lüning K (1979) Isolation of a spermatozoid-releasing and -attracting substance from female gametophytes of Laminaria digitata. Nature 279:430–431
Müller DG, Murúa P, Westermeier R (2019) Reproductive strategies of Lessonia berteroana (Laminariales, Phaeophyceae) gametophytes from Chile: apogamy, parthenogenesis and cross-fertility with L. spicata. J Appl Phycol 31:1475–1481
Murúa P, Westermeier R, Patiño DJ, Müller DG (2013) Culture studies on early development of Lessonia trabeculata (Phaeophyceae, Laminariales): seasonality and acclimation to light and temperature. Phycol Res 61:145–153
Nelson WA (2005) Life history and growth in culture of the endemic New Zealand kelp Lessonia variegata J. Agardh in response to differing regimes of temperature, photoperiod and light. J App Phycol 17:23–28
Oppliger LV, Correa JA, Engelen AH, Tellier F, Vieira V, Faugeron S, Valero M, Gomez G, Destombe C (2012) Temperature effects on gametophyte life-history traits and geographic distribution of two cryptic kelp species. PLoS One 7:e39289
Ratcliff JJ, Soler-Vila A, Hanniffy D, Johnson MP, Edwards MD (2017) Optimisation of kelp (Laminaria digitata) gametophyte growth and gametogenesis: effects of photoperiod and culture media. J Appl Phycol 29:1957–1966
Ridgeway KR (2007) Long-term trend and decadal variability of the southward penetration of the Eastern Australian Current. Geophys Res Lett 34:L12613
Roleda MY (2016) Stress physiology and reproductive phenology of Arctic endemic kelp Laminaria solidungula J. Agardh. Polar Biol 39:1967–1977
Roleda MY, Hurd CL (2019) Seaweed nutrient physiology: application of concepts to aquaculture and bioremediation. Phycologia 58:552–562
Sato Y, Endo H, Oikawa H, Kanematsu K, Naka H, Mogamiya M, Kawano S, Kazama Y (2020) Sexual difference in the optimum environmental conditions for growth and maturation of the brown alga Undaria pinnatifida in the gametophyte stage. Genes 11:1–18
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B (2012) Fiji: an open-source platform for biological-image analysis. Nat Meth 9:676–682
Scott FJ (2017) Marine plants of Tasmania. Tasmanian Museum and Art Gallery, Hobart
Sievert C (2020) Interactive web-based data visualization with R, plotly, and shiny. https://plotly-r.com/
Smale DA (2020) Impacts of ocean warming on kelp forest ecosystems. New Phytol 225:1447–1454
Smale DA, Burrows MT, Moore P, O'Connor N, Hawkins SJ (2013) Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol Evol 3:4016–4038
Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459
Tait LW (2019) Giant kelp forests at critical light thresholds show compromised ecological resilience to environmental and biological drivers. Estuar Coast Shelf Sci 219:231–241
Tatsumi M, Wright JT (2016) Understory algae and low light reduce recruitment of the habitat-forming kelp Ecklonia radiata. Mar Ecol Prog Ser 552:131–143
Thomsen M, Mondardini L, Alestra T, Gerrity S, Tait L, South P, Lilley S, Schiel D (2019) Local extinction of bull kelp (Durvillaea spp.) due to a marine heatwave. Front Mar Sci 6:84
tom Dieck I (1987) Temperature tolerance and daylength effects in isolates of Scytosiphon lomentaria (Phaeophyceae) of the North Atlantic and Pacific Ocean. Helgol Meeresunters 41:307–321
tom Dieck I (1993) Temperature tolerance and survival in darkness of kelp gamtetophytes (Laminariales, Phaeophyta): ecological and biogeographical implications. Mar Ecol Prog Ser 100:253–264
Wade R, Augyte S, Harden M, Nuzhdin S, Yarish C, Alberto F (2020) Macroalgal germplasm banking for conservation, food security, and industry. PLoS Biol 18:e3000641
Wernberg T, Thomsen MS, Tuya F, Kendrick GA, Staehr PA, Toohey BD (2010) Decreasing resilience of kelp beds along a latitudinal temperature gradient: potential implications for a warmer future. Ecol Lett 13:685–694
Wernberg T, Russell BD, Thomsen MS, Gurgel CFD, Bradshaw CJA, Poloczanska ES, Connell SD (2011) Seaweed communities in retreat from ocean warming. Curr Biol 21:1828–1832
Wiencke C (1990) Seasonality of brown macroalgae from Antarctica-a long-term culture study under fluctuating Antarctic daylengths. Polar Biol 10:589–600
Acknowledgements
We thank Damon Britton, Fanny Noisette and Abigail Smith for the help in both the field and IMAS laboratories and Cathy Johnston, Ros Watson and Kim Lee Chang (CSIRO algal laboratory) for use of their temperature gradient table and laboratory support. We also thank Wouter Visch and Bradley Evans for their feedback on the manuscript.
Funding
The project was funded by Tassal Pty Ltd. and the FRDC project ID: 2017-177. MS was supported by funding from Deutsche Forschungsgemeinschaft (DFG, grant ID: SCHM 3335/1-1). JDGE was supported by the Research Grants Council (ECS 27124318) of Hong Kong.
Author information
Authors and Affiliations
Contributions
E. Paine: experimental design, conducted experiments, drafting and editing of manuscript; M. Schmid: experimental design, laboratory assistance, editing manuscript; J. D. Gaitán-Espitia: data analysis and graph creation; J. Castle: analysis of images; Ian Jameson: laboratory facilities and assistance; J. C. Sanderson: original concept, experimental design; C. Hurd: original concept, experimental design, editing manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability
Contact corresponding author.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paine, E.R., Schmid, M., Gaitán-Espitia, J.D. et al. Narrow range of temperature and irradiance supports optimal development of Lessonia corrugata (Ochrophyta) gametophytes: implications for kelp aquaculture and responses to climate change. J Appl Phycol 33, 1721–1730 (2021). https://doi.org/10.1007/s10811-021-02382-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02382-7