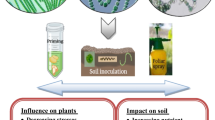
Abstract
Cyanobacteria are Gram-negative oxygenic photosynthetic autotrophic organisms. They are one of the most primitive photosynthetic prokaryotes that have minimal nutritional requirements to grow. Cyanobacteria are used as biofertilizers. Nitrogen is very important for plant growth in many fields, and the effectiveness of this element can be covered by these biofertilizers. In addition to having a healing feature of the soil structure, it has been reported that they facilitate the intake of nutrients and increase the resistance of plants to diseases. Plants are sessile organisms, so it is very difficult to avoid environmental stresses. Abiotic and biotic factors are the most important stresses affecting crop productivity. Biotic factors are the stresses that occur as a result of damage to an organism by other living organisms such as bacteria, viruses, fungi, parasites, insects, weeds, etc. Cyanobacteria are known to have a protective effect under biotic stress.
The purpose of this chapter is to provide detailed information on the use of cyanobacteria as biofertilizers, especially against biotic stresses.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cyanobacteria, also known as blue-green algae, were the first organisms that created molecular oxygen and transformed the biosphere from anaerobic to largely aerobic. Many cyanobacteria have a very wide distribution. Thanks to these features, they are considered as a model organism that enables us to learn about microbial biogeography and evolution (Gupta et al. 2013; Prasanna et al. 2009; Ahmed et al. 2010).
Cyanobacteria have been identified as important inhabitants of many agricultural soils that potentially contribute to biological nitrogen fixation, phosphate dissolution, mineral release to increase soil fertility, and crop productivity (Singh 2014). They produce and secrete a variety of biologically active substances, such as proteins, vitamins, carbohydrates, amino acids, polysaccharides, and phytohormones, which act as signal molecules to support plant growth. So, they protect plants against environmental stress. It is determined that the related bacteria are also found in cultivated fields. Identification of dominant strains effective in plant growth was found important for plant production (Osman et al. 2010; Prasanna et al. 2009).
Cyanobacteria show antagonistic activity against many plant pathogenic fungi. The application of cyanobacteria as biological fertilizers reduced the disease severity caused by the pathogen in many plants (Küçük and Sezen 2019).
2 General Features of Cyanobacteria
Cyanobacteria members are the oldest oxygen-producing photoautotrophs on earth. Plant chloroplasts evolved from cyanobacteria through the process of endosymbiosis. Cyanobacteria are known as blue-green algae, which is commonly confused with algae because it shares traits with algae and bacteria, because of the C-fucocyanin, a blue-green pigment they contain (Yadav et al. 2017) (Table 18.1).
Their cell structures are simple, and individual cells can also exist as spheres, courses, or flat colonies. The most common form of the colonies is a filament. The colonies can contain several cells or several thousands of cells in a mucilage sheath. Threads of cyanobacteria are called “trichomes.” There is no organization or division of labor between cells in the threads. However, it is seen that some cells grow and take a homogeneous appearance, and structures called “heterocyst” occur . A thick wall, enriched with nutrients, surrounds some of the cells, and structures resistant to unfavorable conditions called “akinetes” are formed. In some cells, real branching is seen, while in other cells, false branching is also observed. In some species, it is seen that the trichome thinners from the bottom to the end and there is a heterocyclic at the bottom (Mishra et al. 2013).
Since cyanobacteria cells have a prokaryotic organization, they do not have any membrane organelles. The cell wall is similar in structure and function to Gram-negative bacteria (Whitton and Potts 2012; Mishra et al. 2013). The cytoplasm structure consists of two different layers, namely, chromoplasm and centroplasm. Chromoplasm is a colorful and networked structure with uncertain boundaries around the centroplasm. Generally, it does not have a vacuole and is immobile. As a chemical structure, RNA is dispersed, and assimilation pigments have a lamellar structure. However, they are not homogeneously disperplastics as plastids surrounded by a real membrane. Centroplasm is colourless and located in the centre. Its chemical structure consists of DNA; it contains elements in the form of a stick, reticular, or thread. All of these correspond to the nucleus and are called chromatin devices. There is no real nucleus (Shevela et al. 2013).
There is only chlorophyll-a from chlorophylls in cyanobacteria. Among the carotenoids, they contain β-carotene and E-carotene. Cyanobacteria often have all the types of xanthophylls and lutein. They contain C-fucocyanin and allophycocyanin, which are phycobilins. The color of Cyanophyta is mostly bluish green, olive green, and yellow brown. Cyanobacteria take the blue-green color from fucocyanin. There is also a small amount of phycoerythrin (Takaichi et al. 2009; Singh 2014).
Food storage substances in chromoplasma are glycogen, cyanophilin from proteins, and volutin. Nitrogen constitutes 8% of the dry weight of blue-green algae.
Reproduction in cyanobacteria occurs by dividing the cells into two, as in bacteria. Colony-forming species are seen cell division, and asexual reproduction occurs in a type of fragmentation. In some of the filamentous species, with the death of the cells in between, the thread breaks down into several cells. These parts are called “hormogonium.” Hormogoniums occur in abnormal conditions and develop and form the thread when the conditions are favorable (Cohen and Meeks 1997).
2.1 Ecology and Phylogeny of Cyanobacteria
Cyanobacteria have spread to all parts of the earth. They live in freshwaters and seas. Some of their species are planktonic. Some species are benthic; they live on the grounds of streams, lakes, pond waters, and marshes. In suitable conditions and seasons, some of the planktonic species can over-proliferate and cause the death of fish and other aquatic organisms due to the toxic substances that appear. Some species of cyanobacteria are found in moist soils and on rocks that leak water as a blackish-mucilage cover. They also live on bare rocks on the shores of the seas, bark, and arctic regions (Nagarajan et al. 2011). In addition to their association with plants, they can develop epiphytically on bark, leaves, roots, and stems of submerged areas (Aguiar et al. 2008; Boopathi et al. 2013). They are the most abundant algae after diatoms on the soil surface and below. There are also species living in the dark cave walls as they show chromatic adaptation according to the light intensity. Some species live at 75–85 °C in hot water sources. There are also species living in deserts, poles, snow, rarely in salt waters, and oceans.
Cyanobacteria provide nitrogen for the growth of the plant partner. It has been explained that cyanobacteria can convert atmospheric nitrogen to ammonium form with nitrogenase enzyme, and ATP is used in this conversion (Magnuson 2019):
Species belonging to some blue-green algae genus (Chroococcus, Gloeocapsa, etc.) live symbiotically with fungi and form “lichens.” Some species of Anabaena and Nostoc also live symbiotically with some species of ferns, Gymnosperm and Angiosperms. Cyanobacteria are known to affect tallus morphogenesis in lichens (Singh et al. 2016; Singh 2014). It is known that cyanobacteria, especially those that form symbiotic relationships with plants, secrete protein from carbohydrate-rich arabinogalactan. It has been found that these proteins act as signaling molecules which do not play an important role in the regulation of plant growth and development (Abdel-Raouf et al. 2012). The secretion of phytohormones by cyanobacteria begins with the formation of a symbiotic relationship (Singh et al. 2016).
Nitrogen fixation is an important feature of cyanobacteria. Various species can physiologically detect the free nitrogen of the air. Cyanobacteria are similar to bacteria in these aspects. Apart from cyanobacteria, no other algae group has this feature. The nitrogen-binding species in the structure of lichens give nitrogen they detected to the fungus (Zehr 2011; Stal 2013).
Base compositions of DNA molecules belonging to different cyanobacteria have been determined. GC rates of cyanobacteria with unicellular form vary between 35 and 71%. This ratio indicates that this group includes a very large group of organisms that have very few genetically related relationships. On the other hand, DNA ratios of DNA molecules of the cyanobacteria group that form the heterocysts very much less (between 38 and 46%). Cyanobacteria are grouped with their morphological lines as well as phylogenetic features. Unicellular cyanobacteria are very broad phylogenetic, and different representatives show phylogenetic relationship with different morphological groups (Yadav et al. 2017; Chittora et al. 2020).
3 Biofertilizers
Agricultural systems that use more inputs for high yields cause environmental problems and depletion of natural resources. The rapid production increase caused by the application of chemicals decreases gradually, and a healthy agriculture system becomes inevitable. The production of clean foods without agricultural chemicals is compulsory for the future of humanity and natural resources. Plant nutrients are essential for crop and healthy food production, given the growing population of the world. Today, agricultural strategies are mainly carried out on inorganic chemical-based fertilizers, which pose a serious threat to the environment and human health (Itelima et al. 2018). Biofertilizer is used as an alternative way to increase soil fertility and crop production in sustainable agriculture. The use of beneficial microorganisms as biofertilizers is crucial for the agricultural sector, given their potential in food safety and sustainable crop production (Vessey 2003). Research is ongoing to make biofertilizers an important component of nutritional management. According to a report by the FAO published in 2006, biofertilizer is a substance used for products containing microorganisms that fix atmospheric N or secrete growth-promoting substances that help dissolve soil nutrients (FAO 2006).
Nitrogen fixers (N-fixer), potassium and phosphorus solubilizers, plant growth-promoting rhizobacteria (PGPRs), endo- and ectomycorrhizal fungi, and cyanobacteria are commonly used as biofertilizer components (Fig. 18.1) (Ansari and Mahmood 2017; Zakeel and Safeena 2019). The use of biofertilizers provides improved nutrients and water intake, plant growth, and enhanced plant defense against abiotic and biotic stresses. These properties of biofertilizers play a very important role in soil fertility and environmental protection. Also, their low cost will benefit farmers economically (Itelima et al. 2018).
Classification of biofertilizers. (Adapted from Zakeel and Safeena 2019)
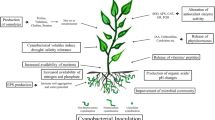
Biofertilizer is an alive, pure, or mixed microorganism formulation that, when applied to seed, plant surface, or soil, colonizes in the rhizosphere or enters the plant tissues, fixes atmospheric nitrogen, and increases soil uptake and plant nutrient uptake and vegetative growth (Chatterjee et al. 2017) (Fig. 18.2). Biofertilizers are cheaper than chemical fertilizers, do not show toxic effects to plants, do not pollute groundwater, do not increase soil acidity, and do not adversely affect plant development. The most prominent features of biofertilizers related to plant development are nitrogen fixation, making plant nutrients available, biological control of diseases, and secretion of plant growth stimulants. While a significant amount of fossil energy is used in chemical fertilizer production, energy is free in biological fertilization. The species that are active among the bacteria generally isolated from the rhizosphere are chosen by considering their adaptability to activity and environmental conditions and are stored for use in single or multiple species containing biological fertilizers. Reducing the use of excessive chemical fertilizers, potential nitrogen fixation and the use of phosphate-dissolving bacteria as biological fertilizers increases productivity in agricultural products. However, it is necessary to develop special plant-microorganism combinations that will show high efficiency in wide environmental conditions (Vessey 2003; Adesemoye and Kloepper 2009; Sinha et al. 2010; Khosro and Yosef 2012; Santos et al. 2012; Raja 2013; Youssef and Eissa 2014; Chun-Li et al. 2014).
As biofertilizers are living content and product content, quality of life and shelf life directly affect the availability or efficiency of biofertilizer.
Biofertilizer:
-
Colonized in the rhizosphere when entering seed, plant surface, or soil or entering plant tissues.
-
Fixing atmospheric nitrogen.
-
A living, pure, or mixed microorganism formulation that increases soil.
These:
-
Cheap cost.
-
Do not show toxic effects to plants.
-
Do not pollute groundwater.
-
Do not increase soil acidity.
-
Biologically controlling soil-borne diseases and secreting substances that stimulate plant growth (increase tolerance to environmental stresses) phosphorus, and uptake of plant nutrients and plant growth (Çakmakçı 2014).
Effective work of microorganisms occurs only when there are favorable and optimal conditions for them to metabolize their substrates. Some of these conditions are adequate water and oxygen (varies depending on whether microorganisms are aerobic or anaerobic), pH, and ambient temperature.
3.1 Types of Biofertilizers
According to the general classification in the FAO’s report entitled “Plant Nutrition for Food Security” published in 2006, biofertilizers can be divided into four main categories:
-
1.
N-fixing biofertilizers : These include Rhizobium, Azotobacter, Azospirillum, Clostridium, and Acetobacter bacteria; cyanobacteria; and fern Azolla (collaborating with cyanobacteria).
-
2.
P-solubilizer/activating biofertilizers : Phosphate-solubilizing bacteria (PSB) and phosphate-solubilizing microorganisms (PSMs), for example, Bacillus, Pseudomonas, and Aspergillus. Mycorrhiza is a nutrient-activating fungus.
-
3.
Composting accelerators: Cellulosic (Trichoderma) and ligninolytic (Humicola).
-
4.
Plant growth-promoting rhizobacteria (PGPRs): Pseudomonas species. PGPRs increase plant growth and performance.
Different types of biological fertilizers and related microorganisms are given in Table 18.2 (Itelima et al. 2018).
Among these, the groups of N-fixing organisms are the most important biological fertilizers used in plant growing. Another important biofertilizer is those containing P-dissolving organism cultures. Unlike industrial nitrogen fixation, biological nitrogen fixation involves the conversion of nitrogen (N2) to ammonia via microorganisms. Many microorganisms (e.g., Rhizobium, Azotobacter, and Cyanobacteria) reduce the atmospheric N2 to ammonia (NH3) using molecular N2 with the help of nitrogen enzyme:
Biological nitrogen fixation is an important nitrogen source for plant life. Biological nitrogen fixation estimates range from 100 to 290 million tons N/year. It is estimated that 40–48 million tons of this total is biologically fixed in agricultural crops and fields. Only nitrogen-fixing microorganisms supply an additional nutrient (N) to the soil plant system. Other biological fertilizers dissolve or activate the nutrients already in the soil. Azolla is an almost unique species when evaluated as a green fertilizer among nitrogen-fixing cyanobacteria. In this process, it does not only add the nitrogen it fixes biologically but also other nutrients it receives from the soil. While Rhizobium is specific to legumes, Cyanobacteria and Azolla are useful in increasing N supplies during flooded rice cultivation as they are abundant in wetlands (FAO 2006).
Some of the biofertilizers promote plant growth through the production of plant hormones. The production of hormones such as auxins, cytokinins, and giberellins has an effect on plant development and quality via direct and/or indirect mechanisms (Eşitken et al. 2003a, b; Elsheikh and Elzidany 1997).
Direct mechanisms:
-
Biological nitrogen fixation.
-
Reducing environmental stress.
-
Harmony in a bacteria-plant relationship.
-
Increasing the inorganic phosphorus solubility.
-
Mineralization of organic phosphorus compounds.
-
Increasing iron intake and increasing the ratio of some trace elements.
-
Vitamin synthesis.
-
Increasing root permeability.
Indirect mechanisms:
-
Taking a role as biocontrol agents, reducing diseases with antibiotic production.
-
In soils contaminated with various organic compounds, it is counted as protecting plants by breaking down barrier xenobiotics.
The main idea in biological fertilization is to reduce the use of chemicals to support agricultural sustainability, to protect natural resources and the environment, and to improve the quality. In its current state, biofertilizers cannot replace agricultural chemicals alone, but they reduce their usage rates and support ecological agriculture (Eşitken et al. 2003a, b; Elsheikh and Elzidany 1997).
4 Biotic Stress
Stress in plants is defined as all external factors that adversely affect the growth, development, or productivity. Plants are constantly subjected to environmental stresses due to their immobile structure. Stresses in plants cause a wide variety of events such as cellular metabolism, gene expression, changes in growth rates, crop yields, etc. Plants developed effective strategies and mechanisms to deal with environmental stresses. Stress response mechanisms contribute to stress resistance or stress tolerance at different morphological, biochemical, and molecular levels (Bakır 2020). The stresses to which plants are exposed are gathered under two important topics. These are “abiotic” and “biotic” stresses (Fig. 18.3). Biotic factors are stresses caused by infection of microorganisms (fungi, bacteria, and virus) and attacks of harmful animals (Lichtenhaler 1996; Büyük et al. 2012). Abiotic stress factors are environmental factors including drought, cold, hot, salt, and nutritional deficiencies and are among the factors that decrease productivity in agricultural production. Biotic and abiotic stresses have been shown to reduce the average crop productivity by 65–87% depending on the crop type (Verma et al. 2013).
Viruses, bacteria, fungi, nematodes, insects, arachnids, and weeds are known as living organisms that cause biotic stress in plants. The organisms that cause biotic stress can lead to the death of plants by depriving their hosts of nutrients directly. Biotic stresses are very important for agriculture due to pre- and postharvest losses. Generally biotic stresses affect photosynthesis, because of chewing insects and virus infections, and reduce the rate of photosynthesis (Gull et al. 2019). The increase in the amount of pests and pathogens in nature can be caused by climate changes. For example, it is known that an increase in temperatures facilitates pathogen spread. At the same time, many abiotic stress conditions weaken the defense mechanisms of plants and thereby increase their susceptibility to pathogen infection (Suzuki et al. 2014).
Three different pathogen attack strategies have been defined (Koeck et al. 2011; Elad et al. 2011):
-
1.
Necrotrophy: Plant cells are killed by pathogen infection (gray mold, Botrytis cinerea).
-
2.
Biotrophy: In biotrophy the plant cells remain alive (powdery mildew, Podosphaera aphanis).
-
3.
Semibiotrophy: The pathogen does not immediately kill the cells, causing them to die later in the infection, in this type (anthracnose, Colletotrichum acutatum).
Some pathogens that cause biotic stress in plants and their effects on the area they infect are given in the table below (Table 18.3) (Kanwar and Jha 2019).
Plants use highly complex defense systems against pathogen attacks. The defense mechanism has two types: innate and systemic plant response. However, the plant in two ways exhibits a natural defense: specific (specific to species/pathogen race) and nonspecific (non-host or general resistance). Nonspecific resistance is based on both structural barriers and inducible responses, including numerous proteins and other organic molecules produced before infection or during a pathogen attack. Structural defenses include morphological and structural barriers, chemical compounds, proteins, and enzymes. These compounds not only protect the plant from invasion but also give the plant strength and hardness, giving it tolerance or resistance to biotic factors (Onaga and Wydra 2016).
5 Usage of Cyanobacteria as Biofertilizer for Biotic Stress
Different microorganism groups associated with plants have been described to produce metabolites with beneficial effects on plants (Berendsen et al. 2012; Mendes et al. 2013). The harmful effects of pathogens on plants have been known for a long time. Studies reveal signals related to microorganisms promoting plant growth (PGPR = plant growth-promoting rhizobacteria) , and plant communications have accelerated in recent years. PGPRs have been reported to release signaling compounds that can bind to receptor sites on the plasma membrane and cause activation of genes, leading to the synthesis of proteins and enzymes or secondary metabolites (Hussain et al. 2013). Many of the signaling compounds included in the phytochemical reaction belonging to the carbohydrate, lipid, glycolipid, or glycoprotein group have been identified (Yamaguchi and Huffaker 2011). Some of these compounds have been found to cause an increase in the accumulation of glucosinolates, alkaloids, polyphenols, flavonoids, flavonoid glycosides, saponins, terpenes, and phytoalexins, when applied to plants as spray or root treatments (Hussain et al. 2013; Rodriguez et al. 2006). These phytochemicals protect plants from biotic and abiotic stress and help plants develop resistance to these stresses (Shan et al. 2012; Sokolova et al. 2011).
When studies on microorganisms that support plant growth are examined, it has been determined that the most researched studies are rhizobacteria, symbiotic rhizobia, and mycorrhizal fungi. In recent studies, it is seen that another group of microorganisms that encourage plant development is cyanobacteria (Mendes et al. 2013; Willis et al. 2013). In recent studies, data affecting the gene expression of host plants have been obtained with the signals produced by cyanobacteria; thus it has been determined that various changes occur in the phytochemical structures of plants (Manjunath et al. 2010; Singh et al. 2016; Yadav et al. 2017). The development of phytochemicals has opened a new field of research that may have significant economic benefits for the agricultural industry. Studies on resistance induced to control plant diseases in laboratory, greenhouse, and field conditions enabled the commercialization of R&D products, thereby providing new-generation microbial fertilizers or product preservatives.
Bioactive compounds produced by cyanobacteria have been found to increase phytohormone levels, which are responsible for triggering the development of the subsoil and aboveground parts of the plant. It is also known that phytohormones regulate the enzymatic activities and metabolic changes that occur during plant growth. Therefore, the increase in the activity of peroxidase and phenylalanine ammonia-lyase enzymes from defense enzymes has also been linked to phytohormone levels (Tvorogova et al. 2013). The presence of jasmonic acid (JA) has been detected in cyanobacteria (Singh 2014). These bacteria have been reported to trigger the accumulation of abscisic acid (ABA), which ensures plant survival in stress conditions such as wilt, water stress, osmotic stress, and salt stress (Khan et al. 2012). Jasmonic acid and its various metabolites are known to be responsible for regulating plant development as well as plant reactions to abiotic and biotic stress (Khan et al. 2012). In addition, members of Synechococcus, Anabaena, Nostoc, Calothrix, Scytonema, and Cylindrospermum can produce ethylene (Singh et al. 2016). Flavonoids and phytohormones have been reported to aid plant-microorganism interactions (Jaiswal et al. 2018); these compounds increased root colonization of microorganisms (Kehr et al. 2011), providing an allelochemical effect on the population of other organisms (Khan et al. 2012). These also served as signal molecules (Kehr et al. 2011; Khan et al. 2012).
Cyanobacteria are used as biological fertilization of some rice cultures. It is known that over a hundred of cyanobacteria species fix N. Common cyanobacteria, Nostoc, Anabaena, Aulosira, Tolypothrix, and Calothrix, are used as biological fertilizers for rice (Chittora et al. 2020). Cyanobacteria also release plant growth substances such as IAA (indoleacetic acid) and GA (gibberellic acid) and improve polysaccharides that help bind soil particles (improving soil structure). These are also used as a soil conditioner and to protect the soil against erosion by entangled bulk formation (FAO 2006). The optimum temperature for cyanobacteria is about 30–35 °C. The pH of the soil is the most important factor in the growth of cyanobacteria and N fixation. The optimal pH for growth of cyanobacteria in the culture medium is 7.5–10, and the lower limit is around 6.5–7. The growth of cyanobacteria is better in neutral to alkaline soils under natural conditions. Cyanobacteria need all plant nutrients to grow and fix nitrogen (N). N-containing fertilizers often inhibit the growth and N fixation of cyanobacteria. Since phosphorus (P) increases the growth and N fixation of cyanobacteria, sufficient phosphorus must be present in irrigation water. Consequently, P deficiency causes a marked decrease in the growth of cyanobacteria and thus N fixation. Cyanobacteria vaccine can be prepared in the laboratory or open areas. The open-air soil culture method is simple, is less expensive, and can be easily adapted by farmers (FAO 2006).
Some cyanobacteria have been found to reduce the occurrence of a disease caused by plant pathogens in plants (Table 18.4), for example, culture filter and ethyl acetate extract of Calothrix elenkinii Kossinskaja; in pot experiments, it has been found that it decreases disease severity on Pythium aphanidermatum (Edson) Fitzp-infected soybean, tomato, and pepper seeds (Manjunath et al. 2010). It was investigated that damping-off disease in tomato seedlings inoculated with a group of fungal pathogens containing Pythium debaryanum R. Hesse, Fusarium oxysporum f. sp. lycopersici W.C. Snyder & H.N. Hansen, Gibberella fujikuroi (Sawada) Wollenw, and Rhizoctonia solani J.G. Kühn decreases with Trichormus variabilis (Kützing ex Bornet & Flahault) Komarek & Anagnostidis and Anabaena oscillarioides Bory ex Bornet & Flahault applications (Chaudhary et al. 2012). Trichormus variabilis and A. laxa A. Braun were found to produce a systemic defense response in tomato plants struggling with Fusarium sp. wilt. Some enzyme activities, phenylalanine ammonia-lyase, polyphenol oxidase, chitosanase, and β-1,3-glucanase, were found high in the tomato roots treated with cyanobacterial formulations. This situation revealed the importance of cyanobacterial interaction with tomato seedlings (Prasanna et al. 2013).
The use of bacteria that promote plant growth as biocontrol agents to be used against soil-borne plant pathogens has become very attractive in recent years for sustainable agriculture. These microorganisms reveal their induced systemic resistance (ISR), which strengthens the physical and mechanical of the cell wall and alters the synthesis of metabolites for defense against pathogens and the physiological and biochemical reaction of the host (Chaudhary et al. 2012).
6 Conclusions
Today, strategies that can help reduce chemicals used for agricultural products, a more economical product to be used instead of chemicals, and environmentally friendly agriculture are demanded. Various methods are tried to increase product yield. Cyanobacteria are abundant in agricultural areas and, especially in rice-cultivated soils, together with microalgae, are considered as microbial photosynthetic agents of the soil. Because of its important roles in nitrogen fixation, cyanobacteria are inevitable to be used in agriculture to increase vegetative production. Although there are several studies on nitrogen fixation abilities, their ecological roles are not fully understood. It has been determined that cyanobacterial inoculation in agricultural areas provides increased yield even in the presence of high doses of nitrogen fertilizers. In addition to increasing the nitrogen content of plants, cyanobacteria can be used to promote plant growth. For this reason, significant progress has been made in recent years in the development and application of cyanobacterial biofertilizers.
Biosynthesis of phytohormones, polysaccharides, vitamins, amino acids, and peptides is considered crucial for plant growth and development. Microorganisms release these active compounds in the rhizosphere where plant roots can absorb.
Cyanobacterial strains have been identified in studies that support the growth of the plant, usually by greenhouse and pot experiments performed under controlled conditions. New studies are needed to try cyanobacterial strains in field conditions. This chapter is expected to shed light on the work to be done in the application of cyanobacteria to agricultural fields.
References
Abdel-Hafez SI, Abo-Elyousr KA, Abdel-Rahim IR (2015) Fungicidal activity of extracellular products of cyanobacteria against Alternaria porri. Eur J Plant Pathol 50:239–245
Abdel-Raouf N, Al-Homaidan AA, Ibraheem IBM (2012) Agricultural importance of algae. Afr J Biotechnol 11:11648–11655
Abo-Shady AM, Al-ghaffar BA, Rahhal MMH, Abd-El Monem HA (2007) Biological control of faba bean pathogenic fungi by three cyanobacterial filtrates. Pak J Biol Sci 10:3029–3038
Adesemoye AO, Kloepper JW (2009) Plant–microbes interactions in enhanced fertilizer-use efficiency. Appl Microbiol Biotechnol 85:1–12
Aguiar R, Fiore MF, Franco MW, Ventrella MC, Lorenzi AS, Vanetti CA, Alfenas AC (2008) A novel epiphytic cyanobacterial species from the genus Brasilonema causin damage to Eucalyptus leaves. J Phycol 44:1322–1334
Ahmed M, Stal LJ, Hasnain S (2010) Association of non-heterocystous cyanobacteria with crop plants. Plant Soil 336:363–375
Ansari RA, Mahmood I (2017) Optimization of organic and bio-organic fertilizers on soil properties and growth of pigeon pea. Sci Hortic 226:1–9
Bakır Ö (2020) Abiotic stress-related miRNAs in Triticeae. Atatürk Univ J Agric Faculty 51:207–218
Balachandran S, Osmond CB, Daley PF (1994) Diagnosis of the earliest strain-specific interactions between tobacco mosaic virus and chloroplasts of tobacco leaves in vivo by means of chlorophyll fluorescence imaging. Plant Physiol 104:1059–1065
Berendsen RL, Pieterse CMJ, Bakker P (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Berger S, Benediktyová Z, Matous K et al (2007) Visualization of dynamics of plant-pathogen interaction by novel combination of chlorophyll fluorescence imaging and statistical analysis: differential effects of virulent and avirulent strains of P. syringae and of oxylipins on A. thaliana. J Exp Bot 58:797–806
Biondi N, Piccardi R, Margheri MC, Rodolfi L, Smith GD, Tredici MR (2004) Evaluation of Nostoc strain ATCC 53789 as a potential source of natural pesticides. Appl Environ Microbiol 70:3313–3320
Bonfig KB, Schreiber U, Gabler A et al (2006) Infection with virulent and avirulent P. syringae strains differentially affects photosynthesis and sink metabolism in Arabidopsis leaves. Planta 225:1–12
Boopathi T, Balamurugna V, Gopinath S, Sundararaman M (2013) Characterization of IAA production by the mangrove cyanobacterium Phormidium sp. MI405019 and its influence on tobacco seed germination and organogenesis. J Plant Growth 32:758–766
Borowitzka MA (1995) Microalgae as source of pharmaceuticals and other biologically active compounds. J Appl Phycol 7:3–15
Büyük İ, Soydam-Aydın S, Aras S (2012) Molecular responses of plants to stress conditions. Turk Hij Den Biyol Derg 69:97–110
Çakmakçı R (2014) Mikrobiyal gübre olarak kullanılabilecek mikroorganizmaların etki mekanizmaları ve özellikleri. In: Mikrobiyal Gübre çalıştayı, pp 5–18 (in Turkish)
Chatterjee A, Singh S, Agrawal C, Yadav S, Rai R, Rai L (2017) Role of algae as a biofertilizer. In: Algal green chemistry. Elsevier, Amsterdam, pp 189–200. https://doi.org/10.1016/b978-0-444-63784-0.00010-2
Chaudhary V, Prasanna R, Nain L, Dubey SC, Gupta V, Singh R, Bhatnagar AK (2012) Bioefficacy of novel cyanobacteria-amended formulations in suppressing damping off disease in tomato seedlings. World J Microbiol Biotechnol 28:3301–3310
Chen J, Zhang H, Feng M et al (2016) Transcriptome analysis of woodland strawberry (Fragaria vesca) response to the infection by strawberry vein banding virus (SVBV). Virol J 13:128–137
Chittora D, Meena M, Barupal T, Swapnil P, Sharma K (2020) Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem Biophys Rep 22:100737
Cho WK, Lian S, Kim S-M et al (2015) Time-course RNA-Seq analysis reveals transcriptional changes in rice plants triggered by rice stripe virus infection. PLoS One 10:e0136736
Chun-Li W, Shiuan-Yuh C, Chiu-Chung Y (2014) Present situation and future perspective of bio-fertilizer for environmentally friendly agriculture. Annu Rep 21:1–5
Cohen MF, Meeks JC (1997) A hormogonium regulating locus, hrmUA, of the cyanobacterium Nostoc punctiforme strain ATCC 29133 and its response to an extract of a symbiotic plant partner Anthoceros punctatus. Mol Plant-Microbe Interact 10:280–289
de Torres Zabala M, Littlejohn G, Jayaraman S et al (2015) Chloroplasts play a central role in plant defence and are targeted by pathogen effectors. Nat Plants 1:1–10
DEQ (n.d.). https://www.deq.ok.gov. Accessed June 2020
Elad Y, Cytryn E, Harel YM, Lew B, Graber ER (2011) The biochar effect: plant resistance to biotic stresses. Phytopathol Mediterr 50:335–349
Elsheikh EA, Elzidany AA (1997) Effects of rhizobium inoculation, organic and chemical fertilizers on yield and physical properties of faba bean seeds. Plant Foods Hum Nutr 51:137–144
Eşitken A, Karlıdağ H, Ercişli S, Turan M, Şahin F (2003a) The effect of spraying a growth promoting bacterium on the yield, growth and nutrient element composition of leaves of apricot (Prunus armeniaca L. cv. Hacihaliloglu). Aust J Agric Res 54:377–380
Eşitken A, Ercişli S, Şevik İ, Şahin F (2003b) Effect of indole-3-butyric acid and different strains of agrobacterium rubi on adventive root formation from softwood and semi-hardwood wild sour cherry cuttings. Turk J Agric For 27:37–42
FAO (2006) Plant nutrition for food security. A guide for integrated nutrient management. FAO Fertilizer and Plant Nutrition Bulletin, Rome
Gull A, Lone AA, Wani NUI (2019) Biotic and abiotic stresses in plants. In: Abiotic and biotic stress in plants. IntechOpen, Rijeka
Gupta V, Ratha SC, Sood A, Chaudhary V, Prasanna R (2013) New insight into the biodiversity and applications of cyanobacteria (blue-green algae)-prospects and challenges. Algal Res 2:79–97
Hagmann L, Juttner F (1996) Fischerllin: a novel with the fungicide Diathane M45 on the control of photosystem II inhibiting allelochemical of the chocolate spot on leaf and pods spot on horse cyanobacterium Fischerella muscicola with beans. Agric Res Rev Cairo 53:123–134
Halitschke R, Hamilton JG, Kessler A (2011) Herbivore-specific elicitation of photosynthesis by mirid bug salivary secretions in the wild tobacco Nicotiana attenuata. New Phytol 191:528–535
Herbers K, Takahata Y, Melzer M et al (2000) Regulation of carbohydrate partitioning during the interaction of potato virus Y with tobacco. Mol Plant Pathol 1:51–59
Hui D, Iqbal J, Lehmann K et al (2003) Molecular interactions between the specialist herbivore Manduca sexta (lepidoptera, sphingidae) and its natural host Nicotiana attenuata: V. microarray analysis and further characterization of large-scale changes in herbivore-induced mRNAs. Plant Physiol 131:1877–1893
Hussain A, Hamayun M, Shah ST (2013) Root colonization and phytostimulation by phytohormones producing entophytic Nostoc sp. AH-12. Curr Microbiol 67:624–630
Itelima JU, Bang WJ, Onyimba IA, Oj E (2018) A review: biofertilizer; a key player in enhancing soil fertility and crop productivity. J Microbiol Biotechnol Rep 2:22–28
Jaiswal A, Das K, Koli DK, Pabbi S (2018) Characterization of cyanobacteria for IAA and siderophore production and their effect on rice seed germination. Int J Curr Microbiol Appl Sci 7:5212–5222
Jaki B, Zerbe O, Heilmann J, Sticher O (2001) Two novel cyclic peptides with antifungal activity from the cyanobacterium Tolypothrix byssoidea (EAWAG 195). J Nat Prod 64:154–158
Jimenez E, Dorta F, Medina C, Ramírez A, Ramírez I, Pena-Cortes H (2011) Anti-phytopathogenic activities of macro-algae extracts. Mar Drugs 9:739–756
Kanwar P, Jha G (2019) Alterations in plant sugar metabolism: signatory of pathogen attack. Planta 249:305–318
Kehr J, Picchi DG, Dittmann E (2011) Natural product biosynthesis in cyanobacteria: a treasure trove of unique enzymes. Beilstein J Org Chem 7:1622–1635
Khan MIR, Syeed S, Nazar R, Anjum NA (2012) An insight into the role of salicylic acid and jasmonic acid in salt stress tolerance. In: Phytohormones and abiotic stress tolerance in plants. Springer, Berlin, p 300
Khosro M, Yosef S (2012) Bacterial biofertilizers for sustainable crop production: a review. J Agric Biol Sci 7:307–316
Kocal N, Sonnewald U, Sonnewald S (2008) Cell wall-bound invertase limits sucrose export and is involved in symptom development and inhibition of photosynthesis during compatible interaction between tomato and Xanthomonas campestris pv vesicatoria. Plant Physiol 148:1523–1536
Koeck M, Hardham AR, Dodds PN (2011) The role of effectors of biotrophic and hemibiotrophic fungi in infection. Cell Microbiol 13:1849–1857
Küçük Ç, Sezen G (2019) Cyanobacteria that promote that plant growth and metabolites. Comm J Biol 3:117–123
Kumar G, Teli B, Mukherjee A, Bajpai R, Sarma BK (2019) Secondary metabolites from cyanobacteria: a potential source for plant growth promotion and disease management. In: Secondary metabolites of plant growth promoting rhizomicroorganisms. Springer, Singapore, pp 239–252
Lichtenhaler HK (1996) Vegetation stress: an introduction to the stress concept in plants. J Plant Physiol 148:4–14
Lohaus G, Heldt HW, Osmond CB (2000) Infection with phloem limited Abutilon mosaic virus causes localized carbohydrate accumulation in leaves of Abutilon striatum: relationships to symptom development and effects on chlorophyll fluorescence quenching during photosynthetic induction. Plant Biol 2:161–167
Magnuson A (2019) Heterocyst thylakoid bioenergetics. Life 9:13
Manjunath M, Prasanna R, Nain L, Dureja P, Singh R, Kumar A, Kaushik BD (2010) Biocontrol potential of cyanobacterial metabolites against damping off disease caused by Pythium aphanidermatum in solanaceous vegetables. Arch Phytopathol Plant Prot 43:666–677
Martin K, Singh J, Hill JH et al (2016) Dynamic transcriptome profiling of bean common mosaic virus (BCMV) infection in common bean (Phaseolus vulgaris L.). BMC Genomics 17:613–619
Mendes R, Garbeva P, Raaijmakers JM (2013) The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37:634–663
Mishra PK, Sailo JL, Mehta SK (2013) Structural, physiological, and ecological adaptations in cyanobacterial mats under stressful environment. In: Stress biology of cyanobacteria: molecular mechanisms to cellular responses. CRC Press, Boca Raton, pp 352–364
Montero R, Pérez-Bueno ML, Barón M et al (2016) Alterations in primary and secondary metabolism in Vitis vinifera “Malvasía de Banyalbufar” upon infection with grapevine leafroll-associated virus 3. Physiol Plant 157:442–452
Mostafa SM, Abdel El-All AAM, Hussien MY (2009) Bioactivity of algal extracellular byproducts on cercospora leaf spot disease, growth performance and quality of sugar beet. In: 4th conference on recent technologies in agriculture, Faculty of Agriculture, Cairo University
Mundt S, Kreitlow S, Jansen R (2003) Fatty acids with antibacterial activity from the cyanobacterium Oscillatoria redekei HUB 051. J Appl Phycol 15(2–3):263–267
Nagarajan M, Maruthanayagam V, Sundararamam M (2011) A review of pharmacological and toxicological potentials of marine cyanobacterial metabolites. J Appl Toxicol 32:153–185
Onaga G, Wydra K (2016) In: Abdurakhmonov IY (ed) Advances in plant tolerance to biotic stresses, plant genomics. IntechOpen, Rijeka. https://doi.org/10.5772/64351
Osman MEH, El-Sheekh MM, El-Naggar AH, Gheda SF (2010) Effect of two species of cyanobacteria as biofertilizers on some metabolic activities, growth, and yield of pea plant. Biol Fert Soils 46:861–875
Pérez-Bueno ML, Ciscato M, VandeVen M et al (2006) Imaging viral infection: studies on Nicotiana benthamiana plants infected with the pepper mild mottle tobamovirus. Photosyn Res 90:111–123
Prasanna R, Babu S, Rana A et al (2013) Evaluating the establishment and agronomic proficiency of cyanobacterial consortia as organic options in wheat–rice cropping sequence. Experimental Agriculture, 49(3), 416–434. https://doi.org/10.1017/S001447971200107X
Prasanna R, Jaiswal P, Nayak S, Sood A, Kaushik BD (2009) Cyanobacterial diversity in the rhizosphere of rice and its ecological significance. Indian J Microbiol 49:89–97
Raja N (2013) Biopesticides and biofertilizers: ecofriendly sources for sustainable agriculture. J Biofertil Biopest 4:112–115
Ralph SG, Yueh H, Friedmann M et al (2006) Conifer defence against insects: microarray gene expression profiling of Sitka spruce (Picea sitchensis) induced by mechanical wounding or feeding by spruce budworms (Choristoneura occidentalis) or white pine weevils (Pissodes strobi) reveals large-scale. Plant Cell Environ 29:1545–1570
Rodriguez AA, Stella AM, Storni MM, Zulpa G, Zaccaro MC (2006) Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Syst 2:7–10
Santos VB, Araújo AS, Leite LF, Nunes LA, Melo WJ (2012) Soil microbial biomass and organic matter fractions during transition from conventional to organic farming systems. Geoderma 170:227–231
Shan X, Yan J, Xie D (2012) Comparison of phytohormone signaling mechanisms. Curr Opin Plant Biol 15:84–91
Shevela D, Pishchalnikov RY, Eichacker LA (2013) Oxygenic photosynthesis in cyanobacteria. In: Stress biology of cyanobacteria: molecular mechanisms to cellular responses. CRC Press, Boca Raton, pp 3–40
Shukla N, Yadav R, Kaur P et al (2017) Transcriptome analysis of root-knot nematode (Meloidogyne incognita)-infected tomato (Solanum lycopersicum) roots reveals complex gene expression profiles and metabolic networks of both host and nematode during susceptible and resistance responses. Mol Plant Pathol 19:615–633
Singh S (2014) A review on possible elicitor molecules of cyanobacteria: their role in improving plant growth and providing, tolerance against biotic abiotic stress. J Appl Microbiol 7:1–19
Singh JS, Kumar A, Rai AN, Singh DP (2016) Cyanobacteria: a precious bioresource in agriculture, ecosystem and environmental sustainability. Front Microbiol 7:1–19
Sinha RK, Valani D, Chauhan K, Agarwal S (2010) Embarking on a second green revolution for sustainable agriculture by vermiculture biotechnology using earthworms: reviving the dreams of Sir Charles Darwin. J Agric Biotechnol Sustain Dev 2:113–128
Sokolova MG, Akimova GP, Vaishlya OB (2011) Effect of phytohormones synthesized by rhizosphere bacteria on plants. Appl Biochem Microbiol 47:274–278
Stal JL (2013) Environmental factors regulating nitrogen fixation in heterocystous and non-heterocystous cyanobacteria. In: Stress biology of cyanobacteria: molecular mechanisms to cellular responses. CRC Press, Boca Raton, pp 291–306
Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R (2014) Abiotic and biotic stress combinations. New Phytol 203:32–43
Takaichi S, Maoka T, Mochimaru M (2009) Unique carotenoids in the terrestrial cyanobacterium Nostoc commune NIES-24: 2-hydroxymyxol 20-fucoside, nostoxanthin and canthaxanthin. Curr Microbiol 59:413–419
Tang JY, Zielinski RE, Zangerl AR et al (2006) The differential effects of herbivory by first and fourth instars of Trichoplusia ni (Lepidoptera: Noctuidae) on photosynthesis in Arabidopsis thaliana. J Exp Bot 57:527–536
Tecsi LI, Smith AM, Maule AJ, Leegood RC (1996) A spatial analysis of physiological changes associated with infection of cotyledons of marrow plants with cucumber mosaic virus. Plant Physiol 111:975–985
Tiwari A, Kaur A (2014) Allelopathic impact of cyanobacteria on pathogenic fungi. Int J Pure Appl Biosci 2(3):63–70
Tvorogova VY, Osipova MA, Doduyeva IY, Lutova LA (2013) Interactions between transcription factors and phytohormones in the regulation of plant meristem activity. Russ J Genet Appl Res 3:325–337
Vega A, Gutiérrez RA, Peña-Neira A et al (2011) Compatible GLRaV-3 viral infections affect berry ripening decreasing sugar accumulation and anthocyanin biosynthesis in Vitis vinifera. Plant Mol Biol 77:261–274
Verma S, Nizam S, Verma PK (2013) Biotic and abiotic stress signalling in plants. In: Sarwat M, Ahmad A, Abdin MZ (eds) Stress signaling in plants: genomics and proteomics perspective, vol 1. Springer, Berlin, pp 25–49
Vessey JK (2003) Plant growth promoting Rhizobacteria as bio-fertilizers. Plant Soil 225:571–586
Whitton BA, Potts M (2012) Introduction to the cyanobacteria. In: Ecology of cyanobacteria II. Springer, Dordrecht, pp 1–13
Willis BF, Rodrigues BF, Harris PJC (2013) The ecology of arbuscular mycorrhizal fungi. Crit Rev Plant Sci 32:1–20
Yadav S, Rai S, Rai R, Shankar A, Singh S, Rai LCR (2017) Cyanobacteria: role in agriculture, environmental sustainability, biotechnological potential and Agroecological impact. In: Singh DP, Singh HB, Prabha R (eds) Plant-microbe interactions in agro-ecological perspectives, Microbial interactions and agro-ecological impacts, vol 2. Springer, Berlin, p 277
Yamaguchi Y, Huffaker A (2011) Endogenous peptide elicitors in higher plants. Curr Opin Plant Biol 14:351–357
Youssef MMA, Eissa MFM (2014) Biofertilizers and their role in management of plant parasitic nematodes. A review. J Biotechnol Pharm Res 5:1–6
Zakeel MCM, Safeena MIS (2019) Biofilmed biofertilizer for sustainable agriculture. In: Plant health under biotic stress. Springer, Singapore, pp 65–82
Zangerl AR, Hamilton JG, Miller TJ et al (2002) Impact of folivory on photosynthesis is greater than the sum of its holes. Proc Natl Acad Sci U S A 99:1088–1091
Zehr JP (2011) Nitrogen fixation by marine cyanobacteria. Trends Microbiol 19:162–171
Zhao D, You Y, Fan H et al (2018) The role of sugar transporter genes during early infection by root-knot nematodes. Int J Mol Sci 19:302–310
Zou J, Rodriguez-Zas S, Aldea M et al (2005) Expression profiling soybean response to Pseudomonas syringae reveals new defense-related genes and rapid HR-specific downregulation of photosynthesis. Mol Plant-Microbe Interact 18:1161–1174
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gören-Sağlam, N. (2021). Cyanobacteria as Biofertilizer and Their Effect Under Biotic Stress. In: Mohamed, H.I., El-Beltagi, H.ED.S., Abd-Elsalam, K.A. (eds) Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management. Springer, Cham. https://doi.org/10.1007/978-3-030-66587-6_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-66587-6_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-66586-9
Online ISBN: 978-3-030-66587-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)