Abstract
Dunaliella sp. (formerly D. salina MACC/C43) was batch-cultured under blue (BL), red (RL), or white light (WL) provided by light-emitting diodes (LED) and white light provided by fluorescent lamps (WF), respectively, to assess growth characteristic. Growth under blue light coupled with red light (BL + RL) was also measured and compared with that under BL, WL, and RL, respectively, in a laboratory-scale photobioreactor. Algae under monochromatic blue light had the highest specific growth rate (μ) with a value of 0.51 day−1, followed by WF (0.49 day−1), WL (0.47 day−1), and RL (0.44 day−1). Red light significantly enhanced Chl a and carotenoid contents, with values of 20.1 and 9.6 pg cell−1, respectively. BL + RL increased the μ (0.20 day−1) compared to those rates under pure BL or RL ranging from 0.17 to 0.18 day−1, in batch culture for 14 days using a photobioreactor with 3.2 L working volume. Cells absorb more photons for photosynthesis in the blue band. Mean extinction coefficient calculated based on the modified Lambert–Bill law at the blue light region (430–480 nm) was 0.885 cm−1, which is 22, 11, and 15% higher than that of the red light region (630–700 nm), green light region (500–560 nm), and photosynthetically active radiation region (400–700 nm), respectively. Blue light also improved the initial slope and the maximum O2 production rate in a photosynthesis–irradiance curve, whereas it lowered the compensation irradiance and dark respiration rate. The photochemistry of photosystem II also displayed its optimal performance under BL. LEDs are a suitable light source for Dunaliella sp. growth, and BL + RL promotes rapid division of algal cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dunaliella salina, a potential food supplement, accumulates β-carotene up to 10% of its dry weight (Ben-Amotz 1995; Borowitzka 1999; Chidambara Murthy et al. 2005; Lam and Lee 2014). Currently, all existing Dunaliella facilities, primarily located in Australia, Israel and China, adopt the outdoor open pond model, using high-salinity brine to produce algal biomass (Del Campo et al. 2007).
A two-stage cultivation process for D. salina β-carotene production has been studied (Ben-Amotz 1995). The alga is first grown in a suitable environment for the rapid growth and biomass accumulation and then the algae are transferred to stress conditions to enhance carotenogenesis, e.g., high irradiance, high salinity, high temperature, or N-deficient medium. This two-stage cultivation is possible in China, India, and Israel where the intensive raceway cultivation process is used. In stage one, a large number of algal cells are critical and necessary for the β-carotene production in the next step (Tafreshi and Shariati 2006; Lamers et al. 2010; Fu et al. 2013). However, in an open pond, where artificial regulation of temperature and light is unlikely, the density of algal cells rarely reached 1 × 106 cells mL−1 (Ben-Amotz 1995). In closed photobioreactors, cell density reach can up to 1 × 108 cells mL−1, according to reported data (Kleinegris et al. 2011; Prieto et al. 2011; Hu and Sato 2017). This means that the number of algal cells in an open pond with 200 m2 in area (whether unmixed shallow open ponds in Australia or raceway-type in India, China and Israel) can be realized through a 600-L reactor. It is very attractive to cultivate D. salina cells in closed reactors for the later stage of carotenoid accumulation, since they offer high values of both photosynthetic efficiency and productivity (Prieto et al. 2011) providing steady and controlled conditions (Tredici and Zitelli 1997; Borowitzka 1999), especially in the context of land requirement.
Several studies have been conducted to explore the feasibility of culturing Dunaliella in closed photobioreactors (Kleinegris et al. 2011; Prieto et al. 2011). There is as yet no report of commercial production of this alga using a photobioreactor, neither a tubular reactor is successfully used for the production of Haematococcus, nor a column reactor suitable for most microalgae (Johnson et al. 2018). It is probable that closed photobioreactors will come to play an important role in the production of carotenoids, either for the growth of the algae or for the β-carotene accumulation stage of Dunaliella (Xu and Harvey 2019a, b). In photobioreactors, the intensity and the quality of the artificial light are two critical factors that must be considered by the designer, especially for the autotrophic growth of microalgae. Light provided by fluorescent lamps with various spectral qualities used to be the most popular lighting source (Blanken et al. 2013). Currently, light-emitting diodes (LEDs) have become one of the most important light sources. LEDs with different light spectra, e.g., red, blue, and white, are commercially available and can be used to illuminate microalgal cultures (Chen et al. 2011; Schulze et al. 2014; Radzun et al. 2015; Molina-Miras et al. 2018). Several studies have been performed to determine the growth of microalgae in response to various selected light regimes (Walter et al. 2011; Kula et al. 2014; Schulze et al. 2014; Vadiveloo et al. 2015). Published data show that the optimal spectra for microalgal growth are highly species-dependent, mainly attributed to the difference of light absorption and conversion efficiency due to inherent properties such as the pigment profile, cellular architecture, and chloroplast arrangement (Li et al. 2013; Moheimani and Parlevliet 2013; Glemser et al. 2016). For the algae of interest, investigating the optimal spectrum composition for their growth aids in the selection of a suitable light source.
To date, little attention has been paid to the effect of different monochromatic light qualities on the growth of this alga. Thus, the objective of this study was to investigate the influence of monochromatic light, e.g., red light, blue light, or the combined blue and red light emitted by LEDs, on the photosynthesis of Dunaliella at the green stage. The light absorption characteristic of this alga for different wavelengths was also measured. We predict that our results will facilitate the construction of more efficient closed photobioreactors, yielding greater biomass accumulation of D. salina. We have carried out further experiments in the reported reactors (Li et al. 2015) to test the batch culture results and find the appropriate artificial light source.
Materials and methods
Microalgal strain and culture media
Dunaliella sp. MACC/C43 (formerly D. salina MACC/C43), from the algal culture collection at Ocean University of China (Qingdao, P.R. China) was inoculated into 250 mL Erlenmeyer flasks containing 125 mL of F/2 medium with salinity of 32 psu (Guillard and Rhyter 1962). It is worth mentioning that this strain can grow both at seawater salinity and 250 psu with optimum salinity ranging from 90 to 150 psu (Chen 2009). However, the carotenoid composition and a systematic examination of this strain are still lacking, and the previous name of this strain D. salina may be incorrect (Borowitzka and Siva 2007; Jang et al. 2009). Therefore, we adopt the name Dunaliella sp., instead of the original name, and provided the optimum salinity, aiming to avoid the potential confusion information. The cultures were maintained at 25 °C and 60 μmol photons m−2 s−1 provided by cool-white fluorescent lamps under a 14-h light/10-h dark cycle. The Erlenmeyer flasks were shaken manually four times per day to avoid sticking. Before the formal experiment, microalgae were cultured in LED white light under 80 μmol photons m−2 s−1 and continuous light for 5 days to exponential growth period. The algal cells at logarithmic growth stage were inoculated into new medium. This process was repeated for five times.
Light treatments in batch cultivation
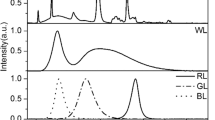
To explore the effect of various light qualities, a batch culture was established under continuous illumination of 80 μmol photons m−2 s−1 in a Precision Constant Temperature Incubator (BPH-9272, Shanghai Yiheng Ltd., Shanghai, P.R. China) at 25 °C. The illumination was provided by four artificial light sources in different spectral regions, i.e., blue LED strips (for blue light, BL), red LED strips (for red light, RL), white LED strips (for white light, WL), and fluorescent lamps (for white light, WF), respectively (Conrad Electronics, Hirschau, Germany). Light spectra were measured with a spectroradiometer (PLA-20, Everfine’s Quality Measurement Instruments, China) between 350 and 800 nm with 1-nm resolution (Fig. 1). LED light, i.e., BL, RL, and WL, was provided by the strips consisting of 80 diodes (5 rows of 16). The incident light hits the vessels from the same one side. The light intensity of each vessel is established by taking the mean of irradiance rate at three points along a vertical facade close to the vessel wall filled with water, measured using a portable light quantum meter (3415F type, pulse photoelectric sensor; Spectrum Technologies, Inc., USA). As for WF, fluorescent tubes arranged on the side of the container illuminated the container. The light intensity of each bottle is consistent with the LED group by adjusting the distance between the vessel and the tube. Five biological replicates were set for each light quality. To guarantee pure light quality, experiments under different light resources were carefully separated using compartments that were impervious to light.
Microalgal cultivation in a photobioreactor with different light qualities
A laboratory-scale flat-plate photobioreactor (PBR) with a working volume of 3.2 L (10 cm long, 8 cm wide, 50 cm high) was used for the cultivation (Li et al. 2015). The design parameters such as the ratio between downcomer and riser cross-sectional area and the ratio of height to diameter were 3:1 and 6:1, respectively. According to our previous report, the reactor used in this study has good mixing, with liquid circulation velocity 40.8 m s−1 and volumetric mass transfer coefficient 5.6 × 10−3 s−1. Samples were obtained from a port 5 cm below the liquid level for further growth or pigment analysis. The aeration was kept constant using mass flow controllers at 0.3 L min−1 and was enriched with 2.5% CO2. The algae were pre-acclimated to light intensity of 120 μmol photons m−2 s−1 for at least 20 days in LED white light as mentioned earlier. In order to investigate the effect of light quality on the growth of cells, all cultures were also maintained in batch growth mode. Growth was repeated three times for each light quality. Three biological replicates were collected every 2 days to measure the cell density. Light was provided via two inner set flexible LED strips on the transparent riser pipe of the PBR (Fig. 2). The growth of strain MACC/C43 under three light qualities, i.e., BL, RL, and RL + BL (with an intensity ratio of 1:1), was recorded at 120 μmol photons m−2 s−1, respectively. LED light sources, i.e., WL, RL, and BL, are all surface-mounted light-emitting diodes. Each LED lamp bead emits light and different light quality lamp beads have a specific light intensity. The irradiance of each WL bead measured by using a 3415F type quantum meter is 1.0 μmol photons m−2 s−1, and both RL and BL are 0.5 μmol photons m−2 s−1. The incident light intensity of microalgal culture under different light qualities was set by controlling the number of light beads for each specific color. We lit 120 white beads, 240 red beads, and 240 blue beads, respectively, to create 120 μmol photons m−2 s−1 of white, red, and blue light. Similarly, 120 red and 120 blue beads were simultaneously lit to provide BL + RL (Li et al. 2019).
Measurement of growth
The specific growth rate in the exponential growth phase (μ) was calculated based on the following equation (Moheimani et al. 2013):
where N2 (final) and N1 (initial) are cell densities (cells mL−1) determined using a hemocytometer at times T2 and T1, respectively.
Quantification of the photosynthetic pigments
Ethanol (99.5%) was used for the extraction of chlorophyll and carotenoids. Chlorophyll a (Chl a), Chl b, and total carotenoids (Car) were extracted and quantified as described by Ritchie (2008) and Jensen (1978) using a dual-beam UV–visible spectrophotometer (Hitachi, U-2900 UV/VIS, Japan) with quartz cell as follows:
where A represents the absorbance at the indicated wavelength. Algal cells on the fifth day were sampled to determine the pigment content.
Photosynthesis–irradiance (P–E) curve of microalga under different light qualities
Oxygen evolution and uptake rates were determined in an iodine number flask (100 mL, total volume) filled with an algal suspension having a cell density of about 1.0 × 105 cells mL−1. First, the microalgae cultured in LED white light as described in the “Microalgal strain and culture media” section were precultured under each light quality for 2 h. Second, the biomass was measured as described by Zhu and Lee (1997). The dissolved oxygen concentration (DO) in the alga solution was then measured using a polarographic sensor with a DO meter (Models 58 and 5100; YSI Inc., USA) every 10 min for 30 min. The net photosynthetic rate (Pnet) was expressed by the rate of variation of the dissolved oxygen concentration with time. After that, the DO concentration was recorded every 10 min for 30 min in the dark. The slope of a linear regression was determined from the data of the second 30-min measurements to estimate the respiratory rates (Rd). Photosynthetic rates were determined at 40, 60, 80, 140, 180, 220, 280, 320, and 460 μmol photons m−2 s−1 (N = 3 replicates/level) at 25 °C. Both the photosynthetic evolution and respiratory O2 uptake rate are expressed as the oxygen release rate per unit dry weight of biomass (μmol O2 g−1 h−1).
The P–E curve was fitted using an exponential equation (Jassby and Platt 1976):
where PGross is the gross O2 production rate, which is the sum of Pnet and Rd. Pm is the maximum O2 production rate and α is the initial slope. Saturation irradiance (Ek) and compensation irradiance (Ec) were calculated as Pm/α and Rd/α, respectively. The fitting was performed using the software origin 8.0 (OriginLab, USA). Curve fit was calculated with the Nonlinear Curve Fit function of this software using the least squares method comparing differences between measured and calculated data (Houlbrèque et al. 2004).
Light absorption of algal cells and chlorophyll fluorescence measurements
The average extinction coefficients at different wavebands, i.e., BL (400–480 nm), green light (GL, 500–560 nm), RL (600–650 nm), and photosynthetic active radiation (PAR, 400–700 nm), were determined as described by Li and Meng (2014). Microalgae precultured in LED white light as described in the “Microalgal strain and culture media” section were used to determine the light absorption in order to avoid the possible influence of light acclimation on light absorption. The transmittances were recorded by scanning the supernatant. The average light transmittance (T) and mean extinction coefficient (a) of algal suspension was calculated with the equation below.
where λd and λu are the lower-limit wavelength and the upper-limit wavelength for each light quality, respectively. T(λ) represents the monochromatic light transmittance of each wavelength (%). S(λ) is the relative spectral power distribution on the corresponding wavelength (W). V(λ) is the efficiency of spectral illumination of the corresponding wavelength. The values of S(λ) and V(λ) can be obtained from the information given by the International Commission on Illumination (CIE) and the National Standardization Technical Committee (2008). According to the CIE, the sampling interval to obtain values of S(λ) and V(λ) is 5 nm in the range 400–700 nm. The specific extinction coefficient of mixed light quality was expressed by the average value of each monochrome light quality.
The chlorophyll fluorescence of photosystem II (PSII) was measured using an FMS-2 pulse-amplitude modulated fluorometer (Hansatech Instruments, UK) as described by Zhang et al. (2015). Algal cells cultivated in BL, RL, WL, and FL, respectively, for 5 days in batch cultivation were sampled to determine the chlorophyll fluorescence. All measurements were performed with microalgae that had been dark-adapted for 20 min at 25 °C.
Statistical analysis
Data in the figures represent the averages of replicates ± standard error of the mean (SEM) and were subjected to one-way ANOVA and Tukey tests performed using SPSS 17.0 software (SPSS Inc., USA). Differences were considered significant at a probability level of P < 0.05.
Results
Batch cultivation of Dunaliella sp. under four light qualities
During the cultivation period of 6 days, strain MACC/C43 exhibited exponential growth under all four light qualities, and the highest cell density was observed in the BL treatment (Fig. 3a). The maximum specific growth rate μ (0.51 ± 0.008 day−1) was also observed under the BL conditions, followed by WF (0.49 ± 0.001 day−1), WL (0.47 ± 0.002 day−1), and RL (0.44 ± 0.014 day−1) (Fig. 3b). RL significantly increased the pigment content, both in the unit volume of algal suspension and in the single cell (Fig. 3c, d). All pigments, i.e., Chl a, Chl b, and total carotenoids (Car) displayed no significant difference between the BL and WF treatments.
The growth curve (a), maximum specific growth rate (b), pigment content per cell (c), and volumetric pigment concentration (d) of Dunaliella sp. MACC/C43 batch-cultivated under blue light (BL), red light (RL), white LED light (WL), and white fluorescent light (WF), respectively. Values are the averages of five biological replicates ± SEM. Car represents total carotenoids. Data of each parameter with different letters (i.e., a, b, c, and d in graph b and a, b, c, ab and bc in graph c and d) are significantly different in each index group (P < 0.05)
P–E curve parameters of Dunaliella sp. under different light qualities
Values of the gross photosynthetic rate increased steeply with increased light intensity under relatively low light conditions, and then basically remained constant under higher light conditions (Fig. 4). The parameters under the four light qualities were determined to be 1.99–2.52 μmol O2 g−1 h−1 for the Pm, 0.016–0.023 μmol O2 g−1 h−1 for α, 0.55–1.68 μmol O2 g−1 h−1 (μmol photons m−2 s−1)−1 for the Rd of the model, and 108.2–122.3 μmol photons m−2 s−1 for Ek. From these parameters, the Ec and the maximum Pnet were calculated to be 23.6–103.4 μmol photons m−2 s−1 and 0.31–1.97 μmol O2 g−1 h−1, respectively. The Pm and the maximum Pnet were both the highest in BL (2.52 and 1.97 μmol O2 g−1 h−1, respectively) and the lowest in RL (1.99 and 0.31 μmol O2 g−1 h−1, respectively). In contrast, α, Ek, and Ec were the lowest under BL and the highest under RL (Table 1).
Light absorption of Dunaliella sp. in different spectral regions
Light is absorbed by D. salina predominantly through Chl a, Chl b, and Car (Fig. 5a). The mean extinction coefficient in the BL region was higher than those of the RL, GL, and PAR regions (Fig. 5b).
Effect of light quality on PSII photochemistry
Data in Fig. 6 shows that (i) the maximum fluorescence yield (Fm) under RL and WL was significantly higher than those of BL and WF; (ii) the maximum quantum yield of PSII photochemistry (Fv/Fm) maintained the highest value under BL, followed by WF, RL, and WL; (iii) the effective quantum yield of PSII (ΦPSII) under both BL and WF was slightly higher than that of RL and WL, although no significant difference was observed between all the treatments; (iv) the photochemical quenching coefficient (qP) showed a pattern similar to that of Fv/Fm, with the highest values found under BL and WF; (v) nonphotochemical quenching (NPQ) under RL and WF light conditions was markedly higher than that under WL, and BL exposure gave the lowest level of all.
The effect of light quality on the maximal fluorescence yield of the dark-adapted state (Fm), maximal quantum yield of PSII photochemistry (Fv/Fm), the effective quantum yield of PSII (ΦPSII), photochemical quenching coefficient (qP), and nonphotochemical quenching (NPQ) of Dunaliella sp. MACC/C43. Values are the averages of six biological replicates ± SEM. Data with different letters (i.e., a, b, and c) are significantly different (P < 0.05). BL, RL, WL, and WF represent blue light, red light, white LED light and white fluorescent light, respectively
Growth response of Dunaliella sp. to light quality in PBR
To further optimize the artificial light source, strain MACC/C43 was cultivated in a PBR with four LED light qualities at the same intensity (120 μmol photons m−2 s−1). As demonstrated in Fig. 7, for growth of algal cells under BL, BL + L, WL, and RL, cell populations increased in all the treatments, with the highest value (4.00 × 107 cells mL−1) observed under combined BL + RL light conditions. For monochromatic light, BL was more suitable for algal growth than RL, which was consistent with the results for batch cultivation in flasks (Fig. 3a). The growth of the algae under WL was slightly faster than that under RL, but significantly lower than those of BL and BL + RL.
Cell density of Dunaliella sp. MACC/C43 in a flat-plate photobioreactor for four light qualities, respectively. BL, RL, and WL represent blue light, red light, and white LED light, respectively. BL + RL represents a mixture of BL and RL at 1:1 light intensity. Data with different letters (i.e., a, b, c, and d) are significantly different (P < 0.05)
Discussion
In the design and operation of a microalgal cultivation system, the use of an appropriate light source is a key factor that requires consideration. The optimal wavelength varies from species to species. For example, Nannochloropsis sp. and Haematococcus pluvialis display the highest biomass yield under red light (Katsuda et al. 2004; Vadiveloo et al. 2017). However, blue light favors the growth and photosynthetic characteristics of D. salina, Isochrysis galbana, Chaetoceros gracilis, and Heterocapsa circularisquama (Gorai et al. 2014) and astaxanthin production in H. pluvialis (Katsuda et al. 2006). Our results also indicate that light quality significantly regulates the growth of Dunaliella, in both batch and closed PBR cultivation.
BL was a promising monochromatic illumination for cell growth in the indoor cultivation of strain MACC/C43, while illumination with RL increased the level of both Chl a and the total carotenoids (Fig. 3). Helena et al. (2016) reported that the growth of D. salina was regulated by LED quality, and the maximum cell densities under BL using the Walne medium and the ZA medium were both higher than those that were illuminated with RL. The current study further indicated that the highest specific growth rate of strain MACC/C43 was obtained using RL + BL together in a laboratory-scale PBR (Fig. 4). Wagner et al. (2016) found that the pigment concentration (Chl a, Chl b, and Car) of Chlamydomonas reinhardtii exposed to a dichromatic illumination of red and blue light at 25 μmol photons m−2 s−1 decreased with the decrease of red light proportion (from 80 to 50%). We can speculate that the enhanced growth of strain MACC/C43 exposed to combined LED light with blue and red wavelengths could be attributed primarily to the high growth rate under BL and higher pigment synthesis under RL. Similarly, Fu et al. (2013) found that combining RL (75%, peak at 470 nm) with BL (25%, peak at 660 nm) with a total incident photon flux of 170 μmol photons m−2 s−1 resulted in a higher growth rate of D. salina compared to the values under RL at the same irradiance.
In green algae, the antenna pigment molecules (e.g., Car and chlorophyll) absorb light in different spectral regions (chlorophyll λ = 450–475 nm and λ = 630–675 nm and Car λ = 400–550 nm) (Jahns and Holzwarth 2012). Chl a acts as a reaction center in the photosystems, though it is also a part of the antenna complex. Carotenoids primarily harvest blue light and transfer energy to the photosystem reaction centers (Gorai et al. 2014; Nisar et al. 2015). When light energy passes through the culture medium and reaches a microalgal cell, the light absorption efficiency of the cell and the spectral distribution of the incident light determine the available light energy for photosynthesis (Dubinsky et al. 1986). In the cells, pigments are contained in chloroplasts within an intricate molecular environment. The constituents of the cells such as proteins, lipids, pigments, and carbohydrates have their own optical properties that reduce the energy of photons reaching the PSII reaction center (Johnsen and Sakshaug 2007). The ratio of light energy absorbed by pigments to that absorbed by Dunaliella cells is unclear, and it is an interesting subject that needs to be investigated further. In this study, the higher growth rate under BL compared to that under WF or WL suggests that more blue photons were absorbed by the cells, which might play a dominant role in driving photosynthesis, although the same quantum flux density was supplied at the blue, red, green, and PAR ranges (Fig. 6). The photosynthetic activity of Dunaliella sp. MACC/C43 exposed to blue light was also enhanced (Table 1, Fig. 6). The highest Pm was observed in BL grown cells, as already reported for some other marine microalgae (Marchetti et al. 2013; Gorai et al. 2014), indicating that the cells expressed higher photosynthetic activity at saturating light intensities than when grown under RL, WL, and WF. The increase in activity can be attributed to an enhanced electron transfer supported by a higher Fv/Fm or qP under BL, as shown in Fig. 7. It is worth noting that our results did not exclude the possibility that blue light enhanced the activity of ribulose-1,5-diphosphate carboxylase/oxygenase (Rubisco), thereby accelerating algal growth. It has been found that blue light increased the synthesis of Rubisco in higher plants and microorganisms, including green algae (Ruyters et al. 1984; Roscher and Zetsche 1986; Eskins et al. 2010). The effective band ranged from 430 to 510 nm, and the maximal effect was at 460 nm. The BL adopted in our study has a spectrum of 400 to 500 nm with a peak of 450 nm (Fig. 1). This question merits further investigation in the future.
Moreover, previous studies have also reported that blue light increased the α in some green or golden microalgae, such as Scenedesmus obliquus (Brinkmann and Senger 1978), Prorocentrum mariae lebouriae (Vogel and Sager 1985), Dunaliella tertiolecta and Cyclotella nana (Wallen and Geen 1971), and Isochrysis sp. (Marchetti et al. 2013). Our finding was consistent with these results. In the model, α is the initial slope of line at low PAR values created by relating PGross to E, which provides a measure of quantum efficiency (Schreiber 2004). Higher quantum efficiency under BL was also supported by the improved Fv/Fm coupled with decreased Fm (Fig. 7) and pigment contents (Fig. 3). Ek is a measure of the point where the light saturation dominates, also known as the minimum saturation level. This parameter was maintained almost unchanged under four light qualities, with the values ranging from 108.2 to 122.3 μmol photons m−2 s−1 (Table 1). Usually, light intensity higher than Ek causes photoinhibition in plants and, thus, results in the decrease of Fv/Fm (Marchetti et al. 2013). The intensity of incident light in our study (80 μmol photons m−2 s−1) was not high enough to induce photoinhibition in the algal cells. Variations in the growth of the cells under different light conditions were primarily caused by light quality. In the literature, the Rd of some microalgae, e.g., S. obliquus (Brinkmann and Senger 1978), Rhodomonas salina (Hammer et al. 2002), and D. tertiolecta (Rivkin 1989), was enhanced by blue light. Our current result was inconsistent with these reports, in that the highest Rd was obtained under RL, with the value of 1.68 μmol O2 g−1 h−1. The Rd calculated in this study includes dark respiration and photorespiration. In view of the former, one is not directly related to light, and it is highly likely that the photorespiration enhanced by RL affects the compensation point of alga, as presumed by Zhang et al. (1997). A higher Rd confirms a higher rate of carbohydrate degradation in RL; consequently, the growth rate is depressed. The compensation irradiance Ec was computed from the ratio of Rd to α. The highest light compensation point was found for RL, and the lowest was found for BL. The changes of Ec under different light qualities could be primarily attributed to changes in the Rd.
Consistent with the previous argument, the light intensity adopted in this study was far lower than the Ek of the plant, indicating that no photoinhibition occurred. Interestingly, significant changes in the chlorophyll a fluorescence parameter were observed under low light intensity (Fig. 7). The fluorescence Fm reflects the total amount of light absorbed by all the pigments in the algal cells (Matorin et al. 2004). The highest and lowest values of Fm were recorded under RL and BL conditions, respectively, which is consistent with the pigment contents, as shown in Fig. 3c. The higher values of Fv/Fm, qP, and ΦPSII under BL suggested that both the photosynthetic activity and the electron transfer efficiency were improved by blue light. In contrast, RL had a significant negative effect on photosynthesis in Dunaliella sp. This negative effect of monochromatic red light has also been reported in higher plants, e.g., cucumber (Wang et al. 2009; Savvides et al. 2012), Cordyline australis, Ficus benjamina, and Sinningia speciosa (Zheng and Van Labeke 2017), despite the fact that red light coincides with the absorbance peak of chlorophyll and is known for its higher relative quantum efficiency than blue light in the instantaneous photosynthetic response (Mccree 1971). Generally, the excess light energy is quenched by photochemical reactions, nonphotochemical reactions, and thermal dissipation. The nonphotochemical quenching of excitation energy, which protects higher plant photosynthetic machinery from photo damage, is triggered by acidification of the thylakoid lumen as a result of light-induced proton pumping, which also drives the synthesis of adenosine triphosphate (Goss and Lepetit 2015). In this study, in addition to the observation of the changes in the photochemical reaction under light-limiting treatment, we also found a significant change in NPQ. The higher total carotenoid content of microalga recorded under RL when compared to BL and WL was in accordance with many previous studies (Xu and Harvey 2019a, b; Han et al. 2019). It also be reported that the zeaxanthin content of alga exposed to RL was higher than that of BL (Xu and Harvey 2019b). The NPQ mechanism is controlled by the trans-thylakoid membrane pH gradient and the special xanthophyll cycle pigments, i.e., zeaxanthin, antheraxanthin, and violaxanthin (Gilmore 1997; Goss and Jakob 2010). The increase of NPQ under red light is probably caused by the enhancement of the xanthophyll cycle components’ pool size. The actual mechanism behind this observation is beyond the scope of this study and would need to be investigated in future studies.
Recently, some studies tried to combine different LEDs with different absorption wavelengths to enhance the growth of plants, since LEDs with a wide variety of emission wavelengths are now available (Fu et al. 2013; Wagner et al. 2016; Hu and Sato 2017; Bian et al. 2018). In the batch cultivation of this study, the results clearly reflected that monochromatic RL increased the pigment contents. Moreover, BL was advantageous to the growth of Dunaliella sp. These results encouraged us to explore the growth response of Dunaliella sp. when these two monochromatic lights were provided simultaneously. The highest growth rate was achieved under dichromatic illumination (RL + BL) in a laboratory-scale PBR (Fig. 4), which could be attributed to increases in the light absorption efficiency or PSII efficiency in BL and pigment contents in RL. It was also reported that green light had positive effects on increasing the productivity of higher plants (Johkan et al. 2012; Wang et al. 2013; Bian et al. 2018). The growth rate under WL was far lower than that of RL + BL. We conclude that the green spectrum in WL has a negative effect on algal growth compared with monochromatic BL or RL, although more experiments are needed to examine this negative impact.
The cultivation employing a two-stage technology is more productive than batch and semicontinuous cultures, both in the tubular reactor and in the open tank, confirming that the two-stage cultures is the best approach for carotenoid production by Dunaliella (Prieto et al. 2011). The cell density of alga initially cultivated in stage one was 0.8 × 106 cells mL−1 (Ben-Amotz 1995). In a two-stage culture carried out by Tafreshi and Shariati (2006) under the climatic conditions of Iran in open ponds, the highest density of algal cells in the first stage was about 4.0 × 106 cells mL−1. Meanwhile, the number of algal cells obtained in the reactor was 10 and 50 times of that in open pond obtained by Tafreshi and Shariati (2006) and Ben-Amotz (1995), respectively, with a biomass of 1.48 g L−1 (data not shown). This increase cannot be attributed solely to the differences in salinity, but to more steady manipulation of environmental factors, e.g., reduced light path, longer light duration, and continuous CO2 supply (especially in the flat-plate PBR) (Borowitzka 1999; Tredici and Zitelli 1997). Our biomass result was lower than that of Prieto et al. (2011) obtained with the closed tubular PBR (2.2 g L−1, also in F/2 medium), which may be attributed to the difference of light path. Many PBR types have been proposed (Tredici 2004), a proper efficiency and economy comparison still awaited for data from their simultaneous operation. It is gratifying to note that several recent studies have confirmed that red light can promote the accumulation of β-carotene in D. salina. For example, Han et al. (2019) proposed a lighting strategy named wavelength-shifting system (B-R system), that is, BL was first used to illuminate D. salina for 6 days (early stage), and then, the illuminating light was shifted to RL. Their results indicate that cells in the early stage showed enhanced density; however, D. salina under the B-R system showed enhanced density and β-carotene productivity compared to those under single LED light wavelengths (BL, WL, and RL). Xu and Harvey (2019a, b) found that red photon flux intensity controls the partitioning of electrons either for carotenoid biosynthesis or for photosynthesis, via energy absorption by chlorophyll and the plastoquinone pool, and further controls phytochrome regulation of the production of gene transcripts for phytoene synthase and β-carotene isomerases. In this study, the LR + LB illumination strategy was proposed for faster cell growth. It can be speculated that LR irradiation in the later period is a method worthy of further study to enhance the accumulation of carotenoids.
Based on the above results, we have drawn a model to summarize the findings of this study (Fig. 8). It should be noted that the results of this study are based on batch culture study and not as a semicontinuous or continuous one. Therefore, extrapolation of our results must be done with caution, and we acknowledge that studies on a chemostat cultivation are needed to verify our hypotheses, as adopted by Vadiveloo et al. (2015, 2016, 2017). Furthermore, the objective of this study was to identify the effect of light quality on the growth of Dunaliella sp. in a batch culture. The cultures that were maintained with various light spectra were not preacclimated to each light. However, our results can reflect the algal growth at different light qualities, which is still significant, whether light acclimation is completed or not (Rivkin 1989; Sánchez-Saavedra et al. 2016).
In conclusion, the photosynthesis of Dunaliella MACC/C43 was regulated by LED light quality. The mean extinction coefficient of this alga in the blue region was higher than those in the red, PAR, and green regions. Growth rate and photosynthesis both increased under BL, with enhanced Fv/Fm and qP values. Enhancement in the initial slope α and Pm and decreases in Ec and Rd, estimated from the P–E curve, were also observed. In contrast, RL-illuminated algae displayed a lower growth rate with a higher pigment content, compared with those under BL. Batch cultivation conducted in a PBR with different LED lights provided additional evidence that dichromatic illumination RL + BL was more suitable than monochromatic light, either RL or BL.
References
Ben-Amotz A (1995) New mode of Dunaliella biotechnology: two-phase growth for β-carotene production. J Appl Phycol 7:65–68
Bian Z, Yang Q, Li T, Cheng R, Barnett Y, Lu C (2018) Study of the beneficial effects of green light on lettuce grown under short-term continuous red and blue light-emitting diodes. Physiol Plant 164:226–240
Blanken W, Cuaresma M, Wijffels René H, Janssen M (2013) Cultivation of microalgae on artificial light comes at a cost. Algal Res 2:333–340
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Borowitzka MA, Siva CJ (2007) The taxonomy of the genus Dunaliella (Chlorophyta, Dunaliellales) with emphasis on the marine and halophilic species. J Appl Phycol 19:567–590
Brinkmann G, Senger H (1978) The development of structure and function in chloroplasts of greening mutants of Scenedesmus IV. Blue light-dependent carbohydrate and protein metabolism. Plant Cell Physiol 19:1427–1437
Chen SX (2009) Studies on selective dominance strains of Dunaliella and Haematococcus pluvialis. MSc thesis, Ocean University of China, China 34 pp. (In Chinese)
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102:71–81
Chidambara Murthy KN, Vanitha A, Rajesha J, Mahadeva Swamy M, Sowmya PR, Ravishankar GA (2005) In vivo antioxidant activity of carotenoids from Dunaliella salina—a green microalga. Life Sci 76:1381–1390
Del Campo JA, García-González M, Guerrero MG (2007) Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotechnol 74:1163–1174
Dubinsky Z, Falkowski PG, Wyman K (1986) Light harvesting and utilization by phytoplankton. Plant Cell Physiol 27:1335–1349
Eskins K, Cai ZJ, Shibles R (2010) Light-quality and irradiance effects on pigments, light-harvesting proteins and Rubisco activity in a chlorophyll- and light-harvesting-deficient soybean mutant. Physiol Plant 83:47–53
Fu WQ, Guðmundsson Ó, Paglia G, Herjólfsson G, Andrésson ÓS, Palsson BØ, Brynjólfsson S (2013) Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Appl Microbiol Biotechnol 97:2395–2403
Gilmore AM (1997) Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol Plant 99:197–209
Glemser M, Heining M, Schmidt J, Becker A, Garbe D, Buchholz R, Brück T (2016) Application of light-emitting diodes (LEDs) in cultivation of phototrophic microalgae: current state and perspectives. Appl Microbiol Biotechnol 100:1077–1088
Gorai T, Katayama T, Obata M, Murata A, Taguchi S (2014) Low blue light enhances growth rate, light absorption, and photosynthetic characteristics of four marine phytoplankton species. J Exp Mar Biol Ecol 459:87–95
Goss R, Jakob T (2010) Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth Res 106:103–122
Goss R, Lepetit B (2015) Biodiversity of NPQ. J Plant Physiol 172:13–32
Guillard RLL, Rhyter JH (1962) Studies on marine planktonic diatoms I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Hammer A, Schumann R, Schubert H (2002) Light and temperature acclimation of Rhodomonas salina (Cryptophyceae): photosynthetic performance. Aquat Microb Ecol 29:287–296
Han SI, Kim S, Lee C, Choi YE (2019) Blue-red LED wavelength shifting strategy for enhancing beta-carotene production from halotolerant microalga, Dunaliella salina. J Microbiol 57:101–106
Helena S, Zainuri M, Suprijanto J (2016) Microalgae Dunaliella salina (Teodoresco, 1905) growth using the LED light (light limiting diode) and different media. Aquatic Procedia 7:226–230
Houlbrèque F, Tambutté E, Allemand D, Ferrier-Pagès C (2004) Interactions between zooplankton feeding, photosynthesis and skeletal growth in the scleractinian coral Stylophora pistillata. J Exp Biol 207:1461–1469
Hu JY, Sato T (2017) A photobioreactor for microalgae cultivation with internal illumination considering flashing light effect and optimized light-source arrangement. Energy Convers Manag 133:558–565
Jahns P, Holzwarth AR (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 1817:182–193
Jang W, Qiao WW, Li Y (2009) Analysis of 5.8S rDNA-ITS sequences and RAPD of seven Dunaliella sp. J Ocean Univ China 39:363–368 (In Chinese)
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Jensen A (1978) Chlorophylls and carotenoids. In: Hellebust J, Craigie JS (eds) Handbook of phycological methods: physiological and biochemical methods. Cambridge University Press, Cambridge, pp 61–64
Johkan M, Shoji K, Goto F, Hahida S, Yoshihara T (2012) Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ Exp Bot 75:128–133
Johnsen G, Sakshaug E (2007) Biooptical characteristics of PSII and PSI in 33 species (13 pigment groups) of marine phytoplankton, and the relevance for pulse-amplitude-modulated and fast-repetition-rate fluorometry. J Phycol 43:1236–1251
Johnson TJ, Katuwal S, Anderson GA, Gu L, Zhou R, Gibbons WR (2018) Photobioreactor cultivation strategies for microalgae and cyanobacteria. Biotechnol Prog 34:811–827
Katsuda T, Lababpour A, Shimahara K, Katoh S (2004) Astaxanthin production by Haematococcus pluvialis under illumination with LEDs. Enzyme Microb Tech 35:81–86
Katsuda T, Shimahara K, Shiraishi H, Yamagami K, Ranjbar R, Katoh S (2006) Effect of flashing light from blue light emitting diodes on cell growth and astaxanthin production of Haematococcus pluvialis. J Biosci Bioeng 102:442–446
Kleinegris DMM, Janssen M, Brandenburg WA, Wijffels RH (2011) Continuous production of carotenoids from Dunaliella salina. Enzyme Microb Tech 48:253–259
Kula M, Rys M, Mozdzeń K, Skoczowski A (2014) Metabolic activity, the chemical composition of biomass and photosynthetic activity of Chlorella vulgaris under different light spectra in photobioreactors. Eng Life Sci 14:57–67
Lam MK, Lee KT (2014) Scale-up and commercialization of algal cultivation and biofuel production. In: Pandey A, Lee DJ, Chisti Y, Soccol CR (eds) Biofuels from algae. Elsevier, Amsterdam, pp 261–286
Lamers PP, Van de Laak CC, Kaasenbrood PS, Lorier J, Janssen M, De Vos RC, Bino RJ, Wijffels RH (2010) Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol Bioeng 106:638–648
Li YF, Meng FP (2014) Type selection of transparent plate for microalgae photo-bioreactor building based on light transmissivity analysis. Acta Opt Sin 1:0123003–0123006
Li YF, Meng FP, Li XL, Ma DD (2013) Effect of illumination on microalgae cultured at high cell density in photo-bioreactor. China Biotechnol 33:103–110 (In Chinese)
Li YF, Meng FP, Wang YJ (2015) Design parameters optimization of internal loop airlift flat plate photobioreactor for Chlorella vulgaris carbon fixation. Chin J Env Eng 9:653–658 (In Chinese)
Li YF, Li RQ, Yi XY (2019) Effects of light quality on growth rates and pigments of Chaetoceros gracilis (Bacillariophyceae). J Oceanol Limnol 1–7. https://doi.org/10.1007/s00343-019-9171-0
Marchetti J, Bougaran G, Jauffrais T, Lefebvre S, Rouxel C, Saint-Jean B, Lukomska E, Robert R, Cadoret JP (2013) Effects of blue light on the biochemical composition and photosynthetic activity of Isochrysis sp. (T-iso). J Appl Phycol 25:109–119
Matorin DN, Antal TK, Ostrowska M, Rubin AB, Ficek D, Majchrowski R (2004) Chlorophyll fluorimetry as a method for studying light absorption by photosynthetic pigments in marine algae. Oceanologia 46:519–531
McCree KJ (1971) The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric Meteorol 9:191–216
Moheimani NR, Parlevliet D (2013) Sustainable solar energy conversion to chemical and electrical energy. Renew Sust Energ Rev 27:494–504
Moheimani NR, Borowitzka MA, Isdepsky A, Fon Sing S (2013) Standard methods for measuring growth of algae and their composition. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 265–284
Molina-Miras A, López-Rosales L, Sánchez-Mirón A, Cerón-García MC, Seoane-Parra S, García-Camacho F, Molina-Grim E (2018) Long-term culture of the marine dinoflagellate microalga Amphidinium carterae in an indoor LED-lighted raceway photobioreactor: production of carotenoids and fatty acids. Bioresour Technol 265:257–267
National Standardization Technical Committee (2008) Standard illuminants and geometric conditions GB/T 3978-2008. China Standard Press, Beijing
Nisar N, Li L, Lu S, Khin N, Pogson B (2015) Carotenoid metabolism in plants. Mol Plant 8:68–82
Prieto A, Cañavate JP, García-González M (2011) Assessment of carotenoid production by Dunaliella salina in different culture systems and operation regimes. J Biotechnol 151:180–185
Radzun KA, Wolf J, Jakob G, Zhang E, Stephens E, Ross I, Hankamer B (2015) Automated nutrient screening system enables high-throughput optimisation of microalgae production conditions. Biotechnol Biofuels 8:65
Ritchie RJ (2008) Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 46:115–126
Rivkin RB (1989) Influence of irradiance and spectral quality on the carbon metabolism of phytoplankton. I. Photosynthesis, chemical composition and growth. Mar Ecol Prog Ser 55:291–304
Roscher E, Zetsche K (1986) The effects of light quality and intensity on the synthesis of ribulose-1,5-bisphosphate carboxylase and its mRNAs in the green alga Chlorogonium elongatum. Planta 167:582–586
Ruyters G, Hirosawa T, Miyachi S (1984) Blue light effects on carbon metabolism in Dunaliella. In: Senger H (ed) Blue light effects in biological systems. Springer, Berlin, pp 317–322
Sánchez-Saavedra MP, Maeda-Martínez AN, Acosta-Galindo S (2016) Effect of different light spectra on the growth and biochemical composition of Tisochrysis lutea. J Appl Phycol 28:839–847
Savvides A, Fanourakis D, Van Ieperen W (2012) Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J Exp Bot 63:1135–1143
Schreiber U (2004) Pulse-amplitude-modulation (PAM) Fluorometry and saturation pulse method: an overview. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence. Advances in photosynthesis and respiration. Springer, Dordrecht, pp 279–319
Schulze PS, Barreira LA, Pereira HG, Perales JA, Varela JC (2014) Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol 32:422–430
Tafreshi AH, Shariati M (2006) Pilot culture of three strains of Dunaliella salina for β-carotene production in open ponds in the central region of Iran. World J Microbiol Biotechnol 22:1003–1006
Tredici MR (2004) Mass production of microalgae: photobioreactors. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell, Oxford, pp 178–214
Tredici MR, Zitelli GC (1997) Cultivation of Spirulina (Arthrospira) platensis in flat plate reactor. In: Vonshak A (ed) Spirulina platensis (Arthrospira): physiology, cell-biology and biotechnology. Taylor and Francis, London, pp 117–130
Vadiveloo A, Moheimani NR, Cosgrove JJ, Bahri PA, Parlevliet D (2015) Effect of different light spectra on the growth and productivity of acclimated Nannochloropsis sp. (Eustigmatophyceae). Algal Res 8:121–127
Vadiveloo A, Moheimani NR, Kosterink NR, Cosgrove JJ, Parlevliet D, Gonzalez-Garcia C, Lubián LM (2016) Photosynthetic performance of two Nannochloropsis spp. under different filtered light spectra. Algal Res 19:168–177
Vadiveloo A, Moheimani NR, Cosgrove JJ, Parlevliet D, Bahri PA (2017) Effects of different light spectra on the growth, productivity and photosynthesis of two acclimated strains of Nannochloropsis sp. J Appl Phycol 29:1765–1774
Vogel H, Sager JC (1985) Photosynthetic response of Prorocentrum mariae-lebouriae (Dinophyceae) to different spectral qualities, irradiances, and temperatures. Hydrobiologia 128:143–153
Wagner I, Steinweg C, Posten C (2016) Mono- and dichromatic LED illumination leads to enhanced growth and energy conversion for high-efficiency cultivation of microalgae for application in space. Biotechnol J 11:1060–1071
Wallen DG, Geen GH (1971) Light quality in relation to growth, photosynthetic rates and carbon metabolism in two species of marine plankton algae. Mar Biol 10:34–43
Walter A, de Carvalho JC, Soccol VT, de Faria ABB, Ghiggi V, Soccol CR (2011) Study of phycocyanin production from Spirulina platensis under different light spectra. Braz Arch Biol Technol 54:675–682
Wang H, Gu M, Cui J, Shi K, Zhou Y, Yu J (2009) Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J Photochem Photobiol B 96:30–37
Wang Y, Maruhnich SA, Mageroy MH, Justice JR, Folta KM (2013) Phototropin 1 and cryptochrome action in response to green light in combination with other wavelengths. Planta 237:225–237
Xu Y, Harvey PJ (2019a) Red light control of β-carotene isomerisation to 9-cis β-carotene and carotenoid accumulation in Dunaliella salina. Antioxidants 8:148
Xu Y, Harvey PJ (2019b) Carotenoid production by Dunaliella salina under red light. Antioxidants 8:123
Zhang X, Brammer E, Pedersén M, Fei X (1997) Effects of light photon flux density and spectral quality on photosynthesis and respiration in Porphyra yezoensis (Bangiales, Rhodophyta). Phycol Res 45:29–37
Zhang LT, He ML, Liu JG, Li L (2015) Role of the mitochondrial alternative oxidase pathway in hydrogen photoproduction in Chlorella protothecoides. Planta 241:1005–1014
Zheng L, Van Labeke MC (2017) Long-term effects of red- and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front Plant Sci 8:1–12
Zhu CJ, Lee YK (1997) Determination of biomass dry weight of marine microalgae. J Appl Phycol 9:189–194
Acknowledgments
The authors would like to thank Dr. Fanping Meng of the Ocean University of China for providing LED light sources. We also thank Dr. Xian Li, CAS Key Laboratory of Experimental Marine Biology, Chinese Academy of Sciences, Institute of Oceanology, for her kind help in measuring the light spectra. The authors also would like to thank Dr. John van der Meer of the Pan-American Marine Biotechnology Association for his assistance with proofreading.
Funding
This work was financially supported by the National Natural Science Foundation of China (grant no. U1706209), the Pilot National Laboratory for Marine Science and Technology (Qingdao) (grant no. MS2019NO01), and the Shandong Provincial Natural Science Foundation, China (grant no. ZR2017QC008).
Author information
Authors and Affiliations
Contributions
YL and JL designed the experiments and wrote the manuscript; YL and LL performed the experiments. YL and RQ analyzed the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Li, L., Liu, J. et al. Light absorption and growth response of Dunaliella under different light qualities. J Appl Phycol 32, 1041–1052 (2020). https://doi.org/10.1007/s10811-020-02057-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02057-9