Abstract
The kelp Laminaria digitata (Hudson) J.V. Lamouroux (Laminariales, Phaeophyceae) is currently cultivated on a small-scale in several north Atlantic countries, with much potential for expansion. The initial stages of kelp cultivation follow one of two methods: either maximising (gametophyte method) or minimising (direct method) the vegetative growth phase prior to gametogenesis. The gametophyte method is of increasing interest because of its utility in strain selection programmes. In spite of this, there are no studies of L. digitata gametophyte growth and reproductive capacity under commercially relevant conditions. Vegetative growth measured by length and biomass, and rate of gametogenesis, was examined in a series of experiments. A two-way fixed-effects model was used to examine the effects of both photoperiod (8:12; 12:12; 16:8, 24:0 L:D) and commonly used/commercially available growth media (f/2; Algoflash; Provasoli Enriched Seawater) on the aforementioned parameters. All media resulted in good performance of gametophytes under conditions favouring vegetative growth, while f/2 clearly resulted in better gametophyte performance and a faster rate of gametogenesis under conditions stimulating transition to fertility. Particularly, the extent of sporophyte production (% of gametophytes that produced sporophytes) at the end of the experiment showed clear differences between treatments in favour of f/2: f/2 = 30%; Algoflash = 9%; Provasoli Enriched Seawater = 2%. The effect of photoperiod was ambiguous, with evidence to suggest that the benefit of continuous illumination is less than expected. Confirmation of photoperiodic effect is necessary, using biomass as a measure of productivity and taking greater account of effects of genotypic variability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many maritime regions, there is increasing interest in developing intensive sea cultivation of macroalgae with the intention of supplying raw material to a number of industries. Potential industrial uses are currently expanding in scope and range from the more traditional, for example phycocolloid production, to newly emerging sectors such as bioactive molecule extraction for nutraceuticals or pharmaceuticals (Holdt and Kraan 2011), or supply of third-generation biofuel feedstock (Kerrison et al. 2015). Cultivation aims to ensure that the supply of raw material is achieved without negatively impacting on natural populations or their associated biodiversity and ecosystem functions. In Europe, much of the focus has centred on a variety of kelp species (Laminariales), of which Laminaria digitata (Hudson) J.V. Lamouroux is a prominent member both in terms of its ecological dominance as a foundation species, and its potential for exploitation, such as harvesting for alginate production as occurs in France (Davoult et al. 2011).
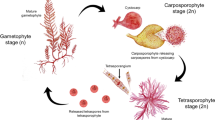
The Laminariales have a heteromorphic life cycle alternating between microscopic gametophytes and conspicuously large sporophytes, both stages requiring management and optimisation in order to maximise production capacity. Cultivation techniques follow one of two methods: either direct release of zoospores onto a culture substrate allowing subsequent development of the gametophyte and gametogenesis to occur unimpeded (the direct method), or release of zoospores into culture vessels and manipulation of conditions to maintain the gametophytes in a vegetative state whereby biomass accumulation can take place prior to induction of fertility (the gametophyte method) (Edwards et al. 2016).
Each method has its own advantages but use of the gametophyte method to cultivate kelp is becoming an increasingly common experimental alternative to the direct release method (Wu et al. 2004; Westermeier et al. 2006; Zhang et al. 2008; Xu et al. 2009). It affords control over the timing of the nursery and sea-transfer stages, freeing farmers from the restrictions of the natural reproductive cycle, while it also allows easy stock maintenance of successful crosses in strain selection programs (Li et al. 1999). For culture purposes, these phases of microscopic growth consisting of the release of zoospores, growth of gametophytes and gametogenesis, are normally carried out in the laboratory. The processes are labour and time intensive, hence the need to improve their efficiency.
Development of the microstages of L. digitata is typical of Laminariales gametophyte development in general and has been described by Kain (1979). They are low light adapted and a photon flux density of ~10 μmol photons m−2 s−1 is sufficient for maintenance of growth. However, they are sensitive to the spectral composition of light, and in order to maintain them in the filamentous vegetative state where biomass increase can take place, growth in predominantly red light is necessary, the omission of violet/blue light (380–500 nm) effectively inhibiting fertility (Saccharina latissima, Laminaria digitata, Laminaria hyperborea, Saccharina japonica, Undaria pinnatifida—Lüning and Dring 1975; L. digitata—Cosson and Gayral 1979; S. latissima—Zhi and Rorrer 1996; Undaria pinnatifida—Xu et al. 2005). Once sufficient biomass has developed, initiation of the development of reproductive structures of gametophytes can be triggered. Altering the light quality to include blue light (main peak of action spectrum 434–452 nm) acts to induce fertility (Lüning and Dring 1975).
Specific nutrient requirements for growth have not been elucidated and reports in the literature are inconsistent due to the many interacting variables. In general, cultivation media provide an excess of the major limiting nutrients nitrogen and phosphorus, alongside trace elements, vitamins and chelating agents, in combinations that are able to sustain maximum growth rates. Effects of nitrogen and phosphorus have been demonstrated such that a change in concentration of one nutrient affects the response of the gametophyte (Lessonia nigrescens) to the other (Hoffmann et al. 1984). Furthermore, the form of nitrogen may affect uptake rate, and preferential uptake of ammonia over nitrate has been demonstrated in some kelps (review: Bartsch et al. 2008).
The existence of the vegetative form of gametophytes has been postulated to be an adaptive response to stressful environments, with transition to fertility triggered when a favourable environment is encountered (Lüning 1980). This is modulated by several factors (day length, irradiance, nutrient status, temperature) and the interactions between them, so that there are no simple thresholds above or below which gametogenesis does or does not occur. In one case, it was found that nitrogen and phosphorus concentrations optimal for vegetative gametophyte growth were also optimal for gametogenesis (S. latissima—Hsiao and Druehl 1973), whereas another study found differences in the response of gametophytes to nitrogen and phosphorus concentrations between the vegetative and reproductive phases (Lessonia nigrescens—Hoffmann and Santelices 1982). Further to this, chelated iron has been demonstrated to be important in stimulating oogenesis in many Laminarian species; in some species oogenesis will not proceed without it, whereas for others, it is simply decreased or delayed (Motomura and Sakai 1984; Lewis et al. 2013; Iwai et al. 2015).
Phase specificity of the microstages of kelps in response to environmental conditions has been demonstrated, even between very close stages, i.e. gametophyte and very early sporophyte (Gerard 1990; Morelissen et al. 2013). In spite of this, there are few studies (Lüning 1980; Hoffmann and Santelices 1982; Choi et al. 2005) that have looked at effects of culture conditions on both vegetative gametophyte growth and the induction of fertility. Furthermore, neither growth nor gametogenesis of L. digitata gametophytes has yet been quantified under cultivation conditions.
The advantages of achieving a maximal growth rate of gametophytes will be negated if the development of their reproductive structures does not occur to its full potential, essentially rendering the percentage of non-reproductive biomass useless in a cultivation sense. Therefore, it was the intention of this work to study the effects of photoperiod and nutrient media on the growth and reproductive development of L. digitata under differing, commercially applicable (but experimental scale) culture conditions.
The objectives if this study were as follows:
-
To compare growth of gametophytes cultured with three commercially available fertilisers: two pre-prepared algal culture media and an off-the-shelf higher plant fertiliser.
-
To compare gametophyte growth under different photoperiod regimes and investigate any interaction between photoperiod and media used.
-
To follow the rate of development of gametogenesis when cultured with each of the media.
Materials and methods
Parent stock
Fertile broodstock (sori) of L. digitata was collected on various occasions between 2008 and 2011, throughout the summer fertile period, from Spiddal (53° 14′ 25″ N, 9° 18′ 44″ W), and Finavarra (53° 09′ 17˝ N, 9° 07′ 22″ W) Co. Galway, Ireland. Different parent individuals were used for each experiment, and in each case, several adults (n = 3–5) were used in order to secure sufficient zoospore density in the final inoculum. Healthy looking, undamaged specimens free from visible epiphytes and herbivore damage were selected as parents.
Zoospore release and pre-experiment conditions
Mature sori were cleaned thoroughly by rubbing and rinsing with tissue and sterilised seawater (SSW), wrapped in tissue and left overnight in the dark and in air at 10 °C to partially dehydrate. Zoospore release was induced by re-immersion in approximately 250 mL SSW in c. 400-mL deep glass petri dishes (10 °C). Sori were removed after 1 h and zoospore cultures were placed in <10 μmol photons m−2 s−1 red light, un-aerated, for settlement of spores. After 2 days, the zoospores had settled and attached to the culture vessels. The SSW was completely replaced; zoospores were returned to <10 μmol photons m−2 s−1 red light for the following 2 weeks to allow germination and formation of the primary cell. Following this period, the gametophytes were gently scraped from the culture vessels and the resulting stock was thoroughly mixed and split evenly to inoculate each of the nutrient solutions and the control, creating a total volume of 2.4 L per treatment. These inoculated media were split into 12 × 200 mL aliquots each contained in 250-mL Erlenmeyer flasks.
Light
Light was provided from below by lightboxes (Medalight LP400) with cold cathode fluorescent bulbs (experiment 1a), or from above by Fillipi 3F Linda 2 × 18 W cool white fluorescent tubes (experiments 1b and 2). The light boxes gave a more direct and less patchy source of light than the flurorescent tubes; however, they were not suited to the the humid environment of the temperature controlled chambers, which resulted in constant monitoring and replacement of delicate lightbulbs to counteract the rapid fall-off in irradiance with time. Later experiments were therefore carried out using the more stable fluorescent tubes. Measurements of photon irradiance were made with a PAR quantum sensor (SKP 215, Skye Instruments, Powys, U.K.).
Nutrients
Three commercially available nutrient media were used: A = Algoflash Tomato (now AlgoPlus Tomato; www.algoplus.net); f/2 (CELL-HI F2P, Varicon Aqua Solutions, Worcestershire, UK) and PES = modified Provasoli Enriched Seawater (Arbona and Molla 2006). These were made up into solution so that the concentration of total available nitrogen (as nitrate, nitrite, ammonium and urea) in all the cultures was approximately equivalent (~ 6 mg L−1); however, only Algoflash contained nitrogen in the form of ammonium-N and uric-N.
Experimental design
Experiment 1a
Each of three nutrient formulations (A, PES, f/2) plus one SSW control was tested in combination with each of four photoperiods (8:16; 12:12; 16:8 and 24:0, L:D), each combination in triplicate. This gave a total of 12, 250-mL Erlenmeyer culture flasks under each light regime (3 replicates × 4 nutrients), giving an overall total of 48 flasks. The light boxes provided cool white fluorescent light from below, adjusted to 10 μmol photons m−2 s−1 red light using tracing paper and coloured cellophane. This gave a spectrum with peaks in the red at 612 nm, and a total of 3.5% emitted light in the blue region, effectively blocking enough blue light to maintain the gametophytes in a vegetative state. The positions of the culture flasks on the light box were rotated on a daily basis to overcome any unevenness of emissions across the light box surface.
Experiment 1b
The experiment was as per experiment 1a but with only two media preparations (f/2 and A) and the SSW control. Exclusion of one treatment was necessary due to time constraints and PES was removed as it was the worst performing medium in experiment 1a. Light was provided by cool white fluorescent tubes from above and adjusted to 10 μmol photons m−2 s−1 red light with a red plastic filter giving a spectrum with peaks in the red at 612 nm and a smaller peak in the green at 545 nm. Blue light totalled 1.4% of all light emitted.
Cultures were maintained at 10 °C ± 2 °C in a walk-in temperature-controlled chamber, and constant aeration was provided by standard aquarium air pumps delivered through silicone tubing and a sterilised glass Pasteur pipette. Aeration supplied a source of carbon, and flow rate was controlled to maintain the gametophytes in constant suspension. 75% of the culture medium was completely replaced every week, and glassware was changed every 2 weeks. If diatoms were observed, GeO2 was added across the treatments at 0.1 mL L−1 of 0.894 g L−1 stock solution (Shea and Chopin 2007), no other problems with contamination were encountered. Measurements of length were taken on Day 0 and weekly thereafter, until the branching growth of the filamentous gametophytes meant that length was no longer representative of overall size/growth. The contents of each individual flask were then thoroughly mixed and split into four 50-mL Erlenmeyer flasks for biomass sampling according to the protocol described below.
Length measurements
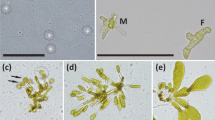
Prior to measurement, the culture vessel walls were scraped to remove any attached gametophytes. The solution was swirled to mix the culture well and a drop was removed from each flask using a sterilised Pasteur pipette and examined systematically using a compound microscope (Leitz Dialux 22). A power analysis had revealed 25 individual gametophyte measurements to be sufficient as a representative sample. Individual gametophytes were measured along their main axis to estimate maximum length using a calibrated ocular micrometre; side branching was discounted. Once gametogenesis had begun and differentiation of the sexes was possible, only the female gametophytes were measured in order that length differences between the sexes did not affect the within sample variability.
Biomass measurements
The initial volume of 200 mL of culture was thoroughly mixed and split between four 50-mL Erlenmeyer flasks. One flask was sampled immediately (week 5) and one flask 2 weeks later (week 7). The entire contents of the flask were emptied onto pre-weighed filter paper (Whatman glass microfibre filter paper GF/C, 55 μm Ø) ensuring any visible clumps of gametophytes adhering to the flask were included. They were then rinsed with distilled water to remove salt, vacuum filtered, freeze-dried and re-weighed to determine their total dry weight.
Experiment 2
A blue light treatment was used to compare the effects of media and photoperiod on gametogenesis. New gametophyte stock made as detailed in experiment 1 was mixed and split between each of the nutrient solutions and the SSW to give a final volume of 200 mL of each inoculated medium and the control. Each medium was then divided between four 50-mL Erlenmeyer flasks covered loosely with Parafilm and cultured at 10 °C ± 2 °C with a 12-h photoperiod, other conditions as described previously. Light was provided from above by cool white fluorescent tubes and adjusted to an average of 31.4 μmol photons m−2 s−1 light using blue plastic filters. This gave a spectrum with peaks in the violet/blue at 436 and 488 nm and in the green at 544 nm. Lüning and Dring (1975) found the main peaks of the spectrum to induce reproductive activity were at 382 nm, ~410 nm, 434–452 nm and ~480 nm; therefore, ‘blue’ light is defined as from the near ultra-violet to the green. In total, 59% of emitted light was in the ‘blue’ range of 380–500 nm, and 19.6% was at the peak of the action spectrum (430–450 nm). Aeration was not provided but the flasks were agitated daily in order to facilitate gas exchange and disrupt any boundary layer that may have formed around settled gametophytes.
Length measurements
Growth was followed as before, along the main axis of female gametophytes, with measurements taken on days 0, 4, 7, 11, 14, 18, 21, 25 and 28. The extent of development of each measured gametophyte (single or multi-cellular) was also noted.
Gametogenesis
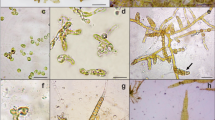
Gametogenesis was first observed on day 11 and counting of reproductive cells began on day 14 and, thereafter, on days 21 and 28. Samples were prepared as for the growth measurements and the first 300 female cells encountered were screened and assigned a status of vegetative (V), developing oogonia (I), mature oogonia or zygote (II), few-celled sporophyte (III), multi-cellular sporophyte (IV). Counts were converted to percentages of cells in each category.
Statistical analysis
Data were tested for homogeneity of variance using Cochran’s test and were reciprocal transformed where necessary (growth data week 3 experiment 1b). Growth under red light (length and biomass) data were then analysed using two-way analysis of variance (ANOVA). Significant differences between treatments were identified a posteriori using Tukey’s pairwise comparisons.
Growth under blue light (length) and gametogenesis data were analysed using one-way ANOVA, at days 21 and day 28, respectively.
All statistical analyses were performed in Minitab.
Results
Summary
The overall trends showed all media to result in good performance of the gametophytes in terms of vegetative growth under red light, while f/2 performed better for growth under blue light and in promoting gametogenesis.
Growth under red light as measured by length (experiments 1a and 1b)
Over the course of the two growth measurement periods, the gametophytes followed an exponential growth curve and achieved maximum lengths of 91–93 μm (1a 24A; 1b 24A, respectively) compared to minima of 25–17 μm in the controls (1a 8C; 1b 8C)—Table 1. Results are reported for, and effects of treatments on lengths of gametophytes were compared at, week 3 (experiment 1a) and week 2 (experiment 1b). This was because length was only found to be a good proxy for growth during the initial development phase while the gametophytes were relatively unbranched, and at week 4, the length data were no longer representative—see the discussion for further comment. Comparing the length of gametophytes at weeks 2 and 3 (Fig. 1a, b) gave significant effects for nutrient type (1a: F3,32 = 34.58, p = <0.001; 1b: F2,24 = 65.23, p = <0.001), photoperiod (1a: F3,32 = 20.47, p = <0.001; 1b: F3,24 = 101.45, p = <0.001), and a significant interaction term for experiment 1b only (1a: F9,32 = 1.64, p = 0.147; 1b: F6,24 = 9.05, p = <0.001).
Growth of vegetative gametophytes under red light as measured by length (μm) in experiment 1a at day 21 (a) and 1b at day 14 (b). Photoperiods—8:12; 12:12; 16:8; 24:0 L:D. Media—C control—sterilised seawater, F f/2, A Algoflash, P Provasoli Enriched Seawater. Error bars = ±1 standard deviation, n = 3
The controls showed uniformly poor or even negative growth under all light regimes in both experiments, although in experiment 1b some growth did occur, and gametophytes were significantly smaller than all other treatments, while no other differences between media were significant. In experiment 1b, all photoperiods differed from all others as expected (i.e. 8 < 12 < 16 < 24) and the interaction term was due to the control group which, however, did not differ between photoperiods (i.e. C8 = C12 = C16 = C24). In experiment 1a, photoperiod 8 resulted in lengths significantly smaller than all others, 12 was equivalent to 16 and smaller than 24 and 16 was equivalent to 24.
Growth under red light as characterised by biomass (experiment 1b)
The biomass results of experiment 1b (Fig. 2) show that at week 7, both nutrient formulation (F2,24 = 38.5, p = < 0.001) and photoperiod (F3,24 = 16.82, p = < 0.001) exerted a significant effect on biomass, and there was a significant interaction term (F6,24 = 3.24, p = 0.018). There was an increase in biomass with increasing length of photoperiod under the media treatments; f/2 regularly, but not significantly, showed the greatest biomass production within each photoperiod. The significant interaction term is accounted for by the fact that there is no difference between the control and f/2 under the 8-h light regime, while under all other light regimes, the control group is significantly smaller.
Growth of vegetative gametophytes under red light as measured by biomass (dry weight g L−1) in experiment 1b at week 5 (a) and week 7 (b). Legend as Fig. 1. Error bars = ±1 standard deviation, n = 3
Similar to the outcome of the effect of photoperiod on length, there were no significant differences between the gametophyte biomass grown in 16- and 24-h photoperiods; however, contrary to the length results, the biomass production in the 12-h photoperiod was significantly smaller than both and equal to the 8-h photoperiod. Significant differences between media treatments were found between the control group and both Algoflash and f/2 under the 24- and 16-h photoperiods, and between the control group and f/2 for the 12-h photoperiod.
Growth under blue light as measured by length (experiment 2)
Growth in all media treatments followed an S-shaped curve achieving a maximum length of 48.3 μm (f/2, day 21), shown in Fig. 3. At day 21, all treatments were significantly greater in length than the controls, and gametophytes growing in f/2 were significantly greater than those growing in either Algoflash or PES (F3,12 = 46.21, p = <0.001). At the end of the experiment (day 25) there was no apparent difference between the lengths of the gametophytes that remained vegetative in any of the media formulations (not tested statistically).
Growth of female gametophytes under blue light as measured by length (μm) over the course of experiment 2. Error bars = ±1 standard deviation, n = 4. Legend as Fig. 1
Female gametophytes under all media treatments were predominantly single-celled but some two-celled and occasional multi-celled individuals were also observed across all treatments. Developing oogonia were first observed in the f/2 treatment on day 11, but counts were first taken on day 14. Comparing the length of cells in all treatments on day 11 gives a highly significant effect of treatment (F3,12 = 37.04, p = <0.001): those in f/2 were larger than those in Algoflash, which were larger than those in PES which were indistinguishable from those in the control treatment (f/2 > A > P = C). By day 18 cells in Algoflash and PES were following a similar growth trajectory, and by day 21, while individuals in f/2 were significantly larger than all other treatments, those in Algoflash and PES were indistinguishable from one another and significantly longer than those in the control treatment (F3,12 = 46.21, p = <0.001).
At day 14, the control treatments were lacking in any visible cellular contents and normal colouration although some growth was still observed. A slight increase in cell size was seen between day 14 and day 21 but these cells failed to develop in any normal capacity.
Gametogenesis
Over the experimental period, all media treatments resulted in gametogenesis, although the degree to which this occurred varied between treatments (Fig. 4). After 7 days, none of the cells in any treatment showed signs of developing reproductive structures, and the cells in the control treatment remained vegetative throughout. By day 14, all media treatments showed signs of reproductive development, with a small percentage (4.7%) of early sporophytes already present in f/2. By day 21, there was little difference between the treatments in the proportion of cells that remained vegetative, but the degree to which oogonia were maturing did vary; a greater number of mature oogonia, zygotes and sporophytes were seen in cultures grown using f/2 medium. By day 28, the difference in proportions of mature fertile cells (Fig. 4: categories II, III and IV—mature oogonia, zygotes and sporophytes) and vegetative or developing fertile cells (categories V and I combined) was significant (F2,9 = 53.64, p = <0.001) with f/2 showing more advanced development than Algoflash or PES, which did not differ from one another (f/2 < A = P).
Discussion
Growth measured by length under red light
This study presents results for growth measured by length, biomass increase and percentage fertility induction of L. digitata gametophytes over the course of 3- , 4- or 7-week experiments. Length was measured along the major gametophyte axis and beginning from primary cells of a mean size of approximately 18 μm. To our knowledge, lengths for vegetative L. digitata gametophytes have never previously been published, so overall performance in this regard cannot be compared. Further to this, within-species variability, dependent on ecotype or even individual, can often be as pronounced as that between species (Han and Kain 1996) and it is of fundamental importance to repeat experiments with the same species, ecotype and perhaps siblings, in order to estimate the extent of the variability and build an accurate picture. This variability is exemplified in the differences between experiments 1a and 1b, and the fact that rapid growth and large size were attained for the 12-, 16- and 24-h photoperiods in experiment 1b, whereas in 1a, there is lower growth overall (lengths at week 3 in experiment 1a approximate those at week 2 in experiment 1b for each photoperiod) and a less defined response to photoperiod.
Examination of graphs of growth rate (not reported) show that the 8- and 12-h photoperiods in experiment 1a produce very similar growth rate patterns for all the media preparations, suggesting that light is limiting growth, whereas in experiment 1b, this only occurs under the 8-h photoperiod. Differences in light acquisition ability between gametophyte populations of S. latissima have been demonstrated by Gerard (1990), dependent upon the depth and turbidity of the environment in which the parent sporophyte was growing—although in that case, they did not translate into differences in gametophyte growth rate. At week 2, lengths achieved under the 12-, 16- and 24-h photoperiods in experiment 1b approximated those in the equivalent photoperiods in experiment 1a at week 3, and if these differences can be attributed to population-specific growth characteristics related to light acquisition, then there are potential implications in terms of maximising efficiency of biomass production. Similarly, the growth of the controls in experiment 1b suggests either a background level of nutrients in the SSW that was not present in 1a, or again an ecotype better adapted to acquisition of nutrients that are present in low concentrations. Ecotypic variation in growth and storage of inorganic nitrogen in response to differing nitrogen availability has been suggested in adult populations of L. longicruris (Gagne et al. 1982) and S. latissima (Laminaria saccharina) (Gerard 1997). As an additional possibility, it has been suggested that season and timing of release may affect zoospore viability and germination success by affecting ‘ripeness’, and the provision of zoospores with sufficient resources to successfully germinate and grow. Differences in kelp gametophyte reproductive success (Lessonia trabeculata, Tala et al. 2004) in growth rate (S. latissima, Nielsen et al. 2016) and in optimal growth and survival (Ecklonia radiata, Mohring et al. 2013) have all been found to relate to season of release of the spores. Sporophytes of L. trabeculata, E. radiata and S. latissima, like L. digitata, have long periods where a sorus is present and they are potentially reproductive. However, the fertility, reproductive success, survival and growth of the gametophytes varied significantly according to month of release of the spores. The present study did not take account of the season of release of the cultured zoospores, and it is possible that ripeness of the zoospores could have accounted for some of the variation between experiments 1a and 1b.
Effect of photoperiod
Collectively, experiments 1a, 1b and 2 show that length is only useful as a proxy for vegetative gametophyte growth in the early stages, prior to extensive branching taking place. At weeks 2 (experiment 1b) and 3 (experiment 1a), the relationship between photoperiod (total irradiance received per day) and length approximates that expected, i.e. it increases roughly in proportion to the total irradiance received per day. By week 4, this relationship has broken down partially in experiment 1a, or wholly in experiment 1b where growth was faster. That the lack of clear relationship between ‘growth’ and photoperiod seen at the end of the experiment is an artefact of measuring technique and not a true representation of growth is strongly suggested by the fact that the relationship does not break down when growth is measured by biomass—compare Fig. 1d with Fig. 2. Biomass is a composite measurement that takes into account the 3-dimensional nature of the gametophyte and is not influenced by the growth form so is arguably more appropriate for measurement of late stage gametophyte growth. We used both techniques in order to evaluate which was most appropriate and we have demonstrated that using dry weight is the more effective at elucidating differences between treatments, even in a small-scale experimental set-up with very low overall biomass.
In spite of the approximately linear relationship between length/biomass and total irradiance, not all differences in length/biomass between photoperiods are significant. In experiment 1a, lengths under 12 h and 16 h are equivalent, as are those under 16 h and 24 h, and in 1b biomass, under the 16-h and 24-h photoperiods are equivalent. Growth saturation points for zoospores or gametophytes are scarce in the literature; however, estimates for gametophyte growth of several Californian kelps (Lüning and Neushul 1978) and for L. ochroleuca (Izquierdo et al. 2002) showed saturation took place between 10 and 20 μmol photons m−2 s−1 (1–2 nmol photons cm−2 s−1 as given in Lüning and Neushul’s paper), and Lüning (1980) estimated growth of the primary cell of species of the European Laminariales under white light to be saturated at 2–3 nmol photons cm−2 s−1 (20–30 μmol photons m−2 s−1, L. digitata 30 μmol photons m−2 s−1). Han and Kain (1996) showed young sporophytes of L. digitata, L. hyperborea and S. latissima to be similarly saturated at 20–30 μmol photons m−2 s−1, although this is a different life phase and may not be directly comparable; indeed sporophyte growth has been demonstrated to be saturated at higher photon flux densities than gametophytes (Gerard 1990). Although 10 μmol photons m−2 s−1 in this study is at the lower boundary of these reported estimates, it is still possible that growth in our experiments was saturated, in which case excess photosynthate could have been carried over to the dark period during which growth could have continued, accounting for lack of a clear effect of photoperiod on length/biomass increase.
Effect of media on growth and gametogenesis
With regard to growth in different media, neither experiment 1a or 1b (growth measured by length and biomass) show evidence of any of the media being more efficient than any other at promoting vegetative gametophyte growth. In experiment 2, slower growth in PES and Algoflash was demonstrated by the results for growth under blue light and there were subsequently lower rates of gametogenesis (see below). Furthermore, although it is beyond the scope of this work to investigate detailed compositional differences between growth media, it is of note that while all the media contain chelated iron, PES and Algoflash also contain boron. While iron has been shown to stimulate gametogenesis in laminarian species ( Motomura and Sakai 1984, Lewis et al. 2013, Iwai et al. 2015); the addition of boron inhibited gametogenesis in S. japonica and S. longissima (L. japonica and L. angustata). The inhibitory effect was moderated by increasing the concentration of iron relative to boron and the authors concluded that the ratio of the concentration of the two elements is important with regard to stimulation of gametogenesis (Motomura and Sakai 1984). It is possible that the same effect also occurred during this experiment, which may have contributed to the increased rate of gametogenesis seen in the f/2 growth medium.
Growth under blue light and rate of gametogenesis
Production of gametophyte biomass is inconsequential without transformation into fertile cells. Of ultimate interest, ecologically and from a cultivation perspective, is the production rate of sporophytes both in terms of time to formation and percentage conversion (gametophyte to sporophyte). There is a trade-off between resources allocated to growth and those allocated to reproduction such that once a gametophyte has become fertile, growth slows and then ceases (Hoffmann and Santelices 1982; Gao et al. 2013). Whether there is a certain minimum cell size below which gametogenesis will not occur is not clear. Lüning (1972) observed that following induction with blue light, S. latissima gametophytes began to become fertile immediately, whereas with newly released zoospores there was a time lag, attributed to the need to acquire sufficient resources before reproduction. In this study, there is a clear relationship between appearance of first mature gametophytes and average size of gametophytes—mature oogonia appear at a gametophyte length of approximately 30 μm. Speed of growth is therefore related to time to maturity as can be seen by the earlier maturation of the gametophytes in f/2, and the approximate parity between Algoflash and PES (gametophytes in Algoflash both grow and mature slightly faster). In this case, f/2 cultures would have been ready for spraying onto cultivation substrate at day 14, those in Algoflash would have been ready at day 21 and gametophytes grown in PES at day 28. This could shorten the labour intensive hatchery growth phase and also facilitate greater control over timing of sea transfer, which would be beneficial in a practical sense.
For S. latissima, the percentage of gametogenesis is proportional to the quantum dose of blue light received (i.e. the photon flux density × total exposure time) up to a saturating value of 400 μmol photons cm−2 but is also dependent on temperature (Lüning and Dring 1975). In low photon fluence rates gametophytes may remain vegetative even under blue light (op. cit.). Under our experimental set-up, 400 μmol photons cm−2 blue light would have been received in 5.26 days, at which point fertility was only just being induced, perhaps suggesting a slightly higher light requirement for stimulation of gametogenesis in L. digitata. By day 28, f/2 had reached 94% induction, and had received a total irradiance of 2120 μmol photons cm−2 of blue light. However, we cannot ascertain from our results the point at which saturation occurred, i.e. the point at which no more blue light was necessary to allow full gametogenesis.
Conclusion
Differences in effect of media formulation on growth were not consistent; under red light gametophytes in all media behaved similarly. Differences became apparent for growth under blue light, gametogenesis and sporophyte production, where f/2 clearly resulted in a shorter time to fertility (e.g. at day 14: f/2 75% of cells developing oogonia; A 37% developing; P 33% developing) and increased production of juvenile sporophytes (e.g. at day 28: f/2 ~ 30%; A ~ 9%; P ~ 2%). Therefore, if rapid transition to fertility is desirable, then f/2 can be recommended. However, based on their comparable growth rates, Algoflash is suitable as an accessible and easy-to-use growth medium for production of vegetative L. digitata gametophyte biomass.
While there were some instances where productivity under the 24-h photoperiod regime was significantly different to that under the 16-h photoperiod regime, the differences were not in proportion to the additional irradiance received. It can be tentatively suggested from these experiments that benefit gained from the 24-h photoperiod regime is not in proportion to the hours of illumination, and that as reduction of hatchery phase energy consumption is of interest, it can be advised that a 16:8 h light: dark regime will not result in significantly lowered biomass yields. As a management strategy, it is likely to be of more benefit to ensure maximum conversion of biomass from vegetative to reproductive condition, and from the results herein, f/2 was more effective in that capacity.
It is recommended that further investigation into gametophyte responses to culture conditions should take account of source population. It would be very interesting to begin to determine the extent of variability in response between sibling gametophyte cultures from parent sporophytes within the same population versus individuals from distinctly different populations, e.g. from sheltered and exposed populations. Experiments should be designed such that potential seasonal effects of time of spore release, and sorus ripeness, on gametophyte growth and fecundity are controlled for.
References
Arbona JF, Molla M (2006) Cultivation of brown seaweed Alaria esculenta. Aquaculture explained series. Bord Iascaigh Mhara, Dublin 50pp
Bartsch I, Wiencke C, Bischof K, Buchholz CM, Buck BH, Eggert A, Feuerpfeil P, Hanelt D, Jacobsen S, Karez R, Karsten U, Molis M, Roleda MY, Schubert H, Schumann R, Valentin K, Weinberger F, Wiese J (2008) The genus Laminaria sensu lato: recent insights and developments. Eur J Phycol 43:1–86
Choi HG, Kim YS, Lee SJ, Park EJ, Nam KW (2005) Effects of daylength, irradiance and settlement density on the growth and reproduction of Undaria pinnatifida gametophytes. J Appl Phycol 17:423–430
Cosson J, Gayral P (1979) Optimal conditions for growth and fertility of Laminaria digitata (Phaeophyceae) gametophytes. Proc Int Seaweed Symp 9:59–65
Davoult D, Engel CR, Arzel P, Knoch D, Laurans M (2011) Environmental factors and commercial harvesting: exploring possible links behind the decline of the kelp Laminaria digitata in Brittany, France. Cah Biol Mar 52:1–6
Edwards MD, Mooney-McAuley K, Gorman E, Champenois J (2016) Standard operating protocol manual for seaweed biomass cultivation and analysis. EnAlgae project report. http://www.enalgae.eu/public-deliverables.htm; Accessed on 29 Nov 2016
Gagne JA, Mann KH, Chapman ARO (1982) Seasonal patterns of growth and storage in Laminaria longicruris in relation to differing patterns of availability of nitrogen in the water. Mar Biol 69:91–101
Gao X, Agatsuma Y, Taniguchi K (2013) Effect of nitrate fertilization of gametophytes of the kelp Undaria pinnatifida on growth and maturation of the sporophytes cultivated in Matsushima Bay, northern Honshu, Japan. Aquacult Int 21:53–64
Gerard VA (1990) Ecotypic differentiation in the kelp Laminaria saccharina: phase-specific adaptation in a complex life cycle. Mar Biol 107:519–528
Gerard VA (1997) The role of nitrogen nutrition in high-temperature tolerance of the kelp, Laminaria saccharina (Chromophyta). J Phycol 33:800–810
Han T, Kain JM (1996) Effect of photon irradiance and photoperiod on young sporophytes of four species of the Laminariales. Eur J Phycol 31:233–240
Hoffmann AJ, Santelices B (1982) Effects of light intensity and nutrients on gametophytes and gametogenesis of Lessonia nigrescens Bory (Phaeophyta). J Exp Mar Biol Ecol 60:77–89
Hoffmann AJ, Avila M, Santelices B (1984) Interactions of nitrate and phosphate on the development of microscopic stages of Lessonia nigrescens Bory (Phaeophyta). J Exp Mar Biol Ecol 78:177–186
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23:543–597
Hsiao SI, Druehl LD (1973) Environmental control of gametogenesis in Laminaria saccharina. II. Correlation of nitrate and phosphate concentrations with gametogenesis and selected metabolites. Can J Bot 51:829–839
Iwai H, Fukushima M, Motomura T, Kato T, Kosugi C (2015) Effect of iron complexes with seawater extractable organic matter on oogenesis in gametophytes of a brown macroalga (Saccharina japonica). J Appl Phycol 27:1583–1591
Izquierdo JL, Pérez-Ruzafa IM, Gallardo T (2002) Effect of temperature and photon fluence rate on gametophytes and young sporophytes of Laminaria ochroleuca Pylaie. Helgol Mar Res 55:285–292
Kain JM (1979) A view of the genus Laminaria. Oceanogr Mar Biol 17:101–161
Kerrison PD, Stanley MS, Edwards MD, Black KD, Hughes AD (2015) The cultivation of European kelp for bioenergy: site and species selection. Biomass Bioenergy 80:229–242
Lewis RJ, Green MK, Afzal ME (2013) Effects of chelated iron on oogenesis and vegetative growth of kelp gametophytes (Phaeophycae). Phycol Res 61:46–51
Li D, Zhou Z, Liu H, Wu C (1999) A new method of Laminaria japonica strain selection and seedling culture by the use of vegetative gametophytes. Hydrobiologia 398/399:473–476
Lüning K (1972) Reproduction induced by blue light in female gametophytes of Laminaria saccharina. Planta 104:252–256
Lüning K (1980) Critical levels of light and temperature regulating the gametogenesis of three Laminaria species (Phaeophyceae). J Phycol 16:1–15
Lüning K, Dring MJ (1975) Reproduction, growth and photosynthesis of gametophytes of Laminaria saccharina grown in blue and red light. Mar Biol 29:195–200
Lüning K, Neushul M (1978) Light and temperature demands for growth and reproduction of laminarian gametophytes in southern and central California. Mar Biol 45:297–309
Mohring MB, Kendrick GA, Wernberg T, Rule MJ, Vanderklift MA (2013) Environmental influences on kelp performance across the reproductive period: an ecological trade-off between gametophyte survival and growth? PLoS One 8:e65310
Morelissen B, Dudley BD, Geange SW, Phillips NE (2013) Gametophyte reproduction and development of Undaria pinnatifida under varied nutrient and irradiance conditions. J Exp Mar Biol Ecol 448:197–206
Motomura T, Sakai Y (1984) Regulation of gametogenesis of Laminaria and Desmarestia (Phaeophyta) by iron and boron. Jap J Phycol 32:209–215
Nielsen MM, Kumar JP, Soler-Vila A, Johnson MP, Bruhn A (2016) Early stage growth responses of Saccharina latissima spores and gametophytes. Part 1: inclusion of different phosphorus regimes. J Appl Phycol 28:387–393
Shea R, Chopin T (2007) Effects of germanium dioxide, an inhibitor of diatom growth, on the microscopic laboratory cultivation stage of the kelp, Laminaria saccharina. J Appl Phycol 19:27–32
Tala F, Edding M, Vásquez J (2004) Aspects of the reproductive phenology of Lessonia trabeculata (Laminariales: Phaeophyceae) from three populations in northern Chile. N Z J Mar Freshw Res 38:255–266
Westermeier R, Patiño D, Piel MI, Maier I, Mueller D (2006) A new approach to kelp mariculture in Chile: production of free-floating sporophyte seedlings from gametophyte cultures of Lessonia trabeculata and Macrocystis pyrifera. Aquac Res 37:164–171
Wu C, Li D, Liu H, Peng G, Liu J (2004) Mass culture of Undaria gametophyte clones and their use in sporeling culture. Hydrobiologia 512:153–156
Xu Z, Dapeng L, Hanhua H, Tianwei T (2005) Growth promotion of vegetative gametophytes of Undaria pinnatifida by blue light. Biotechnol Lett 27:1467–1475
Xu B, Zhang QS, Qu SC, Cong YZ, Tang XX (2009) Introduction of a seedling production method using vegetative gametophytes to the commercial farming of Laminaria in China. J Appl Phycol 21:171–178
Zhang QS, Qu SC, Cong YZ, Luo SJ, Tang XX (2008) High throughput culture and gametogenesis induction of Laminaria japonica gametophyte clones. J Appl Phycol 20:205–211
Zhi C, Rorrer GL (1996) Photolithotrophic cultivation of Laminaria saccharina gametophyte cells in a bubble-column bioreactor. Enzym Microb Technol 18:291–299
Acknowledgements
This study was jointly funded by (1) SuperGen Biomass Biofuels and Energy Crops II under EPSRC grant EP/039995 through Leeds University and (2) The Seaweed Hatchery Project (PBA/SW/07/001 (01), carried out under the Sea Change Strategy with the support of the Marine Institute and the Marine Research Sub-programme of the National Development Plan, 2007–2013.
The authors would also like to acknowledge the help given by Colm Moriarty for the technical assistance in setting up the culture chambers and Jazmin Hernandez-Kantun and Jyotsna Mishra for their help with sample preparation and analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ratcliff, J.J., Soler-Vila, A., Hanniffy, D. et al. Optimisation of kelp (Laminaria digitata) gametophyte growth and gametogenesis: effects of photoperiod and culture media. J Appl Phycol 29, 1957–1966 (2017). https://doi.org/10.1007/s10811-017-1070-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1070-1