Abstract
To improve the production of Kappaphycus plantlets in tissue culture, optimum media concentrations of an Ascophyllum nodosum extract (Acadian Marine Plant Extract Powder, AMPEP), plant growth regulators (PGR), pH–temperature combinations, and explant density were determined. Kappaphycus alvarezii var. tambalang purple (PUR), kapilaran brown (KAP), vanguard brown (VAN), adik-adik (AA), tungawan green (TGR), and K. striatum var. sacol green (GS) were used as explants. Based on the shortest period for shoot emergence and the economical use of AMPEP, the optimum enriched media was 3.0 mg L−1 AMPEP and 0.1 mg L−1 AMPEP + PGR 1 mg L−1 each phenylacetic acid (PAA) and zeatin for PUR, 1.0 mg L−1 AMPEP + PGR for KAP and GS, 0.1 mg L−1 AMPEP + PGR for VAN, and 3.0 mg L−1 AMPEP and 0.001 mg L−1 AMPEP + PGR for AA and TGR. Results showed that the addition of PGR to low concentrations of AMPEP hastened shoot formation. pH–temperature combinations for the most rapid shoot formation were determined for the brown (KAP) and purple (PUR) color morphotypes of K. alvarezii var. tambalang and the green morphotype of K. striatum var. sacol (GS) cultured in 1.0 mg L−1 AMPEP + PGR. The brown morphotype produced the most number of shoots at pH 7.7 at 20°C after as little as 20 days. Purple K. alvarezii showed an increased shoot formation at pH 6.7 at 25°C and the green K. striatum morphotype at pH 8.7 at 25°C. The optimum number of explants added to the culture media was also determined for tungawan green (TGR), brown (KAP), and tambalang purple (PUR) varieties of K. alvarezii in 1.0 mg L−1 AMPEP + PGR. The number of explants and the volume of the culture media combination were also tested. The highest average number of shoots formed occurred in two explants:1 mL culture media (2:1) for KAP and PUR (35.00% and 16.67%, respectively) and 1 explant: 2 mL culture media for the TGR (100.00%) with a range of 0.5–3.0 mm shoot length after 40 days in culture. The earliest shoot formation was observed after 21 days for the brown and 9 days for both the green and purple color morphotypes of Kappaphycus, in all densities investigated. This indicated that within the range tested, the density of explants did not have a significant effect on the rate of shoot formation but did influence the average number generated from the culture. The rate of production of new and improved Kappaphycus explants for a commercial nursery stock was improved through the use of AMPEP with optimized culture media pH, temperature, and density conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Kappaphycus is a red macroalga that is an important source of the industrial gel carrageenan (Bixler and Porse 2010). It is one of the important commercial species cultivated in Southeast Asia. However, dwindling resources of seedstocks, loss of genetic variability, and the onset of fast-spreading diseases has significant negative impacts on the carrageenophyte seaweed industry and associated workers dependent on income from farming. One approach developed to mitigate these effects is the optimization of tissue culture techniques aimed to produce a large quantity of high quality Kappahycus seedstock for nursery purposes. Successful micro-propagation of K. alvarezii and Eucheuma species has been reported by Dawes and Koch (1991), Dawes et al. (1993), Hurtado and Cheney (2003), and Hurtado and Biter (2007). The growth and production media utilized by these studies are complicated to prepare and too expensive for practical applications in large-scale, commercial production.
The use of seaweeds for their soil conditioner properties and to improve growth and yield of land-based crops has been in practice for decades (Chapman and Chapman 1980; Craigie 2010). However, the earliest application was limited to agricultural areas near the coastline as transport cost of raw seaweeds was expensive. The positive effects of seaweed extracts on the growth, yield and quality, pest and disease resistance and environmental stress tolerance of commercial land crops have been reported (Featonby-Smith and van Staden 1987; Crouch 1990; Rayorath et al. 2008a, b; Jayaraj et al. 2008). Conversion of the raw seaweed resources to liquid (Milton 1952) and powder forms (Stephenson 1974; Craigie 2010) made the extract version more accessible to a wider array of applications away from the coast.
The brown alga Ascophyllum nodosum is the most important commercial seaweed harvested in Canada. One extensively studied product developed from this alga is its commercial extract. The composition and consistency, as a commercial fertilizer for crop plants, has recently been determined using 1H NMR profiling (Craigie et al. 2008). It has further been shown to effectively promote the growth of the model plant Arabidopsis (Rayorath et al. 2008a), induce root-tip elongation and shoot growth and enhance germination and seedling vigor in barley (Rayorath et al. 2008b) and also reduce foliar fungal disease in carrots and cucumber (Jayaraj et al. 2008; Jayaraj et al. 2010). There is a first report of plants treated with commercial Ascophyllum extracts improving the health benefits of human consumption (Fan et al. 2010).
For the purpose of this study, the product is labeled Acadian Marine Plant Extract Powder (AMPEP). The application of AMPEP has been extended to laboratory tissue culture techniques for the regeneration of the carrageenophyte Kappaphycus alvarezii varieties for nursery purposes in the Philippines (Hurtado et al. 2009). Loureiro et al. (2009) also reported on a substantial increase in the growth rates of K. alvarezii and a reduction of the overall presence of epiphytes such as Cladophora and Ulva when exposed to AMPEP. Furthermore, AMPEP is 100% water soluble and does not require further addition of expensive reagents which can be complicated to prepare, thereby making it a potentially suitable medium for the large-scale production of carrageenophytes in the major Southeast Asian areas of commercial carrageenophyte production (Hurtado et al. 2009). This study was conducted to determine the optimal AMPEP concentration, pH, temperature, and density of explants for the commercial production of K. alvarezii varieties using tissue culture techniques.
Materials and methods
Seven varieties of K. alvarezii were used as plants in this study. Clean and healthy plants of K. alvarezii var. tambalang (green, TGR) and K. alvarezii var. tambalang (curly top brown, CTB) from Tungawan, Zamboanga Sibugay, Philippines (7°30′29′′ N and 122°22′16′′), K. alvarezii var. tambalang (purple, PUR; kapilaran brown, KAP; and reddish brown, BRN), vanguard brown (VAN), adik-adik (AA), and K. striatum var. sacol green (GS) were collected from a commercial farming site in Tictauan Is., Zamboanga City, Philippines (6°54′53.06′′ N and 122°10′43.33′′). The collection sites have grown the explants commercially recently along the coast of the Zamboanga Peninsula. The plants were placed in an ice chest lined with seawater-moistened cheesecloth and transported to the Aquaculture Department of the Southeast Asian Fisheries Development Center (SEAFDEC/AQD) Tigbauan, Iloilo, Philippines, following the protocol described by Hurtado et al. (2009). The explants were acclimatized in concrete tanks with continuous aeration and a flow-through seawater system for 7 days.
Preparation of explants
Healthy apical stems were selected, cut into 2-cm segments and brushed individually with 0.05% povidone iodine. Segments were further cleaned by shaking three times in 50 mL centrifuge tubes with fine glass beads and sterile filtered seawater. Cleaned segments were then incubated in 9.1% E3 anti-bacterial solution (Bradley et al. 1988) in sterile, filtered seawater at a temperature of 23°C, 13:11 h L:D cycle, and an irradiance of 10–15 μmol photons m−2 s−1. After 3 days, the ends of the segments were discarded and the remaining parts were cut into 2 mm explants. Three different experiments were performed in order to optimize the culture conditions to produce the highest number of emergent shoots over the shortest number of days.
Optimization of AMPEP concentration
Two-millimeter sections of TGR, PUR, KAP, VAN, AA, and GS were thoroughly rinsed in sterile seawater and placed individually in six separate, 48-well culture plates with 1 mL of 0.22 μM-filtered AMPEP-seawater solution at different concentrations (0.001, 0.01, 0.1, 1.0, 2.0, 3.0, 4.0, and 5.0 mg L−1, n = 8 per concentration), with or without a plant growth regulator (PGR (phenylacetic acid, PAA) + zeatin at 1 mg L−1). ESS/2 (half strength of enriched sterile seawater) with E3 (antibiotic mixture) and PGRs was used as a control. Hurtado and Biter (2007) used ESS/2 with E3 as the culture media in the plantlet regeneration of K. alvarezii var. adik-adik and for comparative purposes in the present study, ESS/2 with E3 was used as the control. The use of PAA and zeatin was based on earlier studies on the tissue culture of K. alvarezii (Hurtado and Biter 2007; Hurtado et al. 2009). The culture wells were sealed with Parafilm, completely covered with aluminum foil and incubated in the dark at 23°C for 7 days. The explants were then exposed to an irradiance of 10–15 μmol protons m−2 s−1, with 13:11 h L:D cycle at 23°C. Media changes were performed weekly. Cell and shoot development were observed at every media change using an Olympus IX70 inverted microscope and an Olympus Coolsnap CF Monochrome Imaging Unit.
Transfers of sections to T25 flasks filled with 40 mL of the corresponding AMPEP concentration with, or without, PGR were made after the explants developed shoots of 1–2 mm. The explants were then exposed to an irradiance of 30–45 μmol protons m−2 s−1, 13:11 h L:D cycle at 23°C in combination with gentle shaking. Further increases in shoot length (2–5 mm) required a transfer of the explants to 250 mL culture flasks filled with 200 mL of the corresponding media provided with gentle aeration and exposed to an irradiation of 30–45 μmol protons m−2 s−1, 13:11 h L:D cycle at 23°C. Young plants having >5 mm shoots were placed inside perforated 50-mL plastic tubes and submerged in a 1-t capacity tank with continuous seawater flow, at ambient temperature and irradiance. Cleaning and dipping of the plantlets in 100 mg L−1 AMPEP was performed monthly.
Effect of pH and temperature
Healthy 2.0 mm explants of BRN, GS, and PUR were used in this experiment and were prepared as described above. The explants were placed individually in 96-well culture plates with 200 μL of 1.0 mg L−1 AMPEP + PGR (PAA + Zeatin at 1 mg L−1) in 0.22 μM-filtered sterile seawater with varying pH (6.7, 7.7, 8.7, and 9.7, n = 24). The pH was adjusted using 1 M HCl and 1 N NaOH. Three culture plates were prepared for each variety and were placed at different temperatures (e.g. 20, 23, and 25°C). The plates were sealed with Parafilm and covered with aluminum foil for 7 days and then later exposed to an irradiance of 10–15 μmol photons m−2 s−1, with a 13:11 h L:D cycle. Daily observations of emergence of shoot were made using an Olympus IX70 inverted microscope and Olympus DP12 Imaging Unit. The survival of the explants exposed to the different treatments was determined after 54 days of culture. Culture media were changed weekly
Effect of explant density
Healthy 2.0-mm explants of CTB, TGR, and PUR were used in this experiment and prepared as above. The explants were placed in T25 flasks (n = 3) with 40, 20, and 10 mL of 1.0 mg L−1 AMPEP + PGR (PAA + Zeatin at 1 mg L−1) diluted in 0.22 μM-filtered sterile seawater to make explant densities of: (1) 1 explant per 2 mL of culture media (1:2), (2) 1 explant per 1 mL of culture media (1:1), and (3) 2 explants per 1 mL of culture media (2:1) respectively. The T25 flasks were incubated at 20°C and covered with black cloth for 7 days after which the explants were exposed to an irradiance of 10–15 μmol photons m−2 s−1, 13:11 h L:D cycle. The emergence of meristematic (dome) tissue and shoots was observed using an Olympus IX70 inverted microscope and Olympus DP12 Imaging Unit. Media changes were made weekly.
Statistical analysis
Results were analyzed statistically (one-way ANOVA, Tukey, and Duncan tests) at 1% level of significance using SPSS ver. 10 software.
Results
Optimization of AMPEP concentration
The use of different concentrations of AMPEP with or without PGR led to the development of shoots in six varieties of Kappaphycus. The earliest shoot emergence was observed to occur at low concentrations of AMPEP (e.g., 0.001–1.0 mg L−1) with PGR or at high concentrations of AMPEP (3–4 mg L−1) without PGRs (Table 1). Only the control treatment of PUR formed shoots after 78 days of culture in ESS/2.
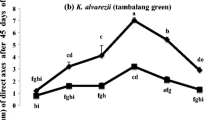
For TGR, explants cultured at all AMPEP concentrations with, or without PGR, produced the first shoots within the same time frame (i.e., 19 days post-culture). An optimum concentration of 3.0 mg L−1 AMPEP and 0.001 mg L−1 AMPEP + PGR was determined for TGR, based on the generated shoot length range (Fig. 1). Significant differences (P < 0.01) were observed between the lengths of shoots under different treatments. However, no definite pattern of response to AMPEP with, or without PGR, was observed.
Effects of pH and temperature
Various pH–temperature combinations elicited shoot growth of PUR, BRN, and GS. After 54 days of incubation, the highest number of shoots formed with the least mortality was pH 8.7 and pH 9.7 at 25°C for GS (11 = 46%), pH 6.7 at 25°C for PUR (15 = 63%) and pH 6.7 and 7.7 20°C for BRN (5 = 21%). All varieties responded positively to almost all pH–temperature combinations except GS, which did not produce shoots at any pH value at 20°C (Table 2).
Effects of explant density
Formation of Kappaphycus shoot primordia was observed as early as day 9 for TGR and PUR and after day 21 for tungawan CTB. The highest average number of new shoots formed was determined at 2:1 density of 1.0 mg L−1 AMPEP + PGR for both brown and purple color morphotypes (35% and 100%, respectively). The green morphotype produced the most number of shoots at 1:2 density of the same culture media (16.7%). Remarkably, no mortality was recorded in either brown or green morphotypes. Mortality was only observed in PUR cultures with the highest value of 53.3% at the 2:1 density. Significant differences (P < 0.01) were observed between the strains and volume of AMPEP:density treatments (Fig. 2).
Discussion
The development of tissue culture techniques was primarily aimed to establish the fundamentals of callus formation, morphogenesis, roles of different bio-active compounds and other parameters for the development and growth of micro-propagated macroalgae (Lawlor et al. 1989, Yokoya et al. 1996, 2002, 2004). With these concepts established, studies on seaweed tissue culture continued to develop the most economical culture media and optimized physico-chemical parameters for large-scale, commercial production of industrially important seaweeds such as Kappaphycus.
Recent studies successfully proved the use of the commercial Ascophyllum extract (AMPEP) as a main, culture media component suitable for the tissue culture of Kappaphycus varieties (Hurtado et al. 2009). Rapid shoot development was observed in explants cultured in AMPEP with or without PGR as compared to ESS/2 with PGR which is a tested and published tissue culture medium for seaweeds (Hurtado and Biter 2007). Furthermore, the present study showed that the use of AMPEP, without expensive and complicated culture media components, e.g., vitamin mix and metal components was sufficient to promote cell growth and development of seaweed explants.
In this study, shoots were successfully developed in all seven Kappaphycus varieties using AMPEP with or without PGR. However, the rate at which the explants responded and produced shoots in the AMPEP concentrations differed for each Kappaphycus variety. In general, earlier shoot development was observed at lower concentrations of AMPEP (0.001–1.0 mg L−1) with PGR, or at higher concentrations of AMPEP (3.0–5.0 mg L−1) without PGRs. PAA and zeatin as PGRs stimulate cell division and elongation and shoot formation. This showed that a small increase in the concentration of the seaweed extract used without the addition of PGR was enough to initiate shoot formation in Kappaphycus seaweeds, making AMPEP a practical and economically viable stand-alone culture medium for large-scale micro-propagation of the carrageenophyte.
Optimization of physical parameters has been described as essential to promote enhanced plant growth and development in bioreactor or large-scale production set-ups (Rorrer and Cheney 2004). These authors showed that macroalgal cells grew best in slightly alkaline seawater culture medium (pH 8–9) at 25°C, where dissolved CO2 speciates to carbonate (CO 2−3 ) and bicarbonate (HCO −3 ) ions essential for photosynthesis. As AMPEP (as used in this study) has an unbuffered pH of 10.0–10.5, pH–temperature optimization was conducted on each variety. The best combinations based on shoot growth and section survival were pH 8.7 at 25°C for GS; pH 6.7 at 25°C for PUR; and pH 7.7 at 20°C for BRN. These results indicated that the different varieties of Kappaphycus responded differently to changes in physico-chemical parameters and therefore optimum conditions need to be established for each variety independently. An increase in shoot formation was observed for green sacol as temperatures were increased at all pH values. This behavior may be correlated to the fact that the Kappaphycus sacol (GS) variety is commonly grown in shallow, coastal areas where seawater temperatures are slightly higher than in the deeper areas where different color morphotypes of K. alvarezii var. tambalang are grown. However, the trends for the two tambalang color morphotypes were contradictory, i.e., shoot formation increased in PUR as temperatures increased, whereas a decrease was observed in BRN. This may indicate some genetic divergence in the morphotypes. Further studies on this difference should be conducted. The occurrence of shoot formation in all three varieties, even as the culture medium became slightly acidic, indicated that favorable growth was still attainable even in these unconventional conditions. This bodes well for practical applications in carrageenophyte nurseries in Southeast Asia.
To maximize the uptake of available nutrients in the culture system, the optimum ratio of the number of seaweed explants to volume of culture media was investigated. An increase in shoot formation was observed in both CTB and PUR as more explants were in the system (i.e., 2:1 explants to media volume ratio). These observations support AMPEP culture medium as economical to use in large-scale production, as more seaweed explants may be accommodated and induced to produce shoots. However, the opposite trend was observed in TGR. This observation indicated that this variety may require more available culture media as compared to the other varieties tested. Mortality in the PUR trial may be attributed to poor quality and age of the explant. Furthermore, formation of shoots occurred at the same time for the different section densities of the different varieties. This observation indicates that the density of explants in the medium did not have an effect on the rate of shoot development.
The use of AMPEP as the main component of a tissue culture medium for commercial micro-propagation of carrageenophytes has been proven successful for different varieties and color morphotypes as used in this study. Optimized physico-chemical parameters such as media concentration, pH, temperature, and density of explants have led to an increase in rate and quantity of shoot formation. The results presented here suggest that improved tissue culture methods using AMPEP may be used for the commercial propagation of various Kappaphycus strains. Feasibility studies on the economic costs, impact, and return on investment of the technique will be pursued.
References
Bixler HJ, Porse H (2010) A decade of change in the seaweed hydrocolloids industry. J Appl Phycology doi:10.1007/s10811-010-9529-3
Bradley PM, Cheney DP, Saga N (1988) One step antibiotic disk method for obtaining axenic cultures of multicellular marine algae. Plant Cell Tissue Organ Cult 12:55–60
Chapman VJ, Chapman DJ (1980) Seaweeds and their uses, 4th edn. Chapman & Hall, London, pp 30–61
Craigie JS (2010) Seaweed extract stimuli in plant science and agriculture. J Appl Phycol doi:10.1007/s10811-010-9560-4
Craigie JS, MacKinnon SL, Walter JA (2008) Liquid seaweed extracts identified using 1H NMR profiles. J Appl Phycol 20:665–671
Crouch IJ (1990) The effect of seaweed concentrate on plant growth. PhD Thesis. University of Natal, South Africa
Dawes CJ, Koch EW (1991) Branch micropropagule and tissue culture of the red alga Eucheuma denticulatum and Kappaphycus alvarezii farmed in the Philippines. J Appl Phycol 6:21–24
Dawes CJ, Trono GC Jr, Lluisma AO (1993) Clonal propagation of Eucheuma denticulatum and Kappaphycus alvarezii for the Philippine seaweed farms. Hydrobiologia 260/261:379–383
Fan D, Hodges DM, Zhang J, Kirby CW, Ji X, Locke SJ, Critchley AT, Prithiviraj B (2010) Commercial extract of the brown seaweed Ascophyllum nnodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem 124:195–202
Featonby-Smith BC, van Staden J (1987) Effects of seaweed concentrate on grain yield in barley. S Afr J Bot 53:125–128
Hurtado AQ, Cheney DP (2003) Propagule production of Eucheuma denticulatum (Burman) Collins and Harvey by tissue culture. Bot Mar 46:338–341
Hurtado AQ, Biter AB (2007) Plantlet regeneration of Kappaphycus alvarezii var. adik-adik by tissue culture. J Appl Phycol 19:783–786
Hurtado AQ, Yunque DA, Tibubos KT, Critchley A (2009) Use of Acadian marine plant extract powder from Ascophyllum nodosum in tissue culture of Kappaphycus varieties. J Appl Phycol 21:633–639
Jayaraj J, Wan A, Rahman M, Punja ZK (2008) Seaweed extracts reduces foliar fungal disease on carrot. Crop Prot 27:1360–1366
Jayaraj J, Norrie J, Punja ZK (2010) Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J Appl Phycol doi:10.1007/s10811-010-9547-1
Lawlor HJ, McComb JA, Borowitzka MA (1989) Tissue culture of Ecklonia radiata (Phaophyceae, Laminariales): effects on growth of light, organic carbon source and vitamins. J Appl Phycol 11:105–112
Loureiro RR, Reis RP, Critchley AT (2009) In vitro cultivation of three Kappaphycus alvarezii (Rhodphyta, Areschougiaceae) variants (green, red and brown) exposed to a commercial extract of the brown alga Ascophyllum nodosum (Fucaceae, Ochrophyta). J Appl Phycol 22:101–104
Milton RF (1952) Improvements in or relating to horticultural and agricultural fertilizers. Br Patent 664:989
Rayorath P, Mundaya JN, Farid A, Wajahatullah K, Ravishankar P, Hankins SD, Critchley AT, Balakrishnan P (2008a) Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L) Heynh. J Appl Phycol 20:423–429
Rayorath P, Khan W, Palanisamy R, MacKinnon SL, Stefanova R, Hankins SD, Critchley AT, Prithiviraj B (2008b) Extracts of the brown seaweed Ascophyllum nodosum induce gibberellic acid (GA3)-independent amylase activity in barley. J Plant Growth Regul 27:370–379
Rorrer GL, Cheney DP (2004) Bioprocess engineering of cell and tissue cultures for marine seaweeds. Aquacult Eng 32:11–41
Stephenson WA (1974) Seaweed in agriculture and horticulture, 3rd edn. Barglya & Gylver Rateaver, CA
Yokoya NS, Handro W (1996) Effects of auxins and cytokinins on tissue culture of Grateloupia dichotoma (Gigartinales, Rhodophyta). Hydrobiologia 326/327:393–400
Yokoya NS, Handro W (2002) Effects of plant growth regulators and culture medium on morphogenesis of Solieria filiformis (Rhodophyta) cultured in vitro. J Appl Phycol 14:97–102
Yokoya NS, West JA, Luchi AE (2004) Effects of plant growth regulators on callus formation, growth and regeneration in axenic culture of Gracilaria tenuistipitata and Gracilaria perplexa (Gracilariales, Rhodophyta). Phycol Res 52:244–254
Acknowledgments
The SEAFDEC/AQD authors are thankful to the Government of Japan Trust Fund for partial financial assistance in the conduct of the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yunque, D.A.T., Tibubos, K.R., Hurtado, A.Q. et al. Optimization of culture conditions for tissue culture production of young plantlets of carrageenophyte Kappaphycus . J Appl Phycol 23, 433–438 (2011). https://doi.org/10.1007/s10811-010-9594-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-010-9594-7