Abstract
Undaria pinnatifida is grown for food and industrial materials worldwide; therefore, advanced breeding is needed to meet quality and productivity requirements. In this study, we examined regional lines of U. pinnatifida from five cultivation sites in Japan with different environmental conditions: Oga (OGA, the northern Sea of Japan coast), Hirota Bay (HRT, the northeastern Pacific coast), Matsushima Bay (MAT, the northeastern Pacific coast), Naruto (the Seto Inland Sea coast) and Shimonoseki (SIM, the southern Sea of Japan coast). The sporophytes of these lines were cultured in a tank culture system under controlled environmental conditions, and their morphological characteristics, nutrient uptake kinetics (V max, K s and V max/K s ), and carbon, nitrogen and phosphorus contents were determined. Sporophytes from MAT grew faster, whereas those from SIM were smaller than those from the other sites. Although the blade thickness of sporophytes cultivated in the sea significantly differs among cultivation sites in the previous study, there was no significant difference in blade thickness among the regional lines cultivated in the tank. Sporophytes from OGA had the greatest V max/K s values and significantly greater nitrogen contents than the other lines. Therefore, the morphological characteristics of MAT and SIM sporophytes, and the nutrient uptake kinetics of OGA sporophytes may have a genetic origin. This indicates that these lines may represent useful resources for selective breeding, with MAT sporophytes providing faster growth and OGA sporophytes being well-adapted to low-nutrient conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The majority of macroalgal species that are globally used as industrial resources are used because they contain abundant minerals, polysaccharides and antioxidant compounds, making them a valuable component of fertilisers, chemicals and animal feed (Holdt and Kraan 2011). Furthermore, macroalgae have recently been considered an ideal feedstock for biofuel production because they do not require arable land, fertiliser applications, or fresh water resources and therefore do not have an adverse impact on food supplies (Aitken and Antizar-Ladislao 2012). The inorganic nutrient uptake ability of macroalgae has also been utilised in ‘Integrated Multi-Trophic Aquaculture’, whereby the amount of waste generated by the fish aquaculture industry is reduced through the aquaculture of bivalves and seaweeds (Buschmann et al. 2001; Troell et al. 2003; Holdt and Edwards 2014). Therefore, macroalgae are an important resource that can contribute to increased food production both directly and indirectly.
Undaria pinnatifida (Wakame) is one of the major species of brown macroalgae cultivated in northeastern Asia; it is commonly used as edible foods (Yamanaka and Akiyama 1993). Although the industrial demand for U. pinnatifida has been increasing globally, its annual production in Japan has been declining (MAFF, Ministry of Agriculture, Forestry and Fisheries 2013). In the coast of Sanriku Region which is a major cultivation centre of U. pinnatifida in Japan, the production decreased from 49,872 t in 1999 to 22,358 t in 2014, and the price fluctuated from 89 Japanese Yen (JPY) kg−1 in 2001 to 239 JPY kg−1 in 2016 (Japan Fisheries Cooperative 2016). In addition, the aging of fishermen has been another major factor responsible for this reduction. For example, the number of fishermen has decreased by approximately 50% during the last 15 years, while the percentage of fishermen aged 65 years and older has increased from 23 to 35.5% (MAFF, Ministry of Agriculture, Forestry and Fisheries 2013). The variable yield, quality and raw material prices are considered to be major factors contributing to the reduction of the market share. Therefore, the use of new cultivars is required to facilitate the development of improved cultivation techniques.
In the macroalgae industry, breeding purposes differ between countries and regions and need to be assessed from the perspectives of fishermen, buyers, and customers. For instance, cultivars with early or late growth and higher yield variation would be useful for extending the harvesting periods, thereby avoiding a concentrated work requirement. In addition, nutrient depletion in early spring recently caused serious damage to sporophytes of U. pinnatifida, resulting in discoloration and reduced quality (Dan et al. 2015). Therefore, the introduction of a cultivar that is tolerant to low-nutrient conditions is also desired.
To improve macroalgal breeding, it is important to distinguish genetic differences from environmental differences between species (Kuckuck et al. 1985). Due to annual fluctuations in natural environmental conditions, it is difficult to determine whether the variation in the morphological characteristics of macroalgae is caused by genetic factors or environmental factors in macroalgae. It also remains unclear whether the characteristics of macroalgae species result from the adaptation or acclimatisation to the environment. In terms of breeding methods of U. pinnatifida, heterosis (Hara and Akiyama 1985) and selective breeding (Pang et al. 1997; Kato et al. 2010; Niwa 2015; Tanada et al. 2015; Niwa and Harada 2016; Shan et al. 2016) have been previously examined. Although the selection of parental sporophytes is important for obtaining a new cultivar with the desired characteristics, the current understanding of the genetic characteristics of regional cultivation lines of U. pinnatifida is limited. We previously developed a tank culture system named ‘circulation and floating culture system’ (CFCS), which allows macroalgae to be cultivated under controlled environmental conditions, including a constant water flow velocity (Sato et al. 2017). This system would therefore also be suitable for common garden experiments in order to understand the morphological and physiological characteristics of U. pinnatifida.
In the present study, we aimed to investigate whether morphological differences observed in elite lines of U. pinnatifida from five regions are caused by plasticity or inheritance. The morphological characteristics and nutrient uptake kinetics of U. pinnatifida sporophytes cultivated at six major aquaculture sites in Japan have been previously examined to improve understanding of the differentiation of these characteristics (Sato et al. 2016). We cultivated sporelings induced from the sporophylls of sporophytes cultivated as elite lines at five of these sites in the CFCS as common garden experiments to compare their morphological characteristics and nutrient uptake kinetics under the same environmental conditions. Faster growth and an adaptation to low-nutrient conditions in the fields were suggested to be predetermined by genetic characteristics; therefore, their applications in selective breeding are discussed.
Materials and methods
Algal sampling
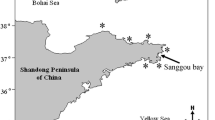
The following five cultivation sites that are major areas for industrial production of U. pinnatifida in Japan were selected on the basis of differences in their environmental conditions (Fig. 1): (1) Hirota Bay (HRT, the northeastern Pacific coast, 38° 98′ N, 141° 71′ E), Iwate Prefecture; (2) Matsushima Bay (MAT, the northeastern Pacific coast, 38° 32′ N, 141° 31′ E), Miyagi Prefecture; (3) Naruto (NAR, the Seto Inland Sea coast, 34° 20′ N, 134° 63′ E), Tokushima Prefecture; (4) Haedomari, Shimonoseki (SIM, the southern Sea of Japan coast, 33° 95′ N, 130° 88′ E), Yamaguchi Prefecture; and (5) the Unosaki Coast, Oga (OGA, the northern Sea of Japan coast, 39° 86′ N, 139° 80′ E), Akita Prefecture. At each cultivation site, an elite line screened from the wild population was used for open sea cultivation. Sporophyte cultivation of an elite line originating from each prefecture from October to December 2013 was conducted using the method described in Sato et al. (2016). The mature sporophytes were harvested from April to June 2014, and one average-sized individual was selected from each site.
Pre-culture
Zoospores were obtained from sporophylls in the laboratory, and male and female gametophytes were cultivated separately for 80 days. Pre-culture for sporophyte induction was performed according to the procedure described previously by Sato et al. (2017). In brief, one male and one female gametophyte were selected and homogenised at 5000 rpm for 2 min using a microblender (T 18 digital Ultra-Turrax; IKA, Japan). Further, to promote maturation, fragments of the gametophytes were then incubated under a 10-h light:14-h dark cycle at 50 μmol photons m−2 s−1 at 20 °C for 2 weeks. Next, germinated sporophytes were cultivated in 3-L Erlenmeyer flasks containing 2 L of quarter-strength PESI medium (Tatewaki 1966) with aeration under a 12-h light:12-h dark cycle at 90 μmol photons m−2 s−1 at 15 °C. The culture medium was changed at 3-day intervals. Once the total length of the sporophytes of each line reached 20 mm, they were sent to Iwate Fisheries Centre in Kamaishi City, Iwate Prefecture and cultivated in a 7-L aquarium with running seawater and aeration. In the present study, this period was defined as day 1 of cultivation.
Cultivation method using the tank system
After 23 days, the 30 longest individuals from each line were selected, tagged with a plastic plate on the holdfast for individual identification, and transferred to the CFCS (Sato et al. 2017) in a volume of 2000 L. The cultivation conditions in the CFCS comprised seawater at a temperature of 10 °C, illumination of approximately 200 μmol photons m−2 s−1, and natural photoperiods. The nutrient levels ranged from 5.2 to 8.0 μM of NO3 -−N, an undetectable level of NH4 +−N, and from 0.3 to 0.5 μM of PO4 −-P, which were the average concentrations at the Iwate Prefecture site during the U. pinnatifida cultivation period. The water velocity was regulated at 12–15 cm s−1 (Sato et al. 2017), which was also the same as that recorded at the U. pinnatifida cultivation site in Iwate Prefecture (Nanba et al. 2011). The water velocity in the CFCS was determined to be the sum of the horizontal and vertical velocities, measured using an electromagnetic current meter (Infinity-Em, JFE Advantec, Nishinomiya, Japan).
Morphological measurement
All sporophytes cultivated in the CFCS were collected approximately once every 15 days from day 23 of cultivation, and their total lengths and sporophyte weights were measured. The sporophytes were then returned to the CFCS, and the cultivation was continued until the next measurement interval. On day 133, the blade thickness was measured with an electronic calliper (NTD13, Mitsutoyo, Kawasaki, Japan) using the method previously described in Sato et al. (2016, 2017).
Measurement of nutrient uptake
On day 89, the five longest sporophytes of each regional line were collected and leaf discs (4.15 cm2) were excised using a cork borer. These were used to measure nutrient uptake rates and to estimate parameters using the methods of Sato et al. (2016). Discs from each regional line were pre-incubated in 1-L flasks containing aerated sterilised seawater at 10 °C and provided with a light intensity of 100 μmol photons m−2 s−1 and a 12-h light:12-h dark photoperiod for 48 h to allow recovery from the shock caused by cutting (Hurd and Dring 1990; Sakanishi and Iizumi 1998). The seawater used for pre-incubation was collected from the Sea of Japan by the Akita Prefectural Institute of Fisheries and contained natural nutrient concentrations that were close to the limits of detection. The seawater was changed at 12 h intervals.
Nutrient uptake was measured on the basis of difference between the initial and final concentrations in the vessels (Hurd and Dring 1990; Li et al. 2007, 2009). Each disc was transferred to a 200-mL flask containing 100 mL of sterilised and filtered (GF/F filters; Whatman, UK) seawater, while flasks that contained seawater without sporophyte discs were used as the control. NO3 −-N and NH4 +-N were added to the flasks as NaNO3 and NH4Cl, respectively, to provide six initial concentrations: 0.5, 2, 5, 10, 15, and 30 μmol L−1. The flasks were then incubated at 10 °C and shaken at 80 rpm on a multi-shaker (MMS-4010; Tokyo Rikakikai, Japan) under an irradiance of 180 μmol photons m−2 s−1 provided by white fluorescent lamps. The incubation time was 60 min because Dean and Hurd (2007) reported no change in the rates of nitrate or ammonium uptake by U. pinnatifida during the first 90 min in time-course experiments (0–3 h).
After 60 min of incubation, seawater samples were collected in plastic tubes and frozen until analysis. The blade discs were dried in a convection oven (NDO–600ND; Tokyo Rikakikai, Japan) at 80 °C for 48 h, following which their dry weights were measured. The uptake rates [V; μmol g−1 dry weight (dw) h−1] of NO3 −-N and NH4 +-N were calculated for each disc according to the difference in the concentration between the control and experimental flasks at each of the six nutrient levels. The nutrient concentrations were determined in an autoanalyser (QuATtro 2-HR; BLTEC, Japan). The seawater used in these experiments was also collected from the Sea of Japan by the Akita Prefectural Institute of Fisheries.
NO3 −-N and NH4 +-N uptake rates were plotted against the mean nutrient concentrations in the control flasks (S, μM) for the five discs from each of the regional lines. A rectangular hyperbola was then fitted to each plot for each line. The curves were fitted using a least-squares regression procedure with JMP software (SAS Institute, USA). The Michaelis–Menten parameter for each individual was also calculated using the following equation:
where V (μmol g−1 dw h−1) is the uptake rate, V max (μmol g−1 dw h−1) is the maximum uptake rate at the saturating concentration, S (μmol L−1) is the saturating concentration and K s (μmol L−1) is the substrate concentration at V max/2. V max, K s and V max/K s are effective indicators of nutrient uptake dynamics that may be used to make cross-comparisons between populations (Harrison et al. 1989). High V max and K s values are indicative of rapid nutrient uptake at high concentrations (Harrison and Hurd 2001), low K s values are indicative of a high affinity at low nutrient concentrations (Hurd et al. 2014) and high V max/K s values are indicative of an enhanced uptake capability at low nutrient concentrations (Healey 1980; Bracken et al. 2011; Bracken and Williams 2013, Hurd et al. 2014).
Measurement of carbon (C), nitrogen (N) and phosphorus (P) contents
For measurement of tissue contents of C, N, and P, the five longest (total length) sporophytes were selected from each regional line on day 89 of cultivation. Using a 2.3-cm-diameter cork borer, one disc (4.15 cm2) per sporophyte was punched from the mid-portion of each divided blade at the point of the maximum width. The discs were dried at 80 °C for 48 h, ground and weighed to determine their dry weights. After weighing, the C and N contents were determined using a CHN analyser (Flash 2000 with Eager Xperience v.1.02 software; Thermo Fisher Scientific, USA). The total P content was analysed according to Menzel and Corwin (1965); the P in a dried sample was extracted by wet persulfate digestion in an autoclave (120 °C, 30 min), and solutions with ammonium molybdate and ascorbic acid were added, followed by measurement of absorbance at a wavelength of 880 nm using a microplate reader (Infinit F200 with I-control software, Tecan, Switzerland).
Statistical analyses
Differences in the total lengths and sporophyte weights between cultivation periods and regional sites, and differences in the blade thickness, C, N, and P contents, and nutrient uptake kinetic parameters (V max, K s and V max/K s ) for NH4 +-N and NO3 −-N between regional lines, were analysed using Kruskal–Wallis test followed by post hoc Scheffe’s test for multiple comparison test (SPSS Statistics v. 23, IBM, USA). A nonparametric procedure was used because not all of the data were normally distributed or homoscedastic.
Results
Morphological characteristics
When U. pinnatifida sporophytes of five regional lines were cultivated in the CFCS, MAT and SIM sporophytes eroded during the cultivation and their morphological characteristics could not be measured on day 152. There were significant differences in the total length between the cultivation periods of all regional lines (Fig. 2a, b; p < 0.01; Supplemental Table 1). The total length reached its maximum on days 133–152 in all lines except for MAT sporophytes, in which the maximum length was reached on day 89, following which the length significantly decreased and the weight remained stable until day 133 (Fig. 2a, b; Supplemental Table 1). In addition, there were also significant differences in the total length between the regional lines in all the cultivation periods (Fig. 2a, b; p < 0.01; Supplemental Table 2). The total length of MAT sporophytes was significantly higher than for the other lines from day 41 to 89 (p < 0.01; Supplemental Table 2), with the exception of HRT sporophytes on day 68 (p > 0.05; Supplemental Table 2), and the total length of SIM sporophytes was significantly smaller than that of the other lines on days 89 and 111 (p < 0.05; Supplemental Table 2), with the exception of OGA sporophytes on day 89 (p > 0.05) and HRT sporophytes on day 111 (p > 0.05; Supplemental Table 2). The total lengths of NAR and OGA sporophytes on day 152 were significantly higher than those for HRT sporophytes (p < 0.05; Supplemental Table 2).
Changes in the total length (a) and sporophyte weight (b) of Undaria pinnatifida that originated from five regional sites and was cultivated in a circulation and floating culture system. Values are means ± standard error; n = 30 individuals. HRT Hirota Bay, MAT Matsushima Bay, NAR Naruto, OGA Oga, SIM Shimonoseki
There were significant differences in sporophyte weight between cultivation periods of all the regional lines (Fig. 2a, b; p < 0.01; Supplemental Table 1). Similar to the total length, the sporophyte weight reached the maximum value on days 133–152 in all lines except MAT sporophytes, which reached a maximum on day 89, following which the length significantly decreased and the weight remained stable until day 133 (Fig. 2a, b; Supplemental Table 1). There were also significant differences in sporophyte weight between the regional lines in all the cultivation periods (Fig. 2a, b; p < 0.05; Supplemental Table 2). The sporophyte weight was significantly higher in MAT sporophytes than in the other lines on days 41 and 89 (p < 0.01; Supplemental Table 2), reaching a value approximately twice as high as that of the weight (64.2 vs. 20.9–37.3 g). In addition, the total sporophyte weights of NAR and OGA sporophytes on day 152 were significantly higher than those for HRT sporophytes (p < 0.05; Supplemental Table 2). The blade thickness of the five regional lines ranged from 0.18 to 0.25 mm on day 133 (Fig. 3). This difference was significant according to the Kruskal–Wallis test (p < 0.05; Supplemental Table 3), but not according to the multiple comparison test (p > 0.05; Supplemental Table 3). The blade of SIM sporophytes had a harder texture and a more undulated surface with wrinkles than other lines. Photographs of the five lines cultivated in the CFCS for 89 days are shown in Fig. 4. These results demonstrate that the SIM sporophytes were smaller than those of the other lines.
Nutrient uptake kinetics
The nutrient uptake kinetic parameters for NO3 −–N and NH4 +–N of the leaf discs from the five regional lines cultivated in the CFCS are shown in Fig. 5. All of these parameters significantly differed among regional lines (p < 0.05; Supplemental Table 4), with the exception of the V max of NO3 −–N (p > 0.05; Supplemental Table 4). The V max for NH4 +–N in SIM sporophytes was significantly higher (212.2 μmol g−1 dw h−1) than the other regional lines (p < 0.05; Supplemental Table 4). The K s values for NO3 −–N and NH4 +–N uptake were also not significantly different according to the multiple comparison test (p > 0.05; Supplemental Table 4). However, the V max/K s ratio for NO3 −–N in OGA sporophytes was 6.33, which was significantly higher than all other regional lines (p < 0.01; Supplemental Table 4), except MAT sporophytes (p > 0.05; Supplemental Table 4). The V max/K s ratio of NH4 +–N for MAT sporophytes was 10.89, which was significantly higher than the other regional lines (p < 0.05; Supplemental Table 4).
Kinetic parameters for nitrate and ammonium uptake on day 89 in Undaria pinnatifida individuals that originated from five regional sites and were cultivated in a circulation and floating culture system. V max maximum uptake rate, K s half-saturation concentration, V max /K s nutrient uptake efficiency. Values are means ± standard error; n = 5 individuals. Different letters indicate significant differences (p < 0.05) between regional lines. dw dry weight, HRT Hirota Bay, MAT Matsushima Bay, NAR Naruto, OGA Oga, SIM: Shimonoseki
C, N and P contents
There was no significant difference in the C content of sporophytes among the regional lines (p > 0.05; Supplemental Table 5), and the all lines had values within the range of 0.31–0.34 μg mg−1 dw (Fig. 6a). In contrast, the N content of OGA sporophytes was higher than the other lines (p < 0.05; Supplemental Table 5), reaching a maximum value of 0.037 μg mg−1 dw, compared with a minimum value of 0.028 μg mg−1 dw for HRT sporophytes (Fig. 6b). The C/N ratio of OGA sporophytes was significantly lower than that of HRT and MAT sporophytes (p < 0.05; Fig. 6c and Supplemental Table 5). There was no significant difference in the P content and N/P ratios of the sporophytes between the regional lines, which ranged from 2.74 to 3.6 μg mg−1 dw and from 8.5 to 12.24 μg−1 mg dw, respectively (Fig. 6d, e).
Carbon (a), nitrogen (b), and phosphorus (d) content, and C/N (c) and N/P (e) ratios on day 89 in Undaria pinnatifida sporophytes that originated from five regional sites and were cultivated in a circulation and floating culture system. Values are means ± standard error; n = 5 individuals. Different letters indicate significant differences (p < 0.05) between regional lines. HRT Hirota Bay, MAT Matsushima Bay, NAR Naruto, OGA Oga, SIM Shimonoseki
Discussion
Morphological characteristics
Five regional lines of U. pinnatifida were cultivated under the same environmental conditions in the CFCS, and significant differences were observed in their total lengths and sporophyte weights (Fig. 2, Supplemental Table 2). The sporophytes of MAT were significantly larger than those of the other regional lines during the early growing period, and their total length and sporophyte weight reached the maximum values on day 89, which was the earliest among the lines. The parent plant of this line was collected from Kinkazan (38° 30′ N, 141° 55′ E), the southern limit of the perturbed area between approximately lat. 38° and 41°. This area lies between the Oyashio and Kuroshio Currents and the axis of the Kuroshio Extension, where steep temperature and salinity gradients prevail (Kawai 1972; Takemoto and Oda 1997; Fig. 1). Therefore, it is likely that the early growth of MAT sporophytes is an adaptation for survival in these unstable environmental conditions, allowing reallocation of resources from the blade to the sporophylls (Skriptsova et al. 2004) in order to enable earlier sporophyll development (Sato et al. 2016). In contrast, on days 89 and 111, the SIM sporophytes cultivated in the CFCS were significantly smaller than those of the other regional lines (Figs. 1 and 4; Supplemental Table 2). The same characteristics were observed in sporophytes cultivated in the open sea, the sporophyte size of SIM was obviously smaller than other lines (Sato et al. 2016; Supplemental Fig. 1). Furthermore, they also had considerably different morphological features, such as a hard texture of the blade and an undulated surface with wrinkles, which are similar to those of the sporophytes collected from the Kagoshima Prefecture, south of Kyushu (Tanada et al. 2015). Therefore, the morphological characteristics of the SIM line also appear to be genetically determined.
There were no significant differences in blade thickness among the five regional lines cultivated under the same water velocity in the CFCS (Fig. 3). The morphological features and biomass of U. pinnatifida are strongly controlled by the hydrodynamic conditions at the cultivation site (Nanba et al. 2011; Peteiro and Freire 2011). This is evidenced by the higher blade thickness of sporophytes cultivated in a commercial cultivation area exposed to wave action than that of sporophytes in an uncultivated area protected from wave action (Nanba et al. 2011). Therefore, we concluded that the differences in blade thickness among regional lines in open sea cultivation were caused by morphological plasticity depending on the wave motion conditions. Because blade thickness is one of the major quality indicators of U. pinnatifida (Saito 1972; Ishikawa 1991; Nanba et al. 2011; Sato 2015), the selection of cultivation sites on the basis of the hydrodynamic environment is important for the production of higher quality sporophytes.
Nutrient uptake kinetics and C, N, and P contents
There were significant differences in the nutrient uptake kinetics of U. pinnatifida sporophytes among the regional lines (Fig. 5). The high V max/K s ratio of NO3 −–N for OGA sporophytes is likely to be an adaptation to low nutrient concentrations because this site is located in the Sea of Japan, which is influenced by the nutrient-poor Tsushima warm current. In contrast, the high V max/K s ratio for NH4 +–N at MAT is likely to be an adaptation to the unstable hydrodynamic conditions in this region. The V max value for NH4 +–N of SIM sporophytes was 212.2 μmol g−1 dw h−1 , which is even higher than the values reported for U. pinnatifida grown in Nuevo Gulf, Argentina (Torres et al. 2004), and Otago Harbour, southern New Zealand (Dean and Hurd 2007).
The C and N contents of U. pinnatifida have been shown to significantly vary between growing periods due to changes in environmental factors (Dean and Hurd 2007). In the present study, there was no significant difference in the C content between regional lines, the same result was observed in sporophytes cultivated in the sea (Sato et al. 2016). However, the N content of OGA sporophytes was significantly higher than that of sporophytes of the other regional lines cultivated with the same levels of nutrients during the same growing period, likely due to the observed differences in NO3 −–N uptake kinetics in this line. Although the N content was significantly different among the regional lines, all values of the C/N ratios were less than 20, the borderline for N limitation for U. pinnatifida growth (Dean and Hurd 2007). Wheeler and Björnsater (1992) developed mean indices for nutrient limitation using tissue N/P ratios, whereby N/P greater than 12 ± 4 indicates N limitation and N/P greater than 17 ± 6 indicates P limitation. Based on these indices, the N/P ratio in the present study indicates a borderline level of N and P limitation. Therefore, nutrient concentrations in the CFCS (5.2–8.0 μM of NO3 -−N and 0.3–0.5 μM of PO4 –-P) were not the limiting factor in the growth of each regional line of U. pinnatifida.
Genetic differentiation
Sato et al. (2016) examined the morphological characteristics of U. pinnatifida sporophytes cultivated on six industrial farms across Japan and observed significant differences among sites. In particular, they found until February that the sporophytes of MAT were significantly larger than those of the other sites, suggesting that the combination of low seawater temperatures and high nutrient concentrations in the period from autumn to winter promoted the growth of sporophytes. During U. pinnatifida cultivation, NO3 -−N fertilisation of sporelings and juvenile sporophytes promotes the earlier growth of sporophytes and sporophylls compared with that of controls (Gao et al. 2013a). The production of Saccharina japonica is influenced by hydrographic conditions; the yield is positively correlated with lower temperature and higher concentrations of dissolved inorganic N (Nishida 1999). A similar tendency has been reported in Saccharina religiosa (Yoshimori et al. 1998). Surprisingly, we observed that MAT sporophytes that were cultivated in the CFCS also exhibited significantly higher growth than the other regional lines, although seawater temperature and nutrients concentration were different between the CFCS and cultivation sites in the sea. These results indicate that the growth of MAT was faster than other lines in the different condition promoting growth of U. pinnatifida (lower temperature and higher nutrients concentration). Therefore, it was suggested that the MAT line has a characteristic of higher growth at an early growing stage independent of environmental conditions. SIM sporophytes were significantly smaller than the other regional lines and had the same characteristics as sporophytes collected from the cultivation site (Fig. 4; Sato et al. 2016). Notably, the sporophytes of U. pinnatifida collected from the southern sites of Japan were smaller and wider than those collected from the northern sites (Kusaka et al. 2007; Tanada et al. 2015). Therefore, the morphological features of SIM sporophytes may also be genetically determined. Although the blade thickness of sporophytes cultivated in the sea significantly differs among natural cultivation sites, there was no significant difference in blade thickness among the regional lines cultivated in the CFCS (Fig. 3), so far the thickness of HRT blades being approximately twice of that of OGA blades in the previous experiments (Sato et al. 2016). Therefore, the thickness of U. pinnatifida sporophytes appears to be an environmental and plasticity characteristic rather than genetically determined.
Sato et al. (2016) argued that the NO3 −-N and NH4 +-N uptake kinetics of sporophytes of U. pinnatifida indicated that separate ecotypes exist in different cultivation sites in Japan. They proposed that the high values of V max at Akashi and Naruto, which are located in the Seto Inland Sea, are adaptations to periodically occurring high levels of nutrients, whereas the high V max/K s ratios of NO3 −-N at OGA, which is located in the Sea of Japan, are an adaptation to low nutrient concentrations. Similarly, although the values of V max and K s of sporophytes of NAR were not significantly different from those of sporophytes of the other regional lines investigated in the present study, the V max/K s ratio of OGA sporophytes was significantly higher (Fig. 5; Supplemental Table 4), with values similar to those of the sporophytes cultivated in the sea (Sato et al. 2016). Therefore, it appears that the adaptation of sporophytes of OGA to low nutrient concentrations is a genetic rather than an environmental characteristic.
Implications for breeding programmes
The Sanriku Region (Iwate and Miyagi Prefectures) is the largest U. pinnatifida production area in Japan. Many studies have been reported for developing a breeding programme in this region (Hara and Akiyama 1985; Ishikawa 1991; Kusaka et al. 2007; Saigusa et al. 2009; Gao et al. 2013b); however, the elite cultivars available for use on an industrial scale are insufficient. Based on the genetic differentiation identified in this study, MAT sporophytes could be used to develop an early growth cultivar. Based on an industrial cultivation trial with F4 and F5 populations, Shan et al. (2016) previously developed an elite cultivar named Haibao No. 1 through gametophyte clone crossing and consecutive selection; the yield of this cultivar exceeded that of the control by 40%. Therefore, as prerequisites for the development of an early growth cultivar suitable for the Sanriku Region in northeastern Japan, MAT sporophytes originating in Kinkazan should be used as the mother sporophyte, and trials using the gametophyte clone crossing method (Shan et al. 2016) will be required. The OGA sporophytes were found to have a high affinity for nutrients at low concentrations, which also appeared to be genetically determined. In recent years, the price and quality of U. pinnatifida in Japan have decreased after early spring due to its discolouration as a result of a reduction in the levels of seawater nutrients (Dan et al. 2015). Therefore, OGA sporophytes could be used for the development of a cultivar that is tolerant to low nutrient concentrations, helping to overcome this issue. An understanding of the genetic characteristics of every regional line or each cultivar will contribute to the development of an improved breeding technique that allows a reduction in the duration of the breeding period and the production of higher quality cultivars. This study demonstrates that the use of CFCS for cultivation will facilitate the advancement of the development of new cultivars.
References
Aitken D, Antizar-Ladislao B (2012) Achieving a green solution: limitation and focus points sustainable algal fuels. Energies 5:1613–1647
Bracken MES, Williams SL (2013) Realistic changes in seaweed biodiversity affect multiple ecosystem functions on a rocky shore. Ecology 94:1944–1954
Bracken MES, Jones E, Williams SL (2011) Herbivores, tidal elevation, and species richness simultaneously mediate nitrate uptake by seaweed assemblages. Ecology 92:1083–1093
Buschmann AH, Troell M, Kautsky N (2001) Integrated algal farming: a review. Cah Biol Mar 42:83–90
Dan A, Ohno M, Matsuoka M (2015) Changes of the research and development on the resources of Undaria and Laminaria in the culture ground of Tokushima coasts. Bull Tokushima Pref Fish Res Inst 10:25–48 (Japanese with English abstract)
Dean PR, Hurd CL (2007) Seasonal growth, erosion rates, and nitrogen and photosynthetic ecophysiology of Undaria pinnatifida (Heterokontophyta) in southern New Zealand. J Phycol 43:1138–1148
Gao X, Agatsuma Y, Taniguchi K (2013a) Effect of nitrate fertilization of gametophytes of the kelp Undaria pinnatifida on growth and maturation of the sporophytes cultivated in Matsushima Bay, northern Honshu, Japan. Aquacult Int 21:53–64
Gao X, Endo H, Taniguchi K, Agatsuma Y (2013b) Genetic differentiation of high-temperature tolerance in the kelp Undaria pinnatifida sporophytes from geographically separated populations along the Pacific coast of Japan. J Appl Phycol 25:567–574
Hara M, Akiyama K (1985) Heterosis in growth of Undaria pinnatifida (Harvey) Suringar. Bull Tohoku Reg Fish Res Lab 47:47–50
Harrison PJ, Hurd CL (2001) Nutrient physiology of seaweeds: application of concepts to aquaculture. Cah Biol Mar 42:71–82
Harrison PJ, Parslow JS, Conway HL (1989) Determination of nutrient uptake kinetic parameters: a comparison of method. Mar Ecol Prog Ser 52:301–312
Healey FP (1980) Slope of the Monod equation as an indicator of advantage in nutrient competition. Microbial Ecol 5:281–286
Holdt SL, Edwards MD (2014) Cost-effective IMTA: a comparison of the production efficiencies of mussels and seaweed. J Appl Phycol 26:933–945
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23:543–597
Hurd CL, Dring MJ (1990) Phosphate uptake by intertidal fucoid algae in relation to zonation and season. Mar Biol 107:281–289
Hurd CL, Harrison PJ, Bischof K, Lobban CS (2014) Seaweed ecology and physiology, second edn. Cambridge University Press, Cambridge, p 551
Ishikawa Y (1991) Analysis of quantitative traits in cultured Wakame for breeding. Fish Genet Breed Sci 16:19–24 (in Japanese)
Japan Fisheries Cooperative (2016) Annual report of Wakame in the Sanriku region
Kato S, Sumitomo T, Dan A (2010) Cultivar improvement of Undaria pinnatifida by crossing gametophytes originated from one zoospore. Algal Resour 3:205–210 (in Japanese with English abstract)
Kawai H (1972) Hydrography of the Kuroshio extension. In: Stommel H, Yoshida K (eds) Kuroshio, physical aspects of the Japan current. University of Washington Press, Seattle, pp 235–352
Kuckuck H, Kobabe H, Gerhard W (1985) Fundamentals of plant breeding. Springer, Berlin, p 236
Kusaka K, Sasaki R, Tsukada T (2007) Growth and morphological characteristics of native Wakame (Undaria pinnatifida) seed collected from eight locations around Japan coast. Miyagi Pref Rep Fish Sci 7:17–28 (in Japanese)
Li JY, Murauchi Y, Ichinomiya M, Agatsuma Y, Taniguchi K (2007) Seasonal changes in photosynthesis and nutrient uptake in Laminaria japonica (Laminariaceae: Phaeophyta). Aquac Sci 55:587–597
Li JY, Agatsuma Y, Nagai T, Sato Y, Taniguchi K (2009) Differences in resource storage pattern between Laminaria longissima and Laminaria diabolica (Laminariaceae; Phaeophyta) reflecting their morphological characteristics. J Appl Phycol 21:215–224
MAFF (Ministry of Agriculture, Forestry and Fisheries) (2013) Annual report of Ministry of Agriculture, Forestry and Fisheries 2013 annual report of catch statistics on fishery and aquaculture. Ministry of Agriculture, Forestry and Fisheries, Tokyo
Menzel DW, Corwin N (1965) The measurement of total phosphorus in seawater based on the liberation of organically bound fractions by persulfate oxidation. Limnol Oceanogr 10:280–282
Nanba N, Fujiwara T, Kuwano K, Ishikawa Y, Ogawa H, Kado R (2011) Effect of water flow velocity on growth and morphology of cultured Undaria pinnatifida sporophytes (Laminariales, Phaeophyceae) in Okirai Bay on the Sanriku coast, northeast Japan. J Appl Phycol 23:1023–1030
Nishida Y (1999) Relation between hydrographic conditions and harvest of konbu kelps. In: Taniguchi K (ed) The ecological mechanism of “Isoyake” and marine afforestation. Kouseisha Kouseikaku, Tokyo, pp 50–61 (in Japanese)>
Niwa K (2015) Experimental cultivation of Undaria pinnatifida for double cropping in Pyropia farms around Akashi Strait, Hyogo prefecture. Jpn J Phycol 63:90–97 (in Japanese with English abstract)
Niwa K, Harada K (2016) Experiment on forcing cultivation of Undaria pinnatifida sporophytes in the Seto Inland Sea by using free-living gametophytes cultured in laboratory. Jpn J Phycol 64:10–18 (in Japanese with English abstract)
Pang SJ, Hu XY, Wu CY, Hirosawa A, Ohno M (1997) Intraspecific crossing of Undaria pinnatifida (Harv.) Sur. – a possible time-saving way of strain selection. Chin J Oceanol Limnol 15:227–235
Peteiro C, Freire O (2011) Effect of water motion on the cultivation of the commercial seaweed Undaria pinnatifida in a coastal bay of Galicia, Northwest Spain. Aquaculture 314:269–276
Saigusa M, Kumano Y, Tsukada T (2009) Growth and morphological characteristics of the six generations of Wakame Undaria pinnatifida from Tsushima cultivated in Kesennuma Bay. Miyagi Pref Rep Fish Sci 9:25–32 (in Japanese)
Saito Y (1972) On the effect of environmental factors on morphological characteristics of Undaria pinnatifida and the breeding of hybrids in the genus Undaria. In: Abbott IA, Kurogi M (eds) Contributions to the systematics of benthic marine algae of the North Pacific. Japanese Society of Phycology, Kobe, pp 117–132
Sakanishi Y, Iizumi H (1998) Photosynthesis–temperature relationship of Laminaria longissima Miyabe (Laminariales, Phaeophyta). Jpn J Phycol 46:105–110 (in Japanese with English abstract)
Sato J (2015) Wakame Nyumon. The Japan Food Journal, Tokyo, p 164 (in Japanese)
Sato Y, Hirano T, Niwa K, Suzuki T, Fukunishi N, Abe T, Kawano S (2016) Phenotypic differentiation in the morphology and nutrient uptake kinetics among Undaria pinnatifida cultivated at six sites in Japan. J Appl Phycol 28:3447–3458
Sato Y, Yamaguchi M, Hirano T, Fukunishi N, Abe T, Kawano S (2017) Effect of water velocity on Undaria pinnatifida and Saccharina japonica growth in a novel tank system designed for macroalgae cultivation. J Appl Phycol 29:1429–1436
Shan TF, Pang SJ, Li J, Gao SQ (2016) Breeding of an elite cultivar Haibao no.1 of Undaria pinnatifida (Phaeophyceae) through gametophyte clone crossing and consecutive selection. J Appl Phycol 28:2419–2426
Skriptsova A, Khomenko V, Isakov V (2004) Seasonal changes in growth rate, morphology and alginate content in Undaria pinnatifida at the northern limit in the sea of Japan (Russia). J Appl Phycol 16:17–21
Takemoto A, Oda M (1997) New Planktic Foraminiferal transfer functions for the Kuroshio : Oyashio current region off Japan. Paleontol Res 1:291–310
Tanada N, Dan A, Kato S, Oka N, Hamano T (2015) Cultivar improvement of Undaria pinnatifida (Harvey) Suringar by reciprocal crossing free-living male/female gametophytes between a Kagoshima natural strain and a Naruto cultivar. Algal Resour 8:103–112 (in Japanese with English abstract)
Tatewaki M (1966) Formation of a crustose sporophyte with unilocular sporangia in Scytosiphon lomentaria. Phycologia 6:62–66
Torres AI, Gil MN, Esteves JL (2004) Nutrient uptake rates by the alien alga Undaria pinnatifida (Phaeophyta) (Nuevo gulf, Patagonia, Argentina) when exposed to dilute sewage effluent. Hydrobiologia 520:1–6
Troell M, Halling C, Neori A, Chopin T, Buschmann AH, Kautsky N, Yarish C (2003) Integrated mariculture: asking the right questions. Aquaculture 226:69–90
Wheeler PA, Björnsater BR (1992) Seasonal fluctuations in tissue nitrogen, phosphorus, and N:P for five macroalgal species common to the Pacific northwest coast. J Phycol 28:1–6
Yamanaka R, Akiyama K (1993) Cultivation and utilization of Undaria pinnatifida (wakame) as food. J Appl Phycol 5:249–253
Yoshimori A, Kono T, Iizumi H (1998) Mathematical models of population dynamics of the kelp Laminaria religiosa, with emphasis on temperature dependence. Fish Oceanogr 7:136–146
Acknowledgements
We sincerely thank Haruo Hosoya (Riken Vitamine Co., Ltd.), and Hironobu Watanabe, Katsunori Ono and Junichi Sato (Riken Food Co., Ltd.) for their guidance throughout this research. We are very grateful to Osamu Sakuma, Tatsuya Musashi and Takahiro Saido (Iwate Prefectural Fisheries Technology Centre), Masaki Yamaguchi (Coastal Regional Development Bureau, Department of Fisheries in Iwate Prefecture), Susumu Ohwada and Osamu Hatakeyama (Hirota Bay Fisheries Cooperative), Hiroshi Akama (Seafoods Akama Co., Ltd.), Akihito Tojyo (Tojyo Shouten), Kenji Yoshitake (Yamaguchi Prefecture Fisheries Cooperative), Nobuyuki Hosoda (Haedomari Fisheries Cooperative), Kazuyoshi Saito and Dr. Nobuyasu Nakabayashi (Akita Fisheries Centre), and Wataru Suzuki (Wataru Suisan) for providing the sporophytes of cultivated Undaria pinnatifida and cultivated Undaria, and for their generous support during the field surveys. We also thank Dr. Nobuyasu Nanba and Misaki Shinozuka (Kitasato University) and Dr. Kinuko Ito (Tohoku University) for their valuable advice and cooperation in the measurement of nutrients concentration, and carbon and nitrogen contents. We thank Yutaka Ito, Miho Mogamiya and Naoko Sasaki (Riken Food Co., Ltd.) for their technical support in measuring the morphological characteristics and cultivation in the CFCS. This study was partly supported by the Formation of Tohoku Marine Science Centre Project (Technical Development That Leads to the Creation of New Industries) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
......
Supplemental Fig. 1
Typical Undaria pinnatifida individuals cultivated at each site for 140–150 days according to the methods described in Sato et al. (2016). HRT: Hirota Bay, MAT: Matsushima Bay, NAR: Naruto, OGA: Oga, SIM: Shimonoseki (PPTX 141 kb)
Supplemental Table 1
(XLSX 37 kb)
Supplemental Table 2
(XLSX 38 kb)
Supplemental Table 3
(XLSX 34 kb)
Supplemental Table 4
(XLSX 35 kb)
Supplemental Table 5
(XLSX 36 kb)
Rights and permissions
About this article
Cite this article
Sato, Y., Hirano, T., Ichida, H. et al. Morphological and physiological differences among cultivation lines of Undaria pinnatifida in a common garden experiment using a tank culture system. J Appl Phycol 29, 2287–2295 (2017). https://doi.org/10.1007/s10811-017-1170-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1170-y