Abstract
This paper compares the biofilter capacity and cost-effectiveness of blue mussels (Mytilus edulis) and seaweed for use in integrated multi-trophic aquaculture (IMTA) based on experiences in Ireland and Denmark. This comparison shows that weight for weight, mussels are a better biofilter than seaweed with regard to the amount of nitrogen assimilated. Furthermore, in optimized systems, areal requirement for mussels is similar to the cultivation of the same tonnage (1,000 t) of seaweed (approximately 8 ha). The cost-effectiveness of a mussel biofilter is €11–30 kg−1 nitrogen (N) removed based on various examples compared to production costs of €209–672 removed and €1,013 kg−1 N removed, respectively, for Laminaria digitata and Alaria esculenta from extrapolated laboratory and field trials. However, commercial seaweed (Saccharina latissima) producers claim that production costs are less than €10–38 kg−1 N removed. These up-scaled and commercial figures make the seaweed cost competitive to mussels for removal of nitrogen. Disadvantages such as predators (e.g. eider ducks) and biofouling should also be taken into account before choice of biofilter is made. These drawbacks can reduce overall biofilter capacity and biomass value as a consequence of biomass spoilage or loss. However, disadvantages may be mitigated by seasonal choice of cultivation and harvest times. Cultivation technologies and harvesting methods may be improved together with breeding to improve the cost-efficiency of the biofilter, especially in the newer European seaweed cultivation. Furthermore, upscaling of IMTA to commercial proportions, other than the Danish example, would allow more real data on production costs and revenues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Western countries, open sea aquaculture is generally performed as a monoculture, with few or no methods to ensure that such an intensive activity has the minimal impact on the marine environment. The production of particulate organic matter (POM) and its effect on the surrounding benthos around and below fish farms has previously been the focus of much debate (Chopin et al. 2001). However, the focus has changed to the production of dissolved inorganic nitrogen (DIN), due to the worldwide phenomenon of nutrient enrichment and eutrophication in coastal waters (Chopin et al. 2001).
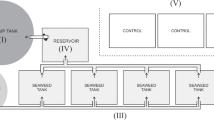
One method that has been proposed to mitigate the effects of nutrient enrichment from aquaculture systems is integrated multi-trophic aquaculture (IMTA). This is accomplished by integrating the production of fed aquaculture species (e.g. fish or shrimp) with extractive organisms (e.g. shellfish, seaweeds, echinoderms, polychaetes, bacteria etc.) whereby extractive and harvested species utilize the particulate and dissolved waste products from the fed organisms (Fig. 1). This combination culture of species occupying different trophic levels attempts to create an innovative, effective and responsible method of aquaculture which maintains the health of the coastal waters (Chopin et al. 2001, 2008; Barrington et al. 2009; MacDonald et al. 2013). The phototrophic and herbivorous conversion of fish waste increases nutrient retention in the culture system (e.g. 20–42 and 29–45 % feed nitrogen (N), respectively), whereas bacteria or worm biomass contributes to much smaller conversions of nutrients (e.g. 7 or 0.06 % feed N, respectively) to the increased overall nutrient retention. However, bacteria and detritivores are rarely integrated into intensive aquaculture systems, and their potential might be underestimated (Schneider et al. 2005).
IMTA is actually a method that has been practised through trial and error for centuries in Asia, while superficially, it appears to be a new concept introduced into Western countries from the 1970s onwards (Chopin et al. 2008; Barrington et al. 2009).
The relationship between the release of nutrients from the fish farms and the potential negative impacts is recognized by environmental protection agencies/departments in many countries. For example, in Denmark, the amount of fish that aquaculture producers are allowed to grow is directly related to a certain limit or quota of released phosphorous (P) and in particular nitrogen (N). This cap on released nutrients is a major limiting factor for the industry; hence, the Danish marine aquaculture enterprises were unable to expand their production of fish for 20 years since 1987 (Holdt et al. 2006; Fig. 2), despite the increasing global demand for aquaculture products (FAO 2011). Between 2007 and 2011, Danish marine fish production increased by 53 % up to almost 11,000 t, but this is far from the goal of the action plans (Fig. 2; Danish Aquaculture Statistics 2013a). This environmental concern and consequent limitations are prevalent despite the fact that the Danish marine fish farms contribute approximately 1 % of the total Danish yearly N release into the Baltic Sea (43,000 t year−1 out of a total N loading of 652,000 t year−1), where terrestrial fertilizer runoff from agriculture is identified as the main input (HELCOM 2012).
The Danish Ministry of Food, Agriculture and Fisheries aquaculture report (Havbrugsudvalget 2003) suggested that fish farms should be moved to the open sea to mitigate for potential eutrophication (‘dilution as a solution’). Furthermore, it was recommended that trials should be initiated with extractive cultures or biofilters in the form of seaweed or mussels (Havbrugsudvalget 2003). The Danish Government action plans for aquaculture included increasing the Danish marine fish production by five times, up to 40,000 t per year by 2013 amongst other goals. However, in addition, a stipulated requirement was made that the environmental impact of 1-kg farmed fish should also be reduced by 40 % to a maximum total Danish fish farm nitrogen release of 2,400 t N (Møhlenberg et al. 2010; Fiskeriudvikling 2007). A revised action plan for aquaculture is expected to be released by the Danish Government in March 2014. In 2008, the Ministry of the Environment demanded that the largest fish farm in Denmark (Musholm Lax A/S) should reduce its nitrogen release by 10 % (i.e. by 10 t N) by more efficient or reduced feed strategies or by the use of biofilters. Farmed trout (and other salmonid fish) diets and feeding techniques have been improved significantly in order to reduce the loss of nutrients (Havbrugsudvalget 2003). These are unlikely to be improved significantly by any further technological developments (Møhlenberg et al. 2010); hence, IMTA practices and the use of biofilters are viewed positively by the industry as a way to decrease their environmental footprint (Havbrugsudvalget 2003). However, fish production will not be increased and may even possibly be reduced in Denmark, if new cultivation methods such as IMTA do not fulfill the environmental targets set (Møhlenberg et al. 2010). In Denmark, the seaweed and mussels are recognized as the best available technique for bioremediation of nutrients at fish farms in the open sea; however, the uptake of nutrients released from the fish farms has to be confirmed (NEBA 2012). While the extractive biofilters of an IMTA system can make a significant difference to the marine fish farm nutrient budget, they cannot yet contribute much to remediation of the total N budget of Denmark due to the scale of this input.
Extractive organisms such as seaweed and mussels must be harvested in order to remove the assimilated nutrients from the marine environment. Efficiency of the biofilter can be calculated by estimating the total N and P in the tissue of the removed biomass (total tonnage of biomass multiplied by concentrations of N and P in tissue as determined by compositional analysis). Uptake efficiency of the biofilters cannot be inferred by water analyses from these open system marine fish farms because there is no defined in and outlet. Dilution of released nutrients can be enormous, and the concentrations fluctuate over time.
This paper compares the production efficiencies and costs of blue mussel (Mytilus edulis) and kelp (large brown seaweeds) cultivation within an IMTA system with figures taken from Danish and Irish aquaculture examples. Unlike some studies (Cranford et al. 2013; Reid et al. 2013b; Cranford et al. 2007), the paper does not follow the fate of N throughout the whole nutrient cycle (e.g. the nutrient losses, filtration and accumulation efficiency of the extractive species) but instead focuses only on the total amount of nitrogen removed as mussel/seaweed biomass at harvest. Predators, grazers, aquaculture acceptance and the value of the end product will also be discussed.
Seaweed productivity and assimilative capacity
Seaweed cultivation may be a recent phenomenon in Europe; however, global seaweed production accounts for 24 % of the total quantity of aquaculture (fresh and marine) worldwide (FAO 2012). Asia has 99.6 % of this share, and China alone accounts for the 58.4 % (FAO 2012). The yield of brown seaweeds on an annual basis is 12–60 t dry weight (dw) ha−1 year−1 by rope cultivation reported from worldwide experiences (Bruton et al. 2009). The highest yields in this report are from Chinese studies, and other yields are from extrapolations from small-scale experiments. Yields from scaled-up experiences are lacking, especially from Northern Europe. For example, extrapolation on yield of Saccharina latissima cultivated near a fish farm in Scotland resulted in 33–51 t dw ha−1 year−1 with 2.5 % N concentration (Sanderson et al. 2012). In this paper, we will use a yield of 120 t wet weight S. latissima per hectare per year (the equivalent of 18 t dw ha−1 year−1 and a value of 15 % dry weight (Bruton et al. 2009; Holdt and Kraan 2011; Handå et al. 2013), which will be able to assimilate 576 kg N (3.2 % N dw (Gevaert et al. 2008); 1.6–3.9 % N dw (Handå et al. 2013)). Production of this conservative yield is considered as a realistic short- to medium-term goal, given current levels of production, investment and experience of kelp cultivation in Northern Europe. It also strives to take into account any potential decreased productivity that may occur with intensification of cultivation due to reduced nutrient flow through intensive longline culture systems.
Several of the European kelp species (Laminaria digitata, S. latissima and Alaria esculenta) have been cultivated on string or ropes (Arbona and Molla 2006; Edwards and Watson 2011). The life cycle, cultivation procedure and yield of A. esculenta, S. latissima and L. digitata are similar and can therefore be compared. Kelp sporophytes develop on rope or string either by directly settling spores on the substrate or by control of sexual reproduction of the gametophyte stage. The settling stages are done in a seaweed hatchery under controlled conditions, and the germinated sporophytes are deployed and cultivated in the sea (Arbona and Molla 2006; Edwards and Watson 2011). In addition to phosphate, growth of kelp sporophytes depends on the availability of DIN, mainly in the form of nitrate and ammonium, which is assimilated at different rates by different kelp species depending on a number of environmental parameters, including the seawater concentration of DIN (Bartsch et al. 2008).
Information on the production costs for intensive seaweed cultivation is scarce, and most available data is based on extrapolations from laboratory experiences (nurseries/hatcheries) and field experiments where, for example, 14.4 km seeded culture string is produced in an Irish report (Edwards and Watson 2011). From this example, the costs of approximately €240,000–326,000 are based on a pessimistic yield average of 7 kg m−1 longline wet weight brown seaweed (L. digitata) which produces a harvest of approximately 100 t wet weight of seaweed (Table 1; Edwards and Watson 2011).
An earlier example of an economic analysis of seaweed cultivation suggests that €50,000 production costs for the cultivation of A. esculenta in Ireland (Arbona and Molla 2006) would produce 10 t of wet weight seaweed (Table 2). Extrapolated production costs are approximately €2,380–3,225 t−1 wet weight seaweed from Edwards and Watson (2011) and €5,000 t−1 wet weight seaweed produced from Arbona and Molla (2006). The Danish commercial seaweed producer, Seaweed Seed Supply (formerly Bluefood), claims that seeded ropes of S. latissima can be produced and later harvested for less than €40 t−1 of wet weight seaweed (Fig. 3). This cost includes all processes until the harvested seaweed is landed on the quay (including depreciation costs, labour, electricity etc.; R. Bjerregaard, Seaweed Seed Supply, Denmark, pers. communication). However, this price is questioned by horticulturist and seaweed aquaculturist J. Schipper (Hortimare BV, The Netherlands, pers. communication), ‘because it is most unlikely that seaweed cultivation can be produced with lower production costs of landcrops’. J. Schipper proposes an average production cost of €180 t−1 (wet) of seaweed S. latissima (for a pilot-scale operation in the Netherlands), but believes it is realistic that this cost can be reduced to €75–90 t−1 (wet) in 10 years’ time (Fig. 3).
The production costs per tonne wet seaweed biomass in Ireland (triangles), Denmark (open circle), and The Netherlands (squares). (Irish example shows recurring annual costs only—does not include cost of capital equipment; Arbona and Molla 2006; Edwards and Watson 2011; R. Bjerregaard, Seaweed Seed Supply, Denmark, pers. communication; J. Schipper, Hortimare BV, The Netherlands, pers. communication)
The historical, recurring production costs (consumables, electricity, labour) of wet seaweed biomass from Arbona and Molla (2006) and Edwards and Watson (2011) and Seaweed Seed Supply are plotted in Fig. 3. The figure uses the production costs presented in Edwards and Watson (2011) combined with increased biomass productivity (10 kg seaweed per metre; M. Murphy, Dingle Bay Seaweeds, Ireland, pers. communication) to provide an estimate of current costs in 2013. The Irish values indicate a steady reduction in production costs as experience of pilot-scale seaweed deployment increases over time, although the figures are still significantly greater than the Danish seaweed example. The upscaling of cultivation is necessary in order to keep costs to a minimum (R. Bjerregaard, pers. communication).
Mussel productivity and assimilative capacity
Total global mussel cultivation is approximately 1,800,000 t, of which, 9.8 % are blue mussels (Mytilus edulis) produced mainly in Europe (84 %; FAO 2013). Juvenile mussels (spat) settle on most surfaces deployed or present in the sea, including collector rope. This process can be exploited for rope mussel cultivation, leaving settled mussels on vertically deployed rope ‘droppers’ until they reach a harvestable size (Buck 2007). Mussels can be cultured in a variety of ways, including bottom culture, on rope droppers on rafts or longline systems (Handå et al. 2011). This paper only considers longlines and Smartfarm™ longlines, because these are the cultivation systems commonly used in Denmark and Ireland. Longlines are preferable in Denmark and Ireland because they are the structures best able to withstand the levels of exposure experienced at designated culture locations (D. Millard, BIM, pers. communication).
As filter feeders, mussels assimilate POM mainly in the form of phytoplankton and when downstream of fish farms, they filter additional particulates such as faeces and fish feed. Mussels can therefore be considered to utilize DIN indirectly through the assimilation of the phytoplankton that directly requires DIN for growth and development (MacDonald et al. 2011). Troell and Norberg (1998) found that the ambient seston concentration is of greater importance in controlling mussel growth in a co-cultivation with salmon, and increases in suspended solids from the fish farm may only contribute significantly during periods of low phytoplankton production. While we acknowledge that N assimilation through mussel culture is complex e.g. due to particle size selection, filtration, losses through faeces and pseudo-faeces (Cranford et al. 2013; Troell and Norberg 1998), this paper only addresses the fate of the measurable, assimilated N in seaweed/mussel tissue that is removed upon harvest.
Modeling and calculations were performed for mussel production near three Danish fish farms in a report conducted for the Danish Aquaculture Organization (Møhlenberg et al. 2010). This report concludes that the most cost-effective production of blue mussels was with a production cost of approximately €0.11 kg−1 mussel which equates to €11.40 kg−1 N removed by the production in the Great Belt in Denmark. The cost-effectiveness of mussel production at the two other localities was significantly less due to slower water currents. The modeling revealed that the water current velocity is an important limiting factor for production of rope-cultured blue mussels and has a direct effect on suspended particles and food availability (Troell and Norberg 1998; Møhlenberg et al. 2010; Cranford et al. 2013). Mussel density is also an important consideration with respect to site location and design of the production facility. The production process also requires that mussels are thinned out to secure a high specific growth rate (Møhlenberg et al. 2010).
The operation of traditional longlines (Fig. 4) supporting rope droppers is 45–60 % more cost-effective (the aforementioned €11.40 kg−1 N removed) than the Smartfarm™ model (longline supporting a vertically suspended net; Fig. 1), when the establishment and operational costs are taken into account (Møhlenberg et al. 2010).
Schematic of a mussel production unit with droppers attached to a horizontal longline (not shown) with the width (W), length of traditional longlines (L), height of droppers (H), distance between longlines (D), the free water current outside the production unit (Co) and the water current between longlines (C I ) (reproduced with permission from Møhlenberg et al. 2010; © M.D. Edwards)
A national Danish funded project MarBioShell has produced blue mussels for two seasons and has concluded that the predictions of the carrying capacity in the Great Belt of Denmark were too pessimistic. The most recent estimations are that 2,500 t of mussels can be produced, therefore, removing 20–25 t N in an area of 250 m × 750 m (a typical cultivation area) with an expenditure of €135,000–160,000 per year (F. Møhlenberg, DHI, Denmark, pers. comm.), assuming mussels are grown in a longline system.
Productivity of commercial rope mussel aquaculture was also modeled in the UISCE project (Understanding Irish Shellfish Culture Environments) for Killary Harbour, Ireland (Dallaghan 2009). Several different models estimated the carrying capacity for the fjord-like sea inlet and estimated that the area could produce an annual maximum mussel biomass of 19–27 t ha−1 (Nunes et al. 2011).
Cost-effectiveness and areal requirements of seaweed and mussels biofilters
While it is recognized that seaweed assimilate DIN and mussels filter phytoplankton and POM, DIN is assumed as the nitrogen source for both organisms for the purposes of making a comparison of the efficiencies of mussel and seaweed biofilters. In addition, an average nitrogenous content of mussels and seaweed has been assumed of 1 % N of wet whole mussel (shell and meat) biomass (Møhlenberg et al. 2010; Petersen et al. 2013). This whole mussel N content is also within the range described by other authors, once their data has been standardized to whole mussel N (Smaal and Vonck 1997; Ricciardi and Bourget 1998; Pérez-Camacho et al. 2013). Average nitrogenous content was assumed to be 0.48 % of wet seaweed biomass (Gevaert et al. 2008; Holdt and Kraan 2011). The cost-effectiveness of the biofilter can be calculated using this average nitrogenous content and the production costs for either mussels or seaweeds (€ kg−1 N removed per tonne wet biomass; Table 3). The cost of assimilating nitrogen using mussels as a biofilter ranges from €11.4 to 19.2 kg−1 N removed (Møhlenberg et al. 2010), whereas the cost of removing nitrogen using seaweeds is significantly higher in the Irish example €209 kg−1 N removed, or €496–672 kg−1 N removed when capital costs are included (Edwards and Watson 2011; Table 3). However, the cost-effectiveness of seaweed as a biofilter is €8 kg−1 N removed when produced by the commercial-scale company in Denmark (Seaweed Seed Supply) and €38 kg−1 N when produced by a comparable company in the Netherlands (Hortimare BV, J. Schipper, pers. communication; Table 3).
The space required to produce 1,000 t of mussel biomass (7.5 ha) and seaweed biomass (approximately 8 ha) is approximately the same (Table 4). However, this assumes a much more efficient seaweed cultivation than has been demonstrated by the Irish example where a larger area of 29–143 ha would be required to produce 1,000 t of seaweed, even though the ∼8 ha example is considered conservative (∼18 t dry equivalent to ∼120 t wet weight seaweed as per Bruton et al. (2009); Table 4). Seaweed Seed Supply can cultivate 1,000 t wet weight seaweed biomass in 40 ha (R. Bjerregaard, pers. communication). This latter number is derived from recent, ongoing commercial experience where the seaweed is cultivated in an IMTA system with rainbow trout (Onchorynchus mykiss), blue mussels (Mytilus edulis) and sugar kelp (Saccharina latissima).
When the cultivation areas required to assimilate 10 % of the dissolved nitrogen excreted from the largest and smallest fish farms in Denmark are compared, mussels require approximately half the space than even the most optimized seaweed estimations (Table 4).
Discussion
Whether on land or at sea, food production (amongst other human activities) has often had multiple negative impacts on the surrounding habitats due to the intensity of the farming or harvesting method and the huge strain this places on resources that are often finite (Green et al. 2005). In recent years, monoculture finfish practices have been criticized for disrupting the natural balance of nutrients present within the surrounding marine ecosystems, whereby the excretion of additional macronutrients (nitrogen and phosphorous) can contribute to increased coastal eutrophication in some areas (European Commission 2013; Wang et al. 2012). Consequently, newer and more environmentally sustainable aquaculture practices are to be considered if the effects of monoculture are to be mitigated. Integrated multi-trophic aquaculture (IMTA) may be one such tool as best available technology (BAT), whereby extractive organisms such as, but not limited to, mussels and seaweed are used as an effective biofilter, and ecological balance is maintained (Neori et al. 2007). Naturally, other extractive organisms should be taken into consideration in an IMTA system, especially species that do not require extra space, for example, sea cucumbers, which can be cultured below existing aquaculture structures (MacDonald et al. 2013). However, while this paper focuses on the comparison of the biofilter capacity of seaweed and mussels, the study could be repeated in the future to include more species as IMTA systems become more complex and efficient.
This study shows that on average, weight for weight, mussels are a more efficient biofilter compared to seaweed with regard to nitrogen assimilation and deposition within the biomass by approximately 50 % (Table 3). While Table 4 shows that optimized mussel and seaweed cultivation will produce the same amount of biomass per approximate area (1,000 t in ∼8 ha), it may be more realistic at present to assume that the space required for seaweed production can be significantly greater than for mussels, based on reported pilot-scale seaweed experiments (Arbona and Molla 2006; Edwards and Watson 2011) and commercial experience in Denmark (Seaweed Seed Supply). For example, the Irish pilot-scale experiments suggest seaweed cultivation may consume 19 times the space required for mussel cultivation, whereas Seaweed Seed Supply in Denmark can produce seaweed somewhat more efficiently in 5 times the space required for mussel cultivation, demonstrating a move towards a more optimized system. Mussel cultivation becomes considerably more attractive than seaweed cultivation for the same amount of assimilated nitrogen, if areal restrictions are made on aquaculture sites. Although directly unaccounted for in this desk-based study, future calculations of areal requirements for mussel and seaweed cultivation should acknowledge any decrease in biomass production that may develop when DIN/POM availability is reduced due to decreased current velocity as rope cultivation per unit area intensifies.
In recent years, the complexity of marine ecosystems and aquaculture activities (including IMTA) has been modeled at a variety of spatial scales to answer a range of questions on carrying capacity, productivity and environmental management (Duarte et al. 2003; Guyondet et al. 2010; Navas et al. 2011). In a recent study, Reid et al. (2013a) calculated the excretion of waste products from a salmon (Salmo salar) farm in the Bay of Fundy, Canada and the amount of seaweeds (A. esculenta and S. latissima) required to assimilate all excreted nitrogen (and other waste products). Nitrogen excretion from the Canadian salmon example was similar to that estimated for the Danish rainbow trout production in this study (2.95 and 3.9 %, respectively), while A. esculenta uptake rates were almost identical to averaged seaweed uptake rates assumed in this study (4.4 and 4.8 kg N removed per tonne of wet seaweed, respectively). However, the biofilter capacity of S. latissima was lower (2.3 kg N removed per tonne of wet seaweed) in the study of Reid et al. (2013a). This compares with a modeled N removal rate of 5 kg N t−1 S. latissima as reported by Broch and Slagstad (2012). Production values of 63 to 95 t ha−1 for the A. esculenta and S. latissima (Reid et al. 2013a) exceed both the Irish and Danish seaweed productivity examples (Table 4), suggesting that Canadian yields are more efficient. Nevertheless, space requirement is becoming more apparent from several recent studies. Huge areas are needed to cultivate enough seaweed to fully assimilate the amount of N released from a medium-sized salmonid farm (1,000 t), with each study reporting very similar areal requirements (Table 5). The cultivation of seaweeds in 50–352 ha per medium-sized salmonid farm may be impractical (Table 5); however, the growth of seaweeds (and mussels) for the Danish model of aquaculture bioremediation of 10 % may still be a feasible option (Table 4).
Mussels and seaweeds both take up nutrients (albeit in different forms) in their role as a biofilter, and whereas seaweeds do not produce dissolved waste nitrogen products, mussels excrete DIN and POM in faeces and pseudo-faeces. The concentration of ammonia increases significantly in mussel areas as noted by Asmus and Asmus (1991) and Retamales and Buschmann (1996). Recent modeled estimates for commercial rope mussel cultivation in Bantry Bay, Ireland confirm that mussel cultivation generally increases DIN in the bay by 25 % as a result of the remineralization of POM (filtered and digested phytoplankton; Dabrowski et al. 2013). The mussels excrete 45 % of the ingested N (equivalent to approximately 0.02 g g−1 mussel tissue day−1). However, despite this, mussels do effectively have a net removal of DIN from the water body by building up biomass (Dabrowski et al. 2013). This suggests a useful phenomenon where seaweed productivity could be enhanced not just by the fish farm DIN but further by the mussel DIN within an IMTA system, especially if algal growth is limited by the lack of availability of inorganic nutrients in other areas (Bartsch et al. 2008). Interestingly, Dabrowski et al. (2013) also reported that part of the DIN produced by the mussels was transported outside of the bay (due to bay current regimes and prevailing weather), which suggests that some or all of the chosen biofilter(s) may not need to be closely positioned to the source of the DIN to assimilate the nutrients produced.
Furthermore, this spatial separation of nutrient source and biofilter within an IMTA system is the concept of geographically decoupled nutrient removal (Birkeland 2011). At the system level, the origin of the assimilated nitrogen is immaterial if the biofilter always represents a net removal of nitrogen, when the biomass is harvested and removed from the water body. This is true of nutrients that originate from the fish farms described here or from water bodies upstream of the fish farm. This decoupling also applies to the DIN released by the mussels because, as mentioned previously, mussels take up more nitrogen than they excrete (Dabrowski et al. 2013). While the decoupled biofilter(s) will balance N at the greater ecosystem budget level, it may still be possible that nearby effects of nutrient enrichment from a fish farm could still persist, potentially leading to localized eutrophication problems. In all situations where decoupling of biofilters is considered, a good understanding of the aquaculture site bathymetry, current flow etc. is required to allow for more accurate siting of biofilter structures, thereby improving the efficiency of the IMTA system near to and further away from the source of nitrogen. However, not all fish farms have a large impact on the local environment, such as in Denmark (Havbrugsudvalget 2003), where the fish farms are located in open and relatively exposed areas (dilution as a solution). In turn, this makes the impact of ambient nutrient levels through seston concentration of more importance as concluded by Troell and Norberg (1998). Potentially, if decoupling of nitrogen source and sink can occur without detriment to the surrounding environment, incentive schemes could be developed, whereby fish farms could purchase nitrogen quotas and trade nitrogen credits in a water quality trading scheme similar to carbon dioxide emissions trading (Birkeland 2011; Chopin et al. 2012).
The concept of decoupling the nitrogen source and sink is, however, open to debate, as it may be claimed that the assimilated nutrients in a biofilter must specifically originate from the fed organism (e.g. finfish) cultivation, even though the amount of recovered N may be the same. Additionally, Danish authorities have argued that marine water bodies should be divided into regulated zones, with the entire IMTA practised within small designated areas, which may not necessarily take the physical and biogeochemical properties of the region into consideration. However, the Danish Aquaculture Organization is frustrated by lack of clear guidelines from the Ministry of Environment, and the trade organization strongly argues that the IMTA coupling does not have to be so ‘tight’/closely located (L.J. Plesner, Danish Aquaculture Organization, Denmark, pers. communication). Ultimately, a clear understanding of nutrient dispersal within and from water bodies will dictate whether decoupling of nitrogen sources and sinks is a practical idea.
Cost-effectiveness of both biofilter production and nitrogen removal was also calculated and compared for mussels and seaweeds (Table 3). Nitrogen removal production costs become a direct proportion of biomass production costs, because variability of the nitrogen content in seaweed and mussel tissue is assumed to be relatively stable at the time of harvest. Consequently, the production costs of biomass need to be reduced as much as possible in order to reduce the costs of removing N. When the revenue of the fish production is taken into account, a Danish fish farmer can earn €33 kg−1 of the N they release from the farm (revenue of the fish farm divided by the total nitrogen released from the fish farm; Holdt et al. 2006). Some of this revenue could be used to install biofilters to clean up or assimilate the nitrogen produced, with benefits including an increased permit to culture finfish and the value of the biofilters themselves as crops that have a nitrogen credit value. For example, in an Irish study, Ferreira et al. (2007) quantified the value of nitrogen bioremediation from bottom culture of blue mussels as being worth approximately ∼€31,300 (£27,000). This was estimated from the amount of nitrogen removed by the mussels (equivalent to the sewage discharge of nitrogen by 135 population equivalents) and the land-based treatment costs of this waste. The cost of nitrogen removal in a wastewater treatment plant is €1.48 kg−1 N removed in Denmark (based on a 4-year average of 22 wastewater treatment plants; Ministry of the Environment 2004). Lindahl et al. (2005) suggest a trading system for a mussel biofilter to reduce nitrogen and improve the coastal waters in a case study with mussel cultivation in the coastal water near a sewage treatment plant in the Lysekil municipality. This cost of nitrogen removal was estimated to cost US$9.70 kg−1, and such a trading company that produced biofilter mussels could in this case charge a reduced cost to the emitter of at least US$5.25 kg−1 of removed nitrogen to take into account the additional social benefits such as job creation.
The large differences between seaweed and mussel production costs in this study may be attributed to the maturity and scale of each respective industry; the rope mussel industry has been established in Denmark and Ireland for at least 35 years (Browne et al. 2008; Dansk Skaldyrcenter 2013), with resulting economies of scale and improvements in production efficiencies, whereas the pilot or commercial-scale seaweed production is only 10–15 years old at the very most (Edwards and Watson 2011).
The productivity or yield of the biofilter also impacts on the production costs, requiring efforts to improve the efficiency of the seaweed and mussel methods for biomass production per metre of longline. For example, the Irish seaweed cultivation examples clearly show a steady decrease in the production costs of biomass (Fig. 3). The decrease in production costs of €300 t−1 within 2 years was due to different yield estimates applied in 2011 and 2013 (7 and 10 kg m−1 cultivation rope, respectively), whilst keeping cost estimates and areal deployments the same. The significant difference between the most efficient Irish production example and the Danish example of €40 t−1 can be explained in part by the deployment method used. Currently, both Irish and Danish average yields of 10 kg m−1 are very similar (M. Murphy, pers. communication; R. Bjerregaard, pers. communication), but the Danish deployment method uses 5-m vertical droppers to grow the seaweeds, whereas in Ireland, the seaweed is deployed horizontally across the length of the header rope. The difference between these two methods result in up to 10 times the productivity (assuming seaweed growth is consistent from top to bottom of the droppers, and droppers are spaced 1 m apart; e.g. Fig. 1). Sanderson et al. (2012) found a decrease in S. latissima biomass by depth of cultivation (from around 8 kg wet weight at 1-m depth, to approximately 2 kg wet weight at 7-m depth), with an average of 4 kg wet weight per metre of dropper, which demonstrates site conditions and longline configurations can affect productivity greatly.
The mechanization and rationalization of the two mussel production methods investigated by Møhlenberg et al. (2010) are significantly different. The two systems (single longline and Smartfarm™) represent a labour intensive and a labour extensive system, respectively. The traditional longline system demands significant manual labour in Denmark, because only a few processes have been mechanized. The cleaning of equipment and the stocking of single droppers are very time-consuming and is, therefore, a cost-intensive process (Møhlenberg et al. 2010). Estimations predict that machines will be developed for both production methods within a few years, and this will reduce the need for much of the manual labour. Significant work on the development of harvest machinery is also in progress, reducing time used for harvesting of mussels on longlines. All of these improvements will eventually lower production costs; however, the traditional longline system will not likely be rationalized and mechanized (cleaning, stocking, harvest efficiency) in the same magnitude as anticipated for the Smartfarm™ system (Møhlenberg et al. 2010).
Likewise, improvements should be made for more efficient cultivation technologies and harvesting methods in the developing European seaweed cultivation. Breeding or species strain enhancement of seaweed has great potential, particularly for European kelp species, although this has yet to be exploited commercially. The species currently with the greatest potential for IMTA in Europe are the large brown seaweeds (‘kelps’) including S. latissima, A. esculenta, Laminaria digitata, L. hyperborea and Polyschides sacchoriza, although water salinity and temperature will restrict particular species to certain areas and latitudes (Lüning 1990). The kelps are suitable for IMTA due to the understanding of their life cycles, suitability for rope culture in open sea cultivation systems, yield (which can be harvested in one growth season of 4–6 months), fast specific growth rate and commercial potential. Breeding of strains with high biomass yields, high protein content or enhanced disease resistance should increase the biofilter capacity of the seaweed near an IMTA system because the biomass will be able to assimilate more nitrogen. Recently, a Danish project was initiated with this intended aim and will include the optimization of the production and harvest season on the species S. latissima (Kombiopdræet 2013).
In addition to the nutrient assimilative capacity, areal requirements and costs, the choice of biofilter should also be made based on the risk of loss, social acceptance and value of the additional crop. Loss of biomass can occur when longlines are damaged by rough weather, entanglement with other structures such as boats or rafts etc.; however, these risks of loss are assumed to be equal for both mussels and seaweed. Broch and Slagstad (2012) furthermore suggest that kelp cultures may have some local negative impacts due to dissolved and particulate matter eroded from fronds. Loss can also occur due to grazers or predators. The blue mussels have a natural enemy which is highly abundant in Denmark (P. Andersen, Orbicon, Denmark, pers. communication). Colonies of eider ducks (Somateria mollissima) have been observed to forage in flocks and have already shown to represent a large loss of biomass, consequently reducing capacity of mussels to function as a biofilter (Ross et al. 2001; P. Andersen, pers. communication). Large-scale problems of grazers damaging seaweed biomass in rope culture have yet to be reported; however, epiphyte and epifaunal fouling (Saier and Chapman 2004; Lüning and Pang 2003) can be a major issue for both types of organisms, especially if the biomass is needed for a high-value end product (e.g. for human consumption). Normally, this fouling is most dominant during the summer, and can be avoided by seasonal choice of cultivation and harvest times, and the maintenance of a high density of mussels or seaweed on the culture ropes (Lüning and Pang 2003; Jansen et al. 2011; R. Bjerregaard, pers. communication).
The value of the extractive organism as a co-product should also be taken into account, as this is one of the most likely incentives for establishing an IMTA system. The total production of 540 t (161 t for live export and 379 t for human consumption) of monoculture blue mussels had a retail value of €269,000 (€0.5 kg−1) in Denmark in 2011 (Danish Aquaculture Statistics 2013b). In Ireland, the total production of blue mussels (rope and bottom culture) in 2007 was 29,470 t, with a retail value of €28.7 million or €0.97 kg−1 (Browne et al. 2008).
Restrictions in the end use of biofilter crops are a risk to be considered. The value or price of the biofilter (e.g. wastewater management and value of the increased fish production by compensating with biofilters) is realized once the biofilter biomass is harvested. However, should the crop be intended as feedstock for further processing/use, there is a risk that the value of the biofilter crop can fluctuate, depending on the individual cultivation circumstances. This can often be due to the differences in the biology of the crops. For example, mussels can filter harmful microalgal blooms and accumulate domoic acid and other harmful toxins, which can cause shellfish poisoning (Hallegraeff 1993). The final value of the mussel biofilter crop can therefore decrease if some or all of the products cannot be sold for human consumption. On the other hand, a similar scenario can occur for seaweeds, which will uptake and accumulate heavy metals if present in the ambient waters (Holdt and Kraan 2011; Besada et al. 2009). These issues are more than likely to be species- and site-specific. Analysis of mussel and seaweed tissue from a Canadian IMTA system suggests that concentrations of heavy metals, PCB’s and pesticides were found to be below the regulatory limits prescribed by the Canadian Food Inspection Agency, the USA Food and Drug Administration and the European Community Directives (Chopin et al. 2008).
Whole seaweeds and their extracts are incorporated into countless products such as health-food snacks, sushi, as a stabilizing agent, cosmetics and health care products, as well as being produced as a fertilizer worldwide (Hafting et al. 2012; Arasaki and Arasaki 1983; Holdt and Kraan 2011; Mouritsen 2013; Craigie 2011). The potential use of seaweed for bioenergy is also being studied including biorefinery concepts (Bruton et al. 2009; Alvarado-Morales et al. 2012; Algal Biorefinery 2013; Energetic Algae 2013). The value of the seaweed product differs widely from biofuel production and fertilizers at the low end of the value range to cosmetics and health care products at the high end (Bruton et al. 2009), and is, therefore, difficult to predict and discuss. However, bioeconomic models for IMTA with predictions on different scenarios of this multi-crop system show that the security of the farm increases, because the harvest are not dependent on one crop and the market value that may be subject to change (Barrington et al. 2009; Ridler et al. 2007). Furthermore, the current net values for both seaweeds and mussels were higher from IMTA than monoculture when investing in aquaculture (Barrington et al. 2009; Ridler et al. 2007). This is before considering the value of the mussels or seaweed in the management of wastewater (i.e. through N quotas as discussed) that may allow more fish to be produced as per the ‘Danish model’. For example, the estimated value of the income loss of Danish fish farming is €1.5 billion, due to the missed opportunities for exploitation of the growth potential of the enterprise (Holdt et al. 2006). The Danish national authority’s recognition of the use of seaweed and mussels as biofilters as a means for better fish production practice is unique in the Western world (Holdt et al. 2006; Havbrugsudvalget 2003; Buschmann et al. 2008). At present, only a few marine fish farmers have initiated this integration (IMTA) in Denmark, but more fish farmers are embracing the concept of IMTA and the production of additional ‘biofilter’ crops and intend to incorporate them in their future fish production licenses. Naturally, as the Danish government requires the reduction in the loss of nitrogen from fish farms to the marine environment, fish farmers in Denmark are primarily interested in IMTA due to their N budget and the effects of bioremediation, whereas the value of the extra crop(s) is a secondary consideration.
The development of the social acceptance of aquaculture should also be taken into account. Whereas traditional agriculture is a widely accepted and essential human activity practised for thousands of years, aquaculture experiences greater societal challenges despite broad recognition that aquaculture has the potential to feed an ever-increasing human population (FAO 2012). While cultivation of biofilters may require large areas and multiple structures in the sea, IMTA is a sustainable concept, which may counteract the ‘visual pollution’ as some people may see it. Farming of the sea should in the future become just as normal as the farmed land habits. Aquaculture and agriculture should strive for environmental sustainability as well as productivity. Promotion of the synergies between the agricultural sector (land-based production system) and seaweed production such as sugar kelp (sea-based production system) could stimulate economic growth and a continuous improvement of the environment (Hansen 2011). The loss of nutrients from agricultural production systems to the marine environment has induced several water protection schemes targeting the agricultural sector (Hansen 2011; Iversen et al. 1998). However, a synergy may be created between the sectors by recycling the land-based lost nitrogen assimilated in the seaweed and reused as fertilizer, possibly incorporated into a biorefinery concept. This would create a theoretical framework that makes the collection of nutrients from neighbouring ecosystems possible (Hansen 2011), in effect, another form of N quota system.
Conclusions
The use of blue mussels and seaweeds (kelps) as biofilters of nutrients (nitrogen) within an integrated multi-trophic aquaculture (IMTA) system provides a possible mechanism for fish farmers to reduce the nitrogen loss from e.g. salmonid cultivation in sea cages. This biofiltration (best available technology) may lead to an increase in fish production in the Danish example presented. However, both seaweed and mussel biofilters require large culture areas, which may be restrictive. At present, mussels are a more efficient and cost-effective biofilter than seaweed, although seaweed cultivation is still in the early stages of development within Europe. The biofilters could also be spatially decoupled from the source of the nutrients (although this is dependent on suitable local bathymetric conditions) and may also be considered as a means of developing the trading of nutrient credits. Risk of loss of the biomass (predation, fouling etc.), value of the biofilter crop, improvements to yield (longline design, breeding etc.) and social acceptances are all important aspects to consider. More upscaling of IMTA to commercial proportions would allow studies of e.g. the environmental impact on the capacity of coastal areas, water and bottom quality, biodiversity and result in more real data on production costs and revenues.
References
Abreu MH, Varela DA, Henriquez L, Villarroel A, Yarish C, Sousa-Pinto I, Buschmann AH (2009) Traditional vs. integrated multi-trophic aquaculture of Gracilaria chilensis C. J. Bird, J. McLachlan & E. C. Oliveira: productivity and physiological performance. Aquaculture 293:211–220
Algal Biorefinery (2013) Retrieved from http://www.algalbiorefinery.org on May 2013
Alvarado-Morales M, Boldrin A, Karakashev DB, Holdt SL, Angelidaki I, Astrup T (2012) Life cycle assessment of biofuel production from brown seaweed in Nordic conditions. Bioresour Technol 129:92–99
Arasaki S, Arasaki T (1983) Low calorie, high nutrition vegetables from the sea to help you look and feel better. Japan Publications, Tokyo, 196 pp
Arbona J-F, Molla M (2006) Aquaculture explained: cultivation of the brown seaweed Alaria esculenta. In: Watson L (ed) Bord Iascaigh Mhara, Dublin 50pp
Asmus RM, Asmus H (1991) Mussel beds: limiting or promoting phytoplankton? J Exp Mar Biol Ecol 148:215–232
Barrington K, Chopin T, Robinson S (2009) Integrated multi-trophic aquaculture (IMTA) in marine temperate waters. In: Soto ID (ed.) Integrated mariculture—a global review. FAO Fisheries and Aquaculture Technical Paper No. 529, Rome, FAO pp 7–46
Bartsch I, Wiencke C, Bischof K, Buchholz CM, Buck BH, Eggert A, Feuerpfeil P, Hanelt D, Jacobsen S, Karez R, Karsten U, Molis M, Roleda MY, Schubert H, Schumann R, Valentin K, Weinberger F, Wiese J (2008) The genus Laminaria sensu lato: recent insights and developments. Eur J Phycol 43:1–86
Besada V, Andrade JM, Schultze F, González JJ (2009) Heavy metals in edible seaweeds commercialised for human consumption. J Mar Syst 75:305–313
Birkeland MJ (2011) Tang som biofilter. Meeting of the Seaweed Network in Denmark, Danisco, Copenhagen, Denmark. February 9 (oral presentation)
Broch OJ, Slagstad D (2012) Modelling seasonal growth and composition of the kelp Saccharina latissima. J Appl Phycol 24:759–776
Browne R, Deegan B, Watson L, Mac Giolla Bhríde D, Norman M, Ó’Cinnéide M, Jackson D, O’Carroll T (2008) Status of Irish aquaculture 2007. A compilation report of information on Irish aquaculture. Marine Institute, Bord Iascaigh Mhara and Údarás na Gaeltachta 142 pp
Bruton T, Lyons H, Lerat Y, Stanley M, Rasmussen MB (2009) A review of the potential of marine algae as a source of biofuel in Ireland. Sustainable Energy Ireland 92pp
Buck BH (2007) Experimental trials on the feasibility of offshore seed production of the mussel Mytilus edulis in the German Bight: installation, technical requirements and environmental conditions. Helgol Mar Res 61:87–101
Buschmann AH, Hernández-González MC, Aranda C, Chopin T, Neori A, Halling C, Troell M (2008) Mariculture waste management. In: Jørgensen SE, Fath B (eds) Encyclopedia of ecology, Elsevier, Oxford, pp 2211–2217
Chopin T, Yarish C, Neefus C, Kraemer G, Zertuche-González J, Belyea E, Carmona R (2001) Aquaculture from a different angle: the seaweed perspective, and the rationale for promoting integrated aquaculture. In: Tlusty M, Bengtson D, Halvorson HO, Oktay S, Pearce J, Rheault R. B. Jr (eds) Marine aquaculture and the environment, a meeting for the stakeholders in the Northeast, Cape Cod Printing, Inc. pp 69–72
Chopin T, Robinson SMC, Troell M, Neori A, Buschmann AH (2008) Multitrophic integration for sustainable marine aquaculture. In: Jørgensen SE, Fath B (eds) Encyclopedia of ecology. Elsevier, Oxford, pp 2463–2475
Chopin T, Cooper JA, Reid G, Cross S, Moore C (2012) Open-water integrated multi-trophic aquaculture: environmental biomitigation and economic diversification of fed aquaculture by extractive aquaculture. Rev Aquac 4:209–220
Craigie J (2011) Seaweed extract stimuli in plant science and agriculture. J Appl Phycol 23:371–393
Cranford PJ, Strain PM, Dowd M, Hargrave BT, Grant J, Archambault M-C (2007) Influence of mussel aquaculture on nitrogen dynamics in a nutrient enriched coastal embayment. Mar Ecol Prog Ser 347:61–78
Cranford PJ, Reid GK, Robinson SMC (2013) Open water integrated multi-trophic aquaculture: constraints on the effectiveness of mussels as an organic extractive component. Aquac Environ Interact 4:163–173
Dabrowski T, Lyons K, Curé M, Berry A, Nolan G (2013) Numerical modelling of spatio-temporal variability of growth of Mytilus edulis (L.) and influence of its cultivation on ecosystem functioning. J Sea Res 76:5–21
Dallaghan B (2009) UISCE Project—virtual aquaculture. Aquaculture Ireland No. 128 pp 6–7
Danish Aquaculture Statistics (2013 a) Aquaculture statistics, Ministry of Food, Agriculture and Fisheries. Retrieved from http://naturerhverv.fvm.dk/akvakulturstatistik.aspx?ID=24357 on July 1 2013 (In Danish)
Danish Aquaculture Statistics (2013 b) Aquaculture statistics, Ministry of Food, Agriculture and Fisheries. Retrieved from http://webfd.fd.dk/stat/Akvakultur_tab/prod_art_str_11.html on July 19 2013 (In Danish)
Dansk Skaldyrcenter (2013) Blåmuslingeprojekt rapport: Skaldyropdræt i Limfjorden, 1.6.1999-30.6.2000 by Christensen JA. (In Danish)
Duarte P, Meneses R, Hawkins AJS, Zhu M, Fang J, Grant J (2003) Mathematical modelling to assess the carrying capacity for multi-species culture within coastal waters. Ecol Model 168:109–143
Edwards M, Watson L (2011) Aquaculture explained no. 26: cultivating Laminaria digitata. Bord Iascaigh Mhara (Irish Sea Fisheries Board), Dublin 72pp
Energetic Algae (2013). Retrieved from http://www.enalgae.eu. on June 14 2013
European Commission (2013) Communication from the commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Strategic Guidelines for the sustainable development of EU aquaculture. Retrieved from http://ec.europa.eu/fisheries/cfp/aquaculture/official_documents/com_2013_229_en.pdf on August 5 2013
FAO (2011) World Aquaculture 2010. FAO Fisheries and Aquaculture Technical Paper 500/1. Food and Agriculture Organization of the United Nations, Rome 120 pp
FAO (2012) Food and Agriculture Organization of the United Nations. The state of the world fisheries and aquaculture 2012. FAO Fisheries and Aquaculture Department. Rome. FAO 209 pp
FAO (2013) Food and Agriculture Organization. Aquaculture statistics. Retrieved from www.fao.org on April 2013
Ferreira JG, Hawkins AJS, Monteiro P, Service M, Moore H, Edwards A, Gowen R, Lourenco P, Mellor A, Nunes JP, Pascoe PL, Ramos L, Sequeira A, Simas T, Strong J (2007) SMILE – sustainable mariculture in northern Irish lough ecosystems – assessment of carrying capacity for environmentally sustainable shellfish culture in Carlingford Lough, Strangford Lough, Belfast Lough, Larne Lough and Lough Foyle. Ed. IMAR – Institute of Marine Research, p 100
Fiskeriudvikling (2007) Strategi for udvikling af den danske Fiskeri- og akvakultursektor. 2007–2013. Ministeriet for Fødevarer, Landbrug og Fiskeri, Den Europæiske Fiskerifond. Retrieved from http://ec.europa.eu/fisheries/cfp/eff/national_plans/list_of_national_strategic_plans/denmark_da.pdf on June 13 (In Danish)
Gevaert F, Janquin MA, Davoult D (2008) Biometrics in Laminaria digitata: a useful tool to assess biomass, carbon and nitrogen contents. J Sea Res 60:215–219
Green RE, Cornell SJ, Scharlemann JPW, Balmford A (2005) Farming and the fate of wild nature. Science 307:550–555
Guyondet T, Roy S, Koutitonsky VG, Grant J, Tita G (2010) Integrating multiple spatial scales in the carrying capacity assessment of a coastal ecosystem for bivalve aquaculture. J Sea Res 64:341–359
Hafting JT, Critchley AT, Cornish ML, Hubley SA, Archibald AF (2012) On-land cultivation of functional seaweed products for human usage. J Appl Phycol 24:385–392
Hallegraeff GM (1993) A review of harmful algal blooms and their apparent global increase. Phycologia 32:79–99
Handå A, Alver M, Edvardsen CV, Halstensen S, Olsen AJ, Øie G, Reitan KI, Olsen Y, Reinertsen H (2011) Growth of farmed blue mussels (Mytilus edulis L.) in a Norwegian coastal area; comparison of food proxies by DEB modeling. J Sea Res 66:297–307
Handå A, Forbord S, Wang X, Broch OJ, Dahle SW, Størseth TR, Reitan KI, Olsen Y, Skjermo J (2013) Seasonal- and depth-dependent growth of cultivated kelp (Saccharina latissima) in close proximity to salmon (Salmo salar) aquaculture in Norway. Aquaculture 414–415:191–201
Hansen S (2011) Tang, en overset ressource? - Nye veje mod et bæredygtigt landbrug. Master thesis. Det Naturvidenskabelige Fakultet, Københavns Universitet (In Danish)
Havbrugsudvalget (2003) Rapport (a. Beskrivelse og anbefalinger). Ministeriet for Fødevarer, Landbrug og Fiskeri: p 1–102 (In Danish). Retrieved from http://www.aquacircle.org/images/pdfdokumenter/udvikling/danmark/havbrug-rap/Havbrug-rapport-21-03-03%5B1%5D.pdf on June 2013
HELCOM (2012) The Fifth Baltic Sea pollution load compilation (PLC-5)—an executive summary. Balt Sea Environ Proc 128A:32
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed; functional food applications and legislation. J Appl Phycol 23:543–597
Holdt S, Møhlenberg F, Dahl-Madsen KI (2006) Polyculture in Denmark; status, feasibility and future. World Aquaculture Society Conference, Florence, Italy, May 9–13. Oral presentation
Iversen TM, Grant R, Nielsen K (1998) Nitrogen enrichment of European inland and marine waters with special attention to Danish policy measures. Environ Pollut 102:771–780
Jansen HM, Strand Ø, Strohmeier T, Krogness C, Verdegem M, Smaal A (2011) Seasonal variability in nutrient regeneration by mussel Mytilus edulis rope culture in oligotrophic systems. Mar Ecol Prog Ser 431:137–149
Kombiopdræet (2013) Retrieved from http://www.kombiopdraet.dk/da/ on June 2013 (In Danish)
Lindahl O, Hart R, Hernroth B, Kollberg S, Loo L-O, Olrog L, Rehnstam-Holm A-S, Svensson J, Svensson S, Syversen U (2005) Improving marine water quality by mussel farming: a profitable solution for Swedish society. Ambio 34:131–138
Lüning K (1990) Seaweeds. Their Environment, biogeography and ecophysiology. Wiley, New York, 527 pp
Lüning K, Pang SJ (2003) Mass cultivation of seaweeds: current aspects and approaches. J Appl Phycol 15:115–119
MacDonald BA, Robinson SMC, Barrington KA (2011) Feeding activity of mussels (Mytilus edulis) held in the field at an integrated multi-trophic aquaculture (IMTA) site (Salmo salar) and exposed to fish food in the laboratory. Aquaculture 314:244–251
MacDonald CLE, Stead SM, Slater MJ (2013) Consumption and remediation of European seabass (Dicentrarchus labrax) waste by the sea cucumber Holothuria forskali. Aquac Int 21:1279–1290
Ministry of the Environment (2004) The Danish Ministry of the Environment. Retrieved from http://www2.mst.dk/common/Udgivramme/Frame.asp?http://www2.mst.dk/Udgiv/publikationer/2004/87-7614-400-3/html/kap05.htm on July 1 2013 (In Danish)
Møhlenberg F, Holtegård LE, Hansen FT (2010) Miljøneutral udvidelse af havbrugsproduktion—Undersøgelse af rentable muligheder for dyrkning og høst af muslinger som kompensation for tab af næringsstoffer fra havbrug, Dansk Akvakultur, rapport, October 2010 (In Danish)
Mouritsen OG (2013) Seaweeds: edible, available & sustainable. University of Chicago Press, Chicago, 304 pp
Navas JM, Telfer TC, Ross LG (2011) Application of 3D hydrodynamic and particle tracking models for better environmental management of finfish culture. Cont Shelf Res 31:675–684
NEBA (2012) Decision report from Nature and Environmental Board of Appeal. Retrieved from http://www.nmknafgoerelser.dk/ShowDoc.aspx?q=NMK-10-00390&docId=nmk20121220-0001-full on June 18, 2013
Neori A, Troell M, Chopin T, Yarish C, Critchley A, Buschmann AH (2007) The need for a balanced ecosystem approach to blue revolution aquaculture. Environment 49:36–43
Nunes JP, Ferreira JG, Bricker SB, O’Loan B, Dabrowski T, Dallaghan B, Hawkins AJS, O’Connor B, O’Carroll T (2011) Towards an ecosystem approach to aquaculture: assessment of sustainable shellfish cultivation at different scales of space, time and complexity. Aquaculture 315:369–383
Pérez-Camacho A, Labarta U, Vinseiro V, Fernández-Reiriz MJ (2013) Mussel production management: raft culture without thinning-out. Aquaculture 406–407:172–179
Petersen JK, Timmermann K, Holmer M, HaslerB, Göke C, Zandersen M (2013) Miljømuslinger Muslinger som supplerende virkemiddel, Notat fra DCE—Nationalt Center for Miljø og Energi, Aarhus Universitet. Retrieved from http://dce.au.dk/fileadmin/dce.au.dk/Udgivelser/NLK/Notat_Miljoemuslinger_april_2013.pdf on January 13, 2014 (In Danish)
Reid GK, Chopin T, Robinson SMC, Azevedo P, Quinton M, Belyea E (2013a) Weight ratios of the kelps, Alaria esculenta and Saccharina latissima, required to sequester dissolved inorganic nutrients and supply oxygen for Atlantic salmon, Salmo salar, in integrated multi-trophic aquaculture systems. Aquaculture 408–409:34–46
Reid CK, Robinson SMC, Chopin T, MacDonald BA (2013b) Dietary proportion of fish culture solids required by shellfish to reduce the net organic load in open-water integrated multi-trophic aquaculture: a scoping exercise with cocultures Atlantic salmon (Salmo salar) and blue mussel (Mytilus edulis). J Shellfish Res 32:509–517
Retamales CA, Buschmann AH (1996) Gracilaria-Mytilus interaction on commercial algal farm in Chile. Hydrobiologia 326–327:355–359
Ricciardi A, Bourget E (1998) Weight-to-weight conversion factors for marine benthic macroinvertebrates. Mar Ecol Prog Ser 163:245–251
Ridler M, Wowchuk M, Robinson M, Barrington K, Chopin T, Robinson S, Page F, Reid G, Szemerda M, Sewuster J, Boyne-Travis S (2007) Integrated multi-trophic aquaculture (IMTA): a potential strategic choice for farmers. Aquac Econ Manage 11:99–110
Ross BP, Lien J, Furness RW (2001) Use of underwater playback to reduce the impact of eiders on mussel farms. ICES J Mar Sci 58:517–524
Saier B, Chapman AS (2004) Crusts of the alien bryozoan Membranipora membranacea can negatively impact spore output from native kelps (Laminaria longicruris). Bot Mar 47:265–271
Sanderson JC, Dring MJ, Davidson K, Kelly MS (2012) Culture, yield and bioremediation potential of Palmaria palmata (Linnaeus) Weber & Mohr and Saccharina latissima (Linnaeus) C.E. Lane, C. Mayes, Druehl & G.W. Saunders adjacent to fish farm cages in northwest Scotland. Aquaculture 354–355:128–135
Schneider O, Sereti V, Eding EH, Verreth JAJ (2005) Analysis of nutrient flows in integrated intensive aquaculture systems. Aquac Eng 32:379–401
Smaal A, Vonck APMA (1997) Seasonal variation in C, N and P budgets and tissue composition of the mussel Mytilus edulis. Mar Ecol Prog Ser 153:167–179
Troell M, Norberg J (1998) Modelling output and retention of suspended solids in an integrated salmon–mussel culture. Ecol Model 110:65–77
Wang X, Olsen LM, Reitan KI, Olsen Y (2012) Review. Discharge of nutrient wastes from salmon farms: environmental effects, and potential for integrated multi-trophic aquaculture. Aquac Environ Interact 2:267–283
Acknowledgments
The authors wish to acknowledge funding from the Energetic Algae project (EU Interreg IVB NWE Strategic Initiative) and the Kombiopdræt project (The Danish AgriFish Agency (GUDP)—3405-11-0375). We would like to thank Rasmus Bjerregaard (Seaweed Seed Supply, Denmark) and Michael Murphy (Dingle Bay Seaweed) for providing figures on commercial seaweed cultivation. We also would like to thank one of the anonymous reviewers for their particularly helpful and detailed comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holdt, S.L., Edwards, M.D. Cost-effective IMTA: a comparison of the production efficiencies of mussels and seaweed. J Appl Phycol 26, 933–945 (2014). https://doi.org/10.1007/s10811-014-0273-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0273-y