Abstract
The photoautotrophic co-culture of Chlorella vulgaris and the yeast Rhodotorula glutinis (MP culture) from industrial wastewater was investigated. Cell numbers, biomass, lipid production, and fatty acid content were measured. Owing to co-culture interaction with yeast and microalgae, the MP culture resulted in the highest number of cells (27.73 × 105 cells mL-1) and biomass (0.808 g L−1). Lipid production in the MP culture (117.73 mg L−1) was fourfold higher than that in the photoautotrophic pure culture (23.1 mg L−1). The content of palmitic acid (C16:0) was 24.65%, whereas that of oleic acid (C18:1) was 56.34% in the MP culture, which was higher than in other cultures. The results of this study indicate that MP cultures can be used to effectively support the growth of microorganisms and as an approach for biodiesel production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Investigations on biodiesel production using oleaginous microorganisms such as algae, bacteria, and fungi have been conducted in attempts to reduce reliance on crude oil (Cooksey et al. 1987; Crabbe et al. 2001). Global biodiesel production was estimated to be around 1.8 billion liters in 2003 (Fulton 2004). Although there was no increase in biodiesel production between 1996 and 1998, a sharp increase in biodiesel production was observed in the past several years, and it is believed that the production of biodiesel will increase greatly in the future owing to the worldwide demand for fuels and clean energy (Ördög et al. 2013; Vello et al. 2014).
However, the high cost of culturing oleaginous microorganisms has hindered development of large-scale microbial cultures. Currently, the price of microbially produced biodiesel is approximately twofold that of conventional crude oil. This high cost is primarily due to the cost of raw materials and that of processing, with the former accounting for 60 to 75% of the total cost (Krawczyk 1996). Raw materials that contain large proportions of fatty acid triglycerides are preferred. Biodiesel is currently produced from plant and animal oils, but not from microalgae. When compared with oleaginous microorganisms, the prices for most plant oils are relatively low and animal fats are even less expensive. However, improving microbial culture techniques used for biofuel production will lead to reductions in the cost of large-scale biodiesel production, which will provide an economic and environmentally friendly fuel option (Chi et al. 2011).
Commercial culture of microalgae has been conducted for over 40 years using photoautotrophs, heterotrophs, co-culture, closed photobioreactors, and large shallow open-air ponds (Borowitzka 2013). Metting and Pyne (1996) and Liu et al. (2008) have suggested that autotrophic microalgae can convert CO2 to biofuels such as oil and biohydrogen via photosynthesis. Additionally, microalgal cells can be cultured under heterotrophic and mixotrophic conditions. Heterotrophic cells utilize organic substrates, whereas mixotrophic cells use light and inorganic and organic substrates as energy and carbon sources (Borowitzka 1999). Yeasts also can produce high amounts of lipid that provide many advantages (Li and Wang 1997; Zhu et al. 2008). The mixed cultivation of the yeast Rhodotorula glutinis and microalga Arthrospira (Spirulina) platensis can boost biomass and lipid accumulation. During co-culture, yeast provides CO2 to the microalgae, whereas microalgae generate oxygen for the yeast. Therefore, the red yeast R. glutinis was considered part of the co-culture (Xue et al. 2010).
The main factor slowing commercialization of lipid production derived from oleaginous microorganisms is the need for inexpensive and environmentally friendly culture approaches. In this study, the biomass and lipid production of a co-culture from winery wastewater were compared with those of pure cultures. Specifically, this study was conducted to compare the lipid and fatty acid composition of Chlorella vulgaris and R. glutinis cultivated under photoautotrophic and heterotrophic conditions to provide scientific evidence to support the biodiesel industry.
Materials and methods
Collection and pretreatment of wastewater
For the wastewater experiments, 50 L of winery effluent (Beijing Dragon Seal Winery, Beijing, China) was deposited and filtered through screens with 196-μm openings at atmospheric pressure. The screened wastewater was autoclaved at 121 °C for 15 min and then stored in airtight containers at room temperature until use.
Microorganisms and growth conditions
Yeast
Rhodotorula glutinis 2.704 (China Microbiological Culture Collection Centre) was grown in a malt extract agar slant (agar 20 g L−1 malt extract) at 26 °C for 48 h. The cells were transferred to a 1000-mL conical flask containing 600 mL of liquid culture medium. Approximately 1 L of liquid culture medium contained 15 g of glucose, 2 g of (NH4)2SO4, 1 g of yeast extract, 7 g of KH2PO4, 2 g of Na2PO4, and 1.5 g of MgSO4. The pH was adjusted to 6.0 using 1 mol L−1 of NaOH/HCl solution. Flasks containing the seed culture were incubated at 26 °C statically for 3 days.
Microalgae
The microalga Chlorella vulgaris FACHB-31 (Freshwater Algae Culture Collection of the Institute of Hydrobiology) was incubated statically in BG11 Medium (Rippka et al. 1979) at 26 °C at a light intensity of 72 μmol photons m−2 s−1 using a GXZ intellectualized illumination incubator (Jiangnan Instrument Factory, Ningbo, China) for 72 h under a 12-h:12-h light:dark cycle for a seed culture.
Pure C. vulgaris cultures under photoautotrophic conditions (PP)
Chlorella vulgaris was incubated statically in 700 mL of pretreated wastewater at 26 °C under a light intensity of 72 μmol photons m−2 s−1 using the illumination incubator with a 12-h:12-h light:dark cycle for 10 days. The initial cell count of the microalgae was 2.3 × 105 cells mL−1. All of the experiments were repeated at least thrice.
Pure C. vulgaris cultures under heterotrophic conditions (PH)
For heterotrophic growth, the seed cells were incubated statically in pretreated wastewater containing 30 g L−1 glucose. Flasks containing 700 mL of medium sterilized in an autoclave were inoculated with 10% seed culture of microalgae. The initial cell count of the microalgae was 2.3 × 105 cells mL−1. The cultures were cultivated for 10 days at 26 °C in the dark, and all of the experiments were repeated at least thrice.
Co-cultures under photoautotrophic conditions (MP)
For co-cultures, the pretreated wastewater was inoculated with a 10% seed culture mixture of yeast and microalgae at initial concentrations of 2.9 × 105 and 2.3 × 105 cells mL−1, respectively. The other conditions of culture cultures were the same with PP culture. All of the experiments were repeated at least thrice.
Co-cultures under heterotrophic conditions (MH)
For heterotrophic growth, the initial cell counts of yeast and microalgae were the same with MP culture. The other conditions of cultures and liquid culture medium were the same with PH culture. All of the experiments were repeated at least thrice.
Microbial cell concentration and dry cell weight
The individual cell counts of yeast and microalgae were determined using a hemocytometer (Cai et al. 2007). Cells were centrifuged at 9000 rpm for 5 min, after which the cells were washed with distilled water three times and freeze dried until a constant weight was obtained. The powdered microorganisms were subsequently cooled to room temperature in a desiccator before the weight was obtained (Kavadia et al. 2001).
Lipid extraction and analysis
Cells were harvested and lyophilized for lipid extraction and analysis. Total lipids were extracted from 300 mg of lyophilized biomass with a solvent mixture of chloroform, methanol, and water (2:1:0.75 v/v) according to the modified Folch procedure (Folch et al. 1957). The extract was dried in a rotary evaporator, weighed, resuspended in chloroform, and stored at 20 °C in nitrogen gas to prevent lipid oxidation.
Fatty acid analysis
At the end of culture experimentation, fatty acid methyl esters (FAMEs) were obtained by acid transesterification (Jham et al. 1982). Briefly, lyophilized cells were incubated overnight with a solvent mixture of toluene and 1% sulfuric acid in methanol (1:2, v/v) at 50 °C to produce FAMEs that were then extracted with hexane. FAMEs were analyzed using an Agilent 6890N capillary gas chromatograph equipped with a flame ionization detector (FID) and an Agilent 19091S-433 HP-5MS capillary column (30 m × 0.25 mm). Helium was used as the carrier gas. The initial column temperature was set at 60 °C and then progressively increased at 10 °C min−1 to 280 °C. The injector was maintained at 250 °C with an injection volume of 2 μL in splitless mode. FAMEs were identified by chromatographic comparison with authentic standards (Sigma, USA). The quantities of individual FAMEs were estimated from the peak areas on the chromatogram using heptadecanoic acid (Sigma) as an internal standard.
Statistical analysis
Significant differences among groups were identified by t tests, with a P < 0.05 considered significant. All data were analyzed in triplicate and are presented as the means ± SD.
Results
Biomass evolution
The co-culture of R. glutinis and C. vulgaris cultivated in effluent from the wine making process in a winery was compared with the pure culture under photoautotrophic and heterotrophic conditions. The biomass evolution of microorganisms grown under four culture conditions was measured in winery wastewater. The microorganisms were then grown under the same culture conditions and in the same wastewater while varying the carbon sources and light conditions to identify the ideal growth conditions (data not shown). Next, the growth and biomass of microalgae and yeast cells at 10 days of cultivation were compared among the four culture methods (Fig. 1).
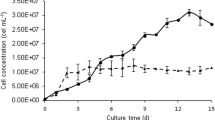
The biomass evolution in pure Chlorella vulgaris cultures under photoautotrophic conditions (PP), pure Chlorella vulgaris cultures under heterotrophic conditions (PH), mixed cultures under photoautotrophic conditions (MP), and mixed cultures under heterotrophic conditions (MH). a Microalgae cell amounts (×105 cells mL−1). b Yeast cell amounts (×105 cells mL−1). c Microorganism biomass (g L−1). d Microorganism culture pH. Results are presented as mean + SD (n = 3)
The biomass evolution is shown in Fig. 1a. Owing to diverse interactions between yeast and microalgae, pure culture of yeast grew faster than pure culture of microalgae. Additionally, the concentration of co-culture increased faster and was higher than that in pure cultures. For the first 4 days of cultivation, the difference in the concentration of microalgae did not differ among culture methods. However, the number of cells in the PH and MP cultures was higher than in other cultures from day 4. The stationary phase of the pure yeast growth curve (Fig. 1b was observed earlier than in the MP yeast growth curve, indicating that the yeast may have dominated the co-culture in terms of the number of cells (Cheirsilp et al. 2012). From days 3 to 6, the difference in the biomass of microorganisms among the four culture methods was not significant (Fig. 1c) possibly due to cell weight accumulation.
The number of MP and PP cells increased continuously until the end of cultivation (Fig. 1a) but the number in the PH and MH groups decreased. Although the biomass was increasing, the amount of cells in the PP group increased slowly. The biomass evolution for yeast in the MP group increased continuously relative to the pure culture (Fig. 1b). Additionally, the co-culture was adjusted to the appropriate pH level to maintain a stable acidic environment favorable for yeast growth (Fig. 1d). Therefore, co-cultures containing yeast were beneficial to both microalgae and yeast growth under more economical culture medium.
Lipid class composition
Lipids were extracted from microbial cells grown in the PP, PH, MP, and MH cultures and observed for 10 days to investigate the lipid content and production (Fig. 2). As biomass concentration in the MP culture increased, the lipid production increased at a greater rate than in the pure cultures.
Lipid production (a: mg L−1) and content (b: mg g−1) of microorganism in pure Chlorella vulgaris cultures under photoautotrophic conditions (PP), pure Chlorella vulgaris cultures under heterotrophic conditions (PH), mixed cultures under photoautotrophic conditions (MP), and mixed cultures under heterotrophic conditions (MH). Results are mean + SD (n = 3)
The highest biomass was determined from the sample with MP culture, indicating that the MP culture was most likely to have a high lipid content and accumulation. The microorganisms grown in the MP culture could accumulate a maximum of 117.73 mg lipids L−1 of medium, and the lipid production was approximately 400% higher than that in the MH culture (23.1 mg L−1) at day 10. The highest lipid content was 194.27 mg g−1 of microorganisms (dry weight). Although the lipid content of the MP culture was slightly higher than that of the other three groups, the lipid production was obviously higher owing to the biomass being the highest. The lipid production of the yeast pure culture under the same culture conditions was 86.31 mg L−1.
Fatty acid composition of individual lipid classes
Esterification with methanol was performed to measure the fatty acid composition of lipids extracted from oleaginous microorganisms grown in wastewater under the four culture conditions. The fatty acids were similar under each culture condition, that is, the main compositions were C16 to C22 fatty acids. The main fatty acids consisted of C16:0 (palmitic acid), C18:1 (oleic acid), and C18:2 (linoleic acid) (Table 1). When compared with the other three groups, the palmitic acid and linoleic acid content of the PP group were the highest (21.26 and 58.04%, respectively), while the oleic acid content of the MH group was the highest (50.47%). The PP group did not have a high content of oleic acid, but its linoleic acid content was significantly higher than the other groups, which resulted in a higher degree of unsaturation when compared with the other groups.
Discussion
The nutritional mode (autotrophs and heterotrophs) and the co-culture of C. vulgaris and R. glutinis in winery wastewater have important functions in microbial biomass evolution, lipid production, lipid content, fatty acid profile, and cell structure.
This higher biomass was attributed to the mutualistic relationship between the two species in the co-culture. Xue et al. (2010) monitored dissolved oxygen in R. glutinis culture after A. platensis was added and found that microalgae could provide additional oxygen for yeast, thereby enhancing aerobic metabolism. CO2 produced during yeast metabolism can be used by microalgae during photosynthesis (Cheirsilp et al. 2012).
The highest biomass was determined from the sample with MP culture, indicating that the MP culture was most likely to have a high lipid content and accumulation. The increased lipid production of the MP group might have occurred owing to co-culture interaction with yeast and microalgae and the extra lipids from the yeast (Zhang et al. 2014).
Activated sludge is a semisolid or solid material produced during biological treatment of industrial wastewaters. As a source of energy, carbon, and nutrients, activated sludge contains a variety of microorganisms that utilize the inorganic and organic compounds in water (Konar et al. 1994). The high costs of medium have hampered the development of lipid production by microorganisms. Large-scale wastewater culture technique is cost-effective, although it exhibited slight improvement of lipid production in proportion to medium culture technique. Nevertheless, biodiesel production based on the photoautotrophic growth of microalgae with wastewater can be an economical and technically feasible solution.
Zhang et al. (2014) investigated the biomass, lipid production, fatty acid content, and other nutrients present in microorganisms by using different culture methods (PP; PH; MP; MH). The four culture methods above were conducted in nutrient medium. When compared with the experimental results in the medium, the microbial lipid fatty acid composition in wastewater was similar to the main ingredients of palmitic acid (C16:0) and oleic acid (C18:1). Palmitic acid and linoleic acid were obviously higher in the PP group than in other groups. The difference between the PP group and the other groups was that the length of the fatty acid chain was between C15 and C19, and that, with eight to ten varieties, there were more types of fatty acid (Table 1). These results indicate that co-cultures under photoautotrophic conditions can potentially be used as biodiesel feedstock.
In general, the co-culture of C. vulgaris and R. glutinis under photoautotrophic conditions resulted in higher cell numbers, biomass, lipid production and content, and oleic acid content than the other culture conditions. Therefore, a co-culture with yeast under photoautotrophic conditions has greater potential in the inexpensive production of biodiesel.
References
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Borowitzka MA (2013) Energy from microalgae: a short history. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 1–15
Cai S, Hu C, Du S (2007) Comparisons of growth and biochemical composition between mixed culture of alga and yeast and monocultures. J Biosci Bioeng 104(5):391–397
Cheirsilp B, Kitcha S, Torpee S (2012) Co-culture of an oleaginous yeast Rhodotorula glutinis and a microalga Chlorella vulgaris for biomass and lipid production using pure and crude glycerol as a sole carbon source. Ann Microbiol 62:987–993
Chi Z, Zheng Y, Jiang A, Chen S (2011) Lipid production by culturing oleaginous yeast and algae with food waste and municipal wastewater in an integrated process. Appl Biochem Biotech 165:442–453
Cooksey KE, Guckert JB, Williams SA, Collis PR (1987) Fluorometric determination of the neutral lipid content of microalgal cells using Nile Red. J Microbiol Meth 6:333–345
Crabbe E, Nolasco-Hipolito C, Kobayashi G, Sonomoto K, Ishizaki A (2001) Biodiesel production from crude palm oil and evaluation of butanol extraction and fuel properties. Process Biochem 37:65–71
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Fulton L (2004) Biomass and agriculture sustainability markets and policies. International Energy Agency (IEA) biofuels study–interim report: result and key messages so far. IEA France OECD Publication Service; pp.105–112
Jham N, Teles F, Campos G (1982) Use of aqueous HCl/MeOH as esterification reagent for analysis of fatty acids derived from soybean lipids. J Am Oil Chem Soc 59(3):132–133
Kavadia A, Komaitis M, Chevalot I, Blanchard F, Marc I, Aggelis G (2001) Lipids and γ-linolenic acid accumulation in strains of Zygomycetes growing on glucose. J Am Oil Chem Soc 78:341–346
Konar S, Boocock D, Maom V, Liu J (1994) Fuels and chemicals from sewage sludge: 3. hydrocarbon liquids from the catalytic pyrolysis of sewage sludge lipids over activated alumina. Fuel 73(5):642–646
Krawczyk T (1996) Biodiesel—alternative fuel makes in roads but hurdles remain. Inform 7:801–829
Li Q, Wang M-Y (1997) Use food industry waste to produce microbial oil. Sci Technol Food Ind 6:65–69
Liu Z-Y, Wang G-C, Zhou B-C (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99:4717–4722
Metting B, Pyne J-W (1996) Biologically active compounds from microalgae. Enzym Microb Technol 8:386–394
Ördög V, Stirk WA, Bálint P, Lovász C, Pulz O, van Staden J (2013) Lipid productivity and fatty acid composition in Chlorella and Scenedesmus strains grown in nitrogen-stressed conditions. J Appl Phycol 25:233–243
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Vello V, Phang S-M, Chu W-L, Abdul Majid N, Lim P-E, Loh S-K (2014) Lipid productivity and fatty acid composition-guided selection of Chlorella strains isolated from Malaysia for biodiesel production. J Appl Phycol 26:1399–1413
Xue F, Miao J, Zhang X, Tan T (2010) A new strategy for lipid production by mix cultivation of Spirulina platensis and Rhodotorula glutinis. Appl Biochem Biotechnol 160:498–503
Zhang K, Sun B, She X, Zhao F, Cao Y, Ren D, Lu J (2014) Lipid production and composition of fatty acids in Chlorella vulgaris cultured using different methods: photoautotrophic heterotrophic and pure and mixed conditions. Ann Microbiol 64:1239–1246
Zhu L-Y, Zong M-H, Wu H (2008) Efficient lipids production with Trichosporon fermentans and its use for biodiesel preparation. Bioresour Technol 99:7881–7885
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos. 31571772, 31671963, and 31201339), the National High Technology Research and Development Program of China (863Program, No. 2013AA102205), and the Major State Research Development Program of China (No. 2016YFD0400604).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, K., Zheng, J., Xue, D. et al. Effect of photoautotrophic and heteroautotrophic conditions on growth and lipid production in Chlorella vulgaris cultured in industrial wastewater with the yeast Rhodotorula glutinis . J Appl Phycol 29, 2783–2788 (2017). https://doi.org/10.1007/s10811-017-1168-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1168-5