Abstract
Biomass and lipid productivities of Isochrysis galbana were optimized using nutrients of molasses (4, 8, 12 g l−1), glucose (4, 8, 12 g l−1), glycerol (4, 8, 12 g l−1) and yeast extract (2 g l−1). Combinations of carbon sources at different ratios were evaluated in which the alga was grown at three different light intensities (50, 100 and 150 μmol m−2 s−1) under the influence of three different photoperiod cycles (12/12, 18/6 and 24/0 h light/dark). A maximum cell density of 8.35 g l−1 with 32 % (w/w) lipid was achieved for mixotrophic growth at 100 μmol m−2 s−1 and 18/6 h light/dark with molasses/glucose (20:80 w/w). Mixotrophic cultivation using molasses, glucose and glycerol was thus effective for the cultivation of I. galbana.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae have attracted considerable attention with advantages of fast growth, short production cycle, efficient oil production and requirement of less agricultural land than is needed for growth of plant oil crops (Guan Hua et al. 2010). It utilizes sunlight efficiently and has important applications as they produce polyunsaturated fatty acids (PUFA; eicosapentaenoic and docosahexaenoic acids), carotenes and phycobiliproteins (Reis et al. 1996). PUFA provides significant health benefits to the human population by reducing the risk of cardiovascular diseases (Romieu et al. 2005).
Accumulation of lipid in the microalgae depends on diverse factors such as temperature, pH, nutritional imbalances (carbon, nitrogen, phosphorous, and silicate), growth regime (autotrophic, mixotrophic and heterotrophic), age of the culture, and the specific microalgal strain (Chisti 2007).
Today, the most common procedure for cultivation of microalgae is photoautotrophic culture. However, this gives low biomass and lipid productivities due to mutual shading of cells and slow growth rate. Other modes of cultivation, such as heterotrophic and mixotrophic, have therefore been investigated. In heterotrophic cultivation, organic carbon sources are used in the absence of light, whereas in mixotrophic cultivation CO2 and an organic carbon source are simultaneously assimilated in the presence of light.
Under mixotrophic conditions, some microalgae have higher growth rates than photoautotrophic conditions (Cheirsilp and Torpee 2012) and produce compounds that are synthesized during both heterotrophic and phototrophic conditions. This has been considered as a potential alternative strategy for producing wide range of economically-viable microalgal products in a short span of time.
Heterotrophic and mixotrophic cultivation of Phaeodactylum tricornutum using glycerol produced maximal cell productivity of 21 mg h−1(Cerón García et al. 2000). Mixotrophic cultivation of Chlorella protothecoides and Nannochloropsis sp. using glucose (Heredia-Arroyo et al. 2009) and acetate (Liang et al. 2009) produced maximum biomasses of 5.9 and 2 g l−1, respectively. The mixotrophic growth of C. protothecoides was reported to produce a 69 % higher lipid yield on glucose when compared to heterotrophic metabolism (Wang and Rischer 2013).
The high cost of glucose limits its commercial feasibility as a carbon source; therefore cheaper alternatives are required. Molasses (by-product from sugarcane industry) and glycerol (by-product from biodiesel industry) can be used as potential alternative carbon sources.
In the current study, effect of different light intensities and photoperiod regimes (exposure to light/dark cycles) on cell growth rate, biomass and lipid productivity of I. galbana were investigated. In addition, fatty acid composition was determined for phototrophic and mixotrophic cultivation.
Materials and methods
Isochrysis galbana was collected from Central Institute of Brackish water Aquaculture (CIBA), Muttukadu, Chennai, India.
Culture medium
The growth medium (Conway medium) contains stock A (in g l−1): NaNO3 (100), NaH2PO4 (20), Na2EDTA (45), H3BO3 (33.6), FeCI3·6H2O (1.3), MnCl2·4H2O (0.36), trace metal solution, stock B (in g l−1): ZnCl2 (4.2), COCl2·6H2O (4), (NH4)6 MO7O2·4H2O (1.8), CuSO4·5H2O (4) and vitamin solution (in 200 ml): B12 (10 mg), B1 (thiamine) (200 mg). Conway medium was prepared by adding 1 ml stock solution A, 0.5 ml stock solution B and 0.1 ml vitamin stock solution in 1 l of filtered-sterilized seawater.
Algal cultivation using different carbon and nitrogen sources
Isochrysis galbana was cultured in 1 l Conway medium and maintained at 22–24 °C, pH 7.3–7.5 under aseptic conditions. Glucose (4, 8 and 12 g l−1), glycerol (4, 8 and 12 g l−1) and molasses (4, 8 and 12 g l−1) were used as carbon sources at light intensity of 70–75 μmol m−2 s−1 with photoperiod 18/6 h (light/dark regimes) until it reached stationary phase. Yeast extract at 2 g l−1 was used as nitrogen source. The amounts of carbon (4–12 g l−1) added were selected based on data available from literature for heterotrophic and mixotrophic cultivation of marine microalgae (Azma et al. 2011; Cerón García et al. 2000). To examine the effect of light intensity on cell growth and total lipid production, the cultures were grown at three different light intensities: 50, 100 and 150 μmol m−2 s−1. The effect of cyclic illumination was also investigated under three different photoperiod conditions, light/dark = 24/0, 12/12 and 18/6 h. All microbiological-grade carbon and nitrogen sources used in the experiments are known to be stable when subjected to sterilization at 121 °C for 15 min. For experiments with bioreactors, mixotrophic cultivation was carried out in 1 l and 5 l closed bubble-column glass photo-bioreactors containing 1 l and 3 l Conway medium with different carbon sources. The photo bioreactor was aerated with CO2-enriched air (2 % v/v CO2) at 0.4 vvm (vessel volume per minute).
Determination of carbohydrate concentration in culture media
Glucose, glycerol, sucrose and fructose concentrations in culture media were determined using HPLC equipped with a refractive index (RI) detector and a 300 × 6.5 mm Chrompack column at 60 °C, with 5 mM H2SO4 as the eluent at 0.5 ml min−1 and a sample volume of 20 μl.
Lipid extraction protocol
Lipid was extracted from lyophilized algal biomass using a modified method of Bligh and Dyer. Freeze-dried cells (100 mg) were weighed accurately in a 15 ml centrifuge tube; 3 ml chloroform:methanol (2:1 v/v) containing 0.5 mg butylated hydroxytoluene (BHT) ml−1 was added and the tube was shaken gently overnight at room temperature. After centrifugation at 2500×g for 5 min, the supernatant containing the extracted lipid was stored at 4 °C throughout the study. The extract was evaporated at 40 °C to remove solvents. The final lipid concentration was determined gravimetrically. Lipid was methylated by direct acid-catalyzed transesterification using 2 ml 4 % (v/v) H2SO4 in methanol (75 °C for 1 h).
Fatty acid analysis
The fatty acid methyl esters (FAMEs) were extracted with hexane and analyzed by Agilent 7890 gas chromatography and a capillary column (60 m × 250 μm × 0.2 μm) with helium as a carrier gas at 0.7 ml min−1. Samples, 1 μl, were injected in the split (20:1) injection mode. The inlet and detector were at 260 °C and the oven was programmed initially at 100 °C and then raised to 250 °C in steps of 10 °C min −1 and thereafter maintained at 250 °C for 3 min. FAMEs were identified by chromatographic comparison with authentic standards.
Statistical analysis
The data are expressed as mean ± SD (standard deviation) and the mean is the average of three test results per experiment. The experiments were repeated thrice to confirm the results.
Results and discussion
Effect of individual carbon sources on mixotrophic growth of Isochrysis galbana
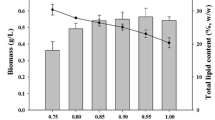
To assess the mixotrophic potential of I. galbana, the effect of different carbon sources on biomass concentration and lipid productivity was evaluated. Conway medium with 2 g of yeast extract l−1 was used as nitrogen source. The biomass concentration was highest for glucose (3.15 g l−1), followed by molasses (2.66 g l−1) and lowest for glycerol (2.35 g l−1) at 4 g l−1 in agreement with experiments on Chlorella vulgaris by Yeh et al. (2012) and decreased with increase in level of carbon sources (Table 1). This could be attributed to substrate inhibition as observed by Mitra et al. (2012). Similar results were achieved for Tetraselmis suecica when grown at high glucose concentration (Azma et al. 2011). Light penetration was not affected for molasses at 4 g l−1 but was affected by further increase in its concentration.
The lipid production was highest for glucose (0.94 g l−1), followed by molasses (0.82 g l−1) and lowest for glycerol (0.7 g l−1) at 4 g l−1(Table 1). Lipid content decreased with increase in carbon sources concentration above 4 g l−1 and this was in accordance with the results obtained by Cheirsilp and Torpee (2012). The complete utilization of glucose, glycerol and molasses were observed for initial concentration of 4 g l−1. Nearly 60 % (w/w) of carbon sources were utilized when at 8 g l−1 and 33 % (w/w) when at 12 g l−1.
Combined effects of carbon sources on biomass and lipid production
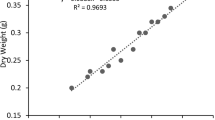
To assess the synergistic effects of carbon sources on biomass production and lipid productivity, three different combinations (glucose/glycerol, molasses/glucose and glycerol/molasses) were tested. Six different ratios (100:0, 80:20, 60:40, 40:60, 20:80, 0:100) were taken for each combination (Fig. 1).
Isochrysis galbana cultivated at different ratios of glucose/glycerol, molasses/glucose and glycerol/molasses concentration under light intensity of 70 μmol m−2 s−1 with photoperiod (18/6 h). (100:0–6:0 g l−1, 80:20–4.8:1.2 g l−1, 60:40–3.2:2.4 g l−1, 40:60–2.4:3.2 g l−1, 20:80–1.2:4.8 g l−1, 0:100–0:6 g l−1)
Maximum biomass concentration of 6.17 g l−1 was achieved for 20:80 % (w/w) molasses/glucose (1.2:4.8 g l−1), followed by 5.34 g l−1 for 20:80 % (w/w) glycerol/glucose (4.8:1.2 g l−1) and 5.14 g l−1 for 40:60 % (w/w) molasses/glucose (2.4:3.6 g l−1) (Fig. 1). Biomass obtained using combined carbon sources (6.17 g l−1) was twice that of individual carbon sources (3.15 g l−1). Similar results were obtained for C. vulgaris with 80:20 % (w/w) of glucose/glycerol (Arroyo et al. 2010) and 20:80 % (w/w) of glucose/glycerol (Kong et al. 2013). However the lipid content (25–30 % w/w) was similar for individual and combined carbon sources. Cell growth inhibition was observed with higher glycerol and molasses concentration (Arroyo et al. 2010).
Effect of photoperiod and light intensity on biomass of Isochrysis galbana
The same species of microalga responds differently to varying light intensities and photoperiods (Danesi et al. 2004). Three best combinations of carbon sources [20:80 % (w/w) molasses/glucose, 80:20 % (w/w) glucose/glycerol, 40:60 % (w/w) molasses/glucose] that gave higher biomass concentration and lipid content were selected for further optimization studies and the results were summarized in Tables 2, 3 and 4. The stationary phase of 120–144 h was observed for all the cell growth patterns (Figs. 2, 3, 4).
Under continuous illumination, the photoperiod of 24:0 h for varying light intensities (50, 100 and 150 μmol m−2 s−1) less biomass productivity was observed (Figs. 2, 3, 4). This is because of the decrease in growth rate observed for a total photoperiod (24:0 h) as reported in earlier studies (Richmond 2004). A similar pattern was observed for all three media combinations (molasses/glucose, glucose/glycerol and molasses/glucose) used in this study.
Availability and intensity of light are major factors controlling productivity of photosynthetic cultures (Qin 2005). We have observed reduced biomass productivity at higher light intensity of 150 μmol m−2 s−1 under all three photoperiods. This may be due to occurrence of photo-inhibition (Wahidin et al. 2012).
The highest biomass, 8.35 g l−1, was achieved with a light intensity of 100 μmol m−2 s−1 under photoperiod of (18/6 h) using molasses/glucose (20:80 w/w) (Alkhamis and Qin 2013; Liu et al. 2013; Wahidin et al. 2012) (Tables 2, 3, 4). This was 4.5 times higher than that (1.88 g l−1) obtained by Picardo et al. (2013). The corresponding lipid productivity was 0.45 g l−1 day−1.
Fatty acid profiling
Fatty acid composition of I. galbana comprised of 25–29 % (w/w) saturated, 23–27 % (w/w) monounsaturated and 36–42 % (w/w) PUFA. The major fatty acids present were palmitic acid (16:0), palmitoleic acid (16:1 n-7), eicosapentaenoic acid (20:5 n-3) and docosahexaenoic acid (22:6 n-3). Docosapentaenoic acid (DPA) (22:5 n-3) at 2–3 % (w/w) was also observed in PUFA (Fig. 5).
Minor variations in fatty acid profile of I. galbana were observed when cultivated under phototrophic and mixotrophic conditions. In mixotrophic condition, the levels of saturated fatty acids increased with decrease in the levels of mono- and poly-unsaturated fatty acids.
Studies reported by Yoshioka et al. (2012) showed that I. galbana cultivated under various light conditions contained 26.2–29.4 % (w/w) saturated, 23.2–25.2 % (w/w) monounsaturated, and 45.5–50.5 % (w/w) PUFA. These results were in accordance with the fatty acid profile observed in our study.
In the present work, effects on biomass and lipid production for three best carbon source combinations were studied under different photoperiods and light intensities. Maximum cell density of 8.35 g l−1 was observed for mixotrophic condition, whereas for phototrophic growth it was only 0.9 g l−1. I. galbana showed maximum lipid productivity of 0.45 g l−1 day−1 at a light intensity (100 μmol m−2 s−1) under a photoperiod of (18/6 h) with molasses/glucose (20:80 w/w). This study demonstrates the potential utilization of low cost and waste by-products as carbon sources. Microalgal biomass produced could be used as a feedstock for production of biofuel, PUFA, aquaculture and for other pharmaceutical applications.
References
Alkhamis Y, Qin JG (2013) Cultivation of Isochrysis galbana in phototrophic, heterotrophic and mixotrophic conditions. Biomed Res Int 1–9
Azma M, Mohamed MS, Mohamad R, Rahim RA, Ariff AB (2011) Improvement of medium composition for heterotrophic cultivation of green microalgae, Tetraselmis suecica, using response surface methodology. Biochem Eng J 53:187–195
Cerón García MC, Fernández Sevilla JM, Molina Grima E, Camacho G (2000) Mixotrophic growth of Phaeodactylum tricornutum on glycerol: growth rate and fatty acid profile. J Appl Phycol 12:239–248
Cheirsilp B, Torpee S (2012) Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour Technol 110:510–516
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Danesi EDG, Rangel Yagui CO, Carvalho JCM, Sato S (2004) Effect of reducing the light intensity on the growth and production of chlorophyll by Spirulina platensis. Biomass Bioenergy 26:29–335
Guan Hua H, Chen F, Wei D, Wu ZX, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87:38–46
Heredia-Arroyo T, Wei W, Hu B (2009) Oil accumulation via heterotrophic/mixotrophic Chlorella protothecoides. Appl Biochem Biotechnol 162:1978–1995
Kong WB, Yang H, Cao YT, Song H, Hua SF, Xia CG (2013) Effect of glycerol and glucose on the enhancement of biomass, lipid and soluble carbohydrate production by Chlorella vulgaris in mixotrophic culture. Food Technol Biotechnol 51:62–69
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Liu J, Sommerfeld M, Hu Q (2013) Screening and characterization of Isochrysis strains and optimization of culture conditions for docosahexaenoic acid production. Appl Microbiol Biotechnol 97:4785–4798
Mitra D, Leeuwen JHV, Lamsal B (2012) Heterotrophic/mixotrophic cutivation of oleaginous Chlorella vulgaris on industrial co-products. Algal Res 1:40–48
Picardo MC, Medeiros JLD, Araujo OQF, Chaloub RM (2013) Effects of CO2 enrichment and nutrients supply intermittency on batch cultures of Isochrysis galbana. Bioresour Technol 143:242–250
Reis A, Gouveia L, Veloso V, Fernandes HL, Empis JA, Novais JM (1996) Eicosapentaenoic acid rich biomass production by the microalgae Phaeodactylum tricornutum in a continuous-flow reactor. Bioresour Technol 55:83–88
Richmond A (2004) Biological principles of mass cultivation. In: Richmond A (ed) Handbook of microalgal mass, culture: biotechnology and applied phycology. CRC Press, Boca Raton
Roe JH (1955) The determination of sugar in the blood and spinal fluid with anthrone reagent. J Biol Chem 212:335–343
Romieu I, Tellez Rojo MM, Lazo M, ManzanoPatino A, Cortez Lugo M, Julien P, Belanger MC, Hernandez-Avila M, Holguin F (2005) Omega-3 fatty acid prevents heart rate variability reductions associated with particulate matter. Am J Respir Crit Care Med 172:1534–1540
Wahidin S, Idris A, Raehanah S, Shaleh M (2012) The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour Technol 129:7–11
Wang Y, Rischer R (2013) Mixotrophic continous flow cultivation of Chlorella protothecoides for lipids. Bioresour Technol 144:608–614
Yeh KL, Chen CY, Chang JS (2012) pH-stat photoheterotrophic cultivation of indigenous Chlorella vulgaris ESP-31 for biomass and lipid production using acetic acid as the carbon source. Biochem Eng J 64:1–7
Yoshioka M, Yago T, Yoshie-Stark Y, Arakawa H, Morinaga T (2012) Effect of high frequency of intermittent light on the growth and fatty acid profile of Isochrysis galbana. Aquaculture 338:111–117
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Babuskin, S., Radhakrishnan, K., Babu, P.A.S. et al. Effect of photoperiod, light intensity and carbon sources on biomass and lipid productivities of Isochrysis galbana . Biotechnol Lett 36, 1653–1660 (2014). https://doi.org/10.1007/s10529-014-1517-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1517-2