Abstract
The genera Kappaphycus Doty and Eucheuma J. Agardh are important sources of carrageenan in Malaysia, offering lucrative revenues to the carrageenan industry, economy, and the local community. The extensive range of morphotypes and the lack of distinct morphological characteristics led to the application of molecular systematics in elucidating this taxonomic confusion. Local varieties of Kappaphycus and Eucheuma, identified using putative external morphology, were analyzed using the mitochondrial cox2–3 spacer and plastid RuBisCO spacer molecular markers. Phylogenetic analysis of these and non-local specimens indicate that Kappaphycus and Eucheuma are genetically distinct. Three main genotypes of Kappaphycus alvarezii were identified, of which two are extant in Hawaii. Morphological and color variations are not supported by molecular data, indicating that most of the local names are not genetically based. Both the cox2–3 spacer and RuBisCO spacer generated phylogenetic trees with similar topology except in variation of nodal supports. The two markers showed clear separation between Kappaphycus and Eucheuma and the existence of three Malaysian Kappaphycus cultivars. Cox2–3 spacer data is more variable and provides better resolution than the RuBisCO spacer, showing that Kappaphycus is more diversified with a larger number of genotypes, strains, and species which are unique to Southeast Asia. Kappaphycus sp. “Aring-aring” appeared to be phenotypically and genotypically different from other Kappaphycus congeners, whereas Kappaphycus striatum exhibited two different genotypes. Our data indicate that Eucheuma denticulatum is the dominant species in Malaysian waters and also suggested paraphyly in Eucheuma which will require further studies. The application of molecular taxonomy on Malaysian Kappaphycus and Eucheuma proves useful, offering valuable insights into the taxonomy and distribution of these commercially important Rhodophytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing demand for carrageenan has been substantial through the years, contributing to the widespread cultivation of carrageenophytes Kappaphycus Doty and Eucheuma J. Agardh, particularly throughout paleotropical regions of the world, with the Philippines and Indonesia currently leading in terms of overall kappa-carrageenan yield (Bindu and Levine 2010; Bixler and Porse 2010; Phang et al. 2010; Pickering 2006). Mariculture of Kappaphycus and Eucheuma are generally well studied (Ask and Azanza 2002; Bindu 2010; Gerung and Ohno 1997; Góes and Reis 2011; Hayashi et al. 2007; Hurtado et al. 1996, 2001, 2008; Thirumaran and Anantharaman 2009), albeit cultivation still relies heavily on vegetative propagation of cultivated variants despite efforts to introduce carpospores (Azanza and Ask 2001; Luhan and Sollesta 2010), tetraspore cultures (Bulboa et al. 2007, 2008; Paula et al. 1999) and even hybrids (Cheney et al. 1998).
Possible misidentification of cultivated strains which led to inevitable loss in production potential is widespread due to the morphologically plastic nature of Kappaphycus and Eucheuma (Bindu and Levine 2010; Conklin et al. 2009; Doty 1985; Doty and Norris 1985; Paula et al. 1999; Phang et al. 2010; Zuccarello et al. 2006). Several approaches have been used in addressing the taxonomic problems of these seaweeds, where associated genetic variation studies were carried out via random amplified DNA polymorphic markers (Dang et al. 2008) and sequences of various molecular markers (Conklin et al. 2009; Zhao and He 2011; Zuccarello and West 2006; Zuccarello et al. 1999a, 1999b, 2006). The genetic approach has proven to be generally more reliable and useful than identification solely based on macroscopic and microscopic morphology of Kappaphycus and Euchuema. Molecular taxonomy studies of these carrageenophytes based on the mitochondrial-encoded partial cox1 and cox2–3 spacer, nuclear-encoded ribosomal internal transcribed spacer (ITS) and partial 28S large subunit, plastid-encoded rbcL, RuBisCO spacer and 23S universal plastid amplicon (UPA), have shown promising results (Conklin et al. 2009; Zhao and He 2011; Zuccarello et al. 1999a, b, 2006). Wider applications of the molecular approach would be useful where superior strains could be identified and commercially utilized and where potential bioinvasive individuals could be verified and curtailed accordingly.
In Malaysia, the cultivation of Kappaphycus and Eucheuma is concentrated mainly along the Sabah coastline, and recently on a small scale at Pangkor Island, west coast Peninsular Malaysia (Phang 2006). Increasing demands have led to the rapid increase in the farming of these carrageenophytes, which are processed into semi-refined carrageenan chips, or are consumed as condiments by the locals. Malaysia produced 15,000 t of dried carrageenan in year 2010, a 2,000 t increase from year 2009 (personal communication with Adibi Rahiman B. Md. Nor, officer from Department of Fisheries Malaysia). Currently, twelve varieties of Kappaphycus, locally named Buaya (Crocodile), Tambalang Brown, Tambalang Giant, Tambalang Green, Tangan-tangan (Loving Beauty), Black Durian, Red Durian, Yellow Durian, Green Flower, Yellow Flower, Aring-aring; and a variety of Eucheuma, locally named Spinosum have been reported as cultivars in Malaysian waters. Varieties are named based on external morphology and color. Local names are believed to be unreliable and are incapable of distinguishing varieties accurately. This leads to the inability to select good cultivars for farming and the local farmers ended up growing a mixed population of Kappaphycus and Eucheuma, resulting in non-optimal production yields. The application of molecular systematics allows the correct identification of cultivars and hence the selection of the best varieties for farming. This study aims to: (1) elucidate the taxonomic confusion associated with the varieties of Kappaphycus and Eucheuma in Malaysia; (2) determine the phylogenetic relationship between Malaysian varieties of Kappaphycus and Eucheuma and those from within and outside Malaysia.
Materials and methods
Kappaphycus and Eucheuma samples were collected from various locations around the Pangkor islands, Sandakan, and Semporna (Selakan, Sabangkat, Bum-bum Island, Omadal, Sisipan, and Karindingan). Cultivated samples were obtained from various seaweed farms whereas wild samples were collected via snorkeling and scuba diving with assistance from the locals. Wild samples in this context refer to samples collected from locations away from seaweed farms. General physical appearance of collected samples were observed and (1) cultivated samples were classified and named based on putative, external morphological criteria differentiating local varieties; and (2) wild samples were individually described.
DNA work
A small portion of the fresh specimen thallus (approximately 1–2 cm of the tip apex) was excised and preserved in silica gel for DNA isolation, with regular change of silica gel to ensure dryness. Remaining plant specimens were subsequently dried and kept as herbarium specimens for future reference. Details of samples are summarized in Table 1. Dried specimens were subjected to the DNA extraction protocol via the i-genomic Plant DNA Extraction Mini Kit (iNtRON Biotechnology, Korea).
PCR amplification of the mitochondrial cox2–3 spacer and plastid RuBisCO spacer genes was carried out using primers suggested by Zuccarello et al. (1999a, b; 2006): (1) Cox2–3 spacer primers (Forward: 5′-GTACCWTCTTTDRGRRKDAAATGTGATGC-3′; Reverse: 5′-GGATCTACWAGATGRAAWGGATGTC-3′); (2) RuBisCO spacer primers (Forward: 5′-TGTGGACCTCTACAAACAGC-3′; Reverse: 5′-CCCCATAGTTCCCAAT-3′). Amplification of the DNA markers was performed with a Labnet MultiGene™ Gradient Thermalcycler (Labnet, USA) using an i-Taq™ Plus DNA Polymerase Kit (iNtRON Biotechnology, Korea). The parameters used were as described in Lim et al. (2007) with only changes on the annealing temperature of 50 °C for the cox2–3 spacer and 52 °C for the RuBisCO spacer. Amplicons were electrophoresed through a 1.0 % agarose gel stained with SYBR®Safe DNA gel stain (Invitrogen, USA). DNA purification was conducted using LaboPass™ (Cosmo Gentech, Korea) gel and PCR purification kits. The purified PCR products were sent to 1st Base Laboratories (Malaysia) for DNA sequencing.
Phylogenetic analyses
Resulting DNA electropherograms for both molecular markers were truncated and processed via ChromasPro V1.5 (Technelysium Pty Ltd) and multiple sequence alignments encoded in NEXUS format were generated using ClustalX V2.0 (Larkin et al. 2007). Maximum parsimony (MP) analyses were carried out using PAUP 4.0b10 (Swofford 2003); where the MP tree was inferred based on the heuristic search algorithm. MP parameters were as follows: 100 random sequence addition, tree bisection reconnection branch swapping and all characters unordered and unweighted. Nodal bootstrap supports were computed using 1,000 bootstrapping replications.
Kakusan v.3 (Tanabe 2007) was utilized to output best fit models for (1) maximum likelihood (ML) analysis via TreeFinder October 2008 version (Jobb et al. 2004); and (2) Bayesian (BI) analysis via Mr. Bayes 3.1.2 (Huelsenback et al. 2001). These models were selected using the corrected Akaike information Criterion for ML (Akaike 1973) and the Bayesian Information Criterion for BI (Schwarz 1978). Phylograms were initially constructed using TreeFinder prior to the annotations of bootstrap values generated via 1,000 ML bootstrap replicates. BI analyses were performed using the Markov chain Monte Carlo (MCMC) method. Two independent runs of 2,000,000 generations with four chains were conducted, with trees sampled every 500th generation. Parameters of each MCMC run were subsequently checked for convergence using Tracer v1.5 (http://tree.bio.ed.ac.uk/software/tracer/). Conservatively, the first 100,000 generations from each run were discarded as burn-in prior to the construction of a 50 % majority-rule consensus tree. Associated phylogenetic trees were viewed and processed with Treev32 (Page 1996).
Alternative phylogenetic hypotheses were tested via the Shimodaira–Hasegawa (SH) test under the maximum likelihood criterion using PAUP4.0b10 (Shimodaira and Hasegawa 1999). The parameters were set using resampling of estimated log-likelihoods with 1,000 bootstrap replicates; where comparisons were performed between optimal trees and topologically constraint trees. The alternative hypothesis was a tree topology constructed by constraining Eucheuma samples to be monophyletic.
Results
A total of 31 samples (Figs. 1 and 2) were examined and morphological descriptions are summarized in Table 2.
Malaysian Kappaphycus and E. denticulatum varieties. a Yellow/Brown Flower. b Green Flower. c Wild, cystocarpic K. striatum, isolate 105. d Wild K. striatum, isolate 31. e Wild, cystocarpic K. striatum, isolate 98. f Wild, cystocarpic K. striatum, isolate 83. g Aring-aring. h Wild, cystocarpic Aring-aring. i Spinosum. j Wild E. denticulatum, isolate 56. k Wild E. denticulatum, isolate 57. l Wild E. denticulatum, isolate 99. [Scale bar = 5 cm]
Phylogenetic analyses
Cox2–3 spacer sequences were truncated until a length of 341 bp to avoid ambiguities at sequence terminals. Within the dataset, a total of 142 sites (41.6 %) were phylogenetically informative, and 171 characters (50.1 %) were constant within the ingroups. The informative variation was randomly distributed throughout the entire length of the gene and indels were observed at nucleotide sites 164, 304, and 305. An adenine insertion at site 164 separates Madagascar and Tanzanian Kappaphycus alvarezii isolates E16 and E130 from the rest of the sequences; whereas indels at sites 304 and 305 separate Kappaphycus sequences from that of Eucheuma. Parsimony analysis of the cox2–3 spacer generated six most parsimonious trees with a consistency index of 0.607 and a retention index of 0.9113. The aligned RuBisCO spacer sequences consisted of 264 characters, where 44 (16.7 %) characters are phylogenetically informative, again randomly scattered. Indels were observed at nucleotide sites 78 and 88, with an insertion of Thymine and Adenine respectively. The former is apparent in a majority of Eucheuma, including those from Malaysia. Phylogenetic analyses produced six most parsimonious trees with a consistency index of 0.7363 and a retention index of 0.92.
Likelihood values for the Bayesian analysis, as indicated by Tracer v1.5, reached apparent stationary (burned-in) prior to 50,000 and 60,000 generations for cox2–3 spacer and RuBisCO spacer, respectively. This observation indicated that the conservative discard of the first 100,000 generations was sufficient to exclude non-stationary estimates.
SH test results (Shimodaira and Hasegawa 1999) have shown that resulting cox2–3 spacer trees from enforced topological constraints were significantly inferior to the optimal, unconstrained ones. Conversely, SH test on the RuBisCO spacer constraint and optimal trees showed that they are not significantly different from each other.
Systematics
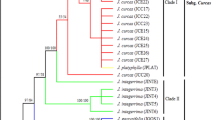
The respective phylogenetic trees based on the mitochondrial-encoded cox2–3 spacer (Fig. 3) and plastid-encoded RuBisCO (Fig. 4) DNA markers are quite similar in terms of tree topology but are not identical. Better resolution was observed for the cox2–3 spacer. The basic tree topology of both trees remains congruent with that of Zuccarello et al. (2006) and Conklin et al. (2009), where Kappaphycus samples are basically distinct from Eucheuma samples, despite some minor deviations.
Maximum likelihood 50 % majority-rule consensus tree based on the cox2–3 spacer. -Ln likelihood score was 1,952.259. (Substitution rate parameters: TC = 0.4528395; TA = 0.01014707; TG = 0.0182113; CA = 0.06420285; CG = 0.001759723; AG = 0.4528395). Numeric values at nodes are arranged in an order of ML bootstrap support/MP bootstrap support/Bayesian posterior probabilities. Local samples are denoted according to isolate no./variety/color/origin/cultivated [C] or wild [W]. Asterisks (*) indicate cystocarpic plants
Maximum likelihood 50 % majority-rule consensus tree based on the RuBisCO spacer. Ln likelihood score was 795.2914. (Substitution rate parameters: TC = 0.5043717; TA = 0.007914078; TG = 0.07270443; CA = 0.07270443; CG = 0.007914078; AG = 0.3343913). Numeric values at nodes are arranged in an order of ML bootstrap support/MP bootstrap support/Bayesian posterior probabilities. Local samples are denoted according to isolate no./variety/color/origin/cultivated [C] or wild [W]. Asterisks (*) indicate cystocarpic plants
Clade annotations for both molecular markers are synchronized for better data presentation. The cox2–3 spacer-based phylogenetic tree showed moderate support for the monophyly of all Malaysian K. alvarezii varieties (A1), both wild and cultivated ones along with an unidentified Hawaiian Kappaphycus (2614) and a Venezuelan K. alvarezii (E3) (ML = 80.9 %; MP = 75 %; BI = 0.71). K. alvarezii (E16 and E130) collected from Madagascar and Tanzania were respectively grouped together, forming a distinctive clade, denoted A2 (ML = 81.3 %; MP = 71 %; BI = 1.00). Hawaiian K. alvarezii (E57, E71, 919, and 3955) formed a distinct, highly supported clade A3 (ML = 99.8 %; MP = 97 %; BI = 1.00) which is resolved as a polytomy with the previous K. alvarezii samples and the K. striatum samples. The K. striatum clade (B) was divided into two subclades, where a majority of cultured and wild Malaysian Flower varieties (1, 31, 59, and 60) were grouped together (B1) with a cultivated K. striatum collected from the Philippines (E89) (ML = 85.4 %; MP = 65 %; BI = 0.56); whereas several wild Malaysian specimens (83, 98, and 105) were resolved with two wild Indonesian K. striatum (E48 and E117) as indicated by Clade B2 (ML = 84.2 %; MP = 63 %; BI = 0.76). Clade C depicts a monophyletic clade consisting solely of Aring-aring varieties (14 and 49), including a wild one (93) from Malaysian waters (ML = 99.8 %; MP = 93 %; BI = 0.80). This clade formed a sister group to the K. alvarezii and K. striatum samples. All the mentioned clades (A, B, and C) eventually constitute a sister group, with no support, to a lone Kappaphycus cottonii sample (E108).
Eucheumoid samples Eucheuma denticulatum, Eucheuma platycladum, and several unidentified Eucheuma were collectively grouped in clades E and F. The phylogenetic relationship between these two main clades is again poorly supported. For E. denticulatum, specimens of different origin constitute a major monophyletic clade E with high support that subdivides into three subclades. Malaysian Spinosum, be it wild or cultivated, jointly formed clade E1, along with a cultivated Indonesian E. denticulatum (ML = 94.7 %; MP = 81 %; BI = −). Clade E2 comprises of two Hawaiian and one Indonesian E. denticulatum (ML = 99.4 %; MP = 81 %; BI = 0.80); whereas clade E3 consisted of E. denticulatum from Southeastern Africa—Madagascar, Mauritius, and Tanzania. The taxonomic position of Betaphycus philippinensis (E118) remains unresolved due to the weak support. Eucheuma isiforme (G) appeared as the basal group to the ingroups (ML = 99.6 %; MP = 100 %; BI = 1.00), with Solieria sp. being the outgroup of this study.
The RuBisCO spacer phylogenetic tree demonstrated a relatively simpler topology, also with clear separation between the Kappaphycus and Eucheuma except for two sequences attributed to Kappaphycus from the Philippines. For K. alvarezii (A), Malaysian varieties were grouped together with the K. alvarezii samples from Madagascar (E16), Venezuela (E3), and the Philippines (2009C and 2009N). One K. alvarezii from Hawaii (E57), being slightly different from its congeners, appeared as the sister group to the K. striatum clade (B) which consisted of all K. striatum specimens and a probably misidentified “K. alvarezii” (2002H; AF489868), regardless of locality (ML = 95.3 %; MP = 87 %; BI = 1.00). Aring-aring samples, both cultivated and wild (14, 49, and 81) from Sabah, clustered together with moderate support (C), and appeared distinct from their Kappaphycus counterparts (ML = 88 %; MP = 63 %; BI = 0.80). The RuBisCO spacer tree grouped the available Philippine K. cottonii samples (AF489869 and AY687409) in clade D (ML = 91.7 %; MP = 95 %; BI = 1.00) which is resolved as a polytomy to the aforementioned clades. Remaining E. denticulatum, E. platycladum, and several unidentified Eucheumoid samples were grouped into two distinct clades E and F. Malaysian Spinosum varieties, both cultured and wild, were claded (E1) together with two Indonesian E. denticulatum samples (E13 and E45) (ML = 94.6 %; MP = 89 %; BI = 1.00), jointly forming a sister group, with low support to another E. denticulatum (E60) collected from Mauritius. Clade G consists of E. isiforme samples which were used as outgroups. Solieria sp. was not used as an outgroup in this particular phylogenetic analysis.
Discussion
Morphological studies
Morphological analysis of cultivated Kappaphycus and Eucheuma (Table 2) samples based on plant size, color, branch diameter, branching patterns, and thalli texture was shown capable of differentiating five varieties of K. alvarezii, two varieties of K. striatum, one variety of Kappaphycus sp. (Aring-aring) and one variety of E. denticulatum; as commonly suggested by local farmers. Cultivated samples, grown under better farm management, are often more robust and healthy in terms of growth. Wild specimens proved difficult to differentiate morphologically due to thalli deformities caused probably by adverse environmental conditions or herbivory. Fertile plants may also exhibit vast morphological differences compared to cultivated ones. It is therefore difficult to meaningfully compare morphologies of wild and cultivated specimens, worsen by the fact that wild plants may be residuals drifted from nearby farms.
Identification of cultivated and undamaged K. alvarezii, K. striatum, Kappaphycus sp. (Aring-aring), and E. denticulatum is fairly easy, with branch diameter, branching patterns, and thalli texture being the three main differentiating criteria. Despite the irregular branching patterns, K. alvarezii tend to exhibit the largest branch diameters, followed by K. striatum, Kappaphycus sp. (Aring-aring), and E. denticulatum (Table 2). Branching frequency is highest in K. striatum, where the degree of branching may be quinary or more and the length of branches seldom exceeding 2 cm, thus giving a compact, bunch-like overall appearance. Kappaphycus sp. (Aring-aring) is recognized via the relatively slimmer branch diameters as compared to K. alvarezii and K. striatum, and also the long, slim, tapered, and pointed branch apices. Unlike the fleshy and cartilaginous nature of Kappaphycus, the thallus of E. denticulatum is brittle and inflexible and covered with whorls of characteristic pinnate or pectinate spines.
The existence of different local varieties of Kappaphycus is not supported by molecular data (to be discussed later) despite the differences in terms of color and putative external morphologies. The variation in terms of color and morphological characteristics may be attributed to the interaction between light, water currents, water depth as well as nutrient availability (Góes and Reis 2011; Munoz et al. 2004; Santelices 1999; Thirumaran and Anantharaman 2009). For instance, K. alvarezii domesticated around the Pangkor Islands (Peninsular Malaysia) are generally more robust under the relatively nutrient rich and strong currents in the area, but are in turn more exposed to severe grazing, seasonal epiphyte, and sediment problems. All these factors contribute to morphologies different than those usually observed under proper farm management. Morphological studies indicate a marked difference between cultivated and wild K. striatum. Phylogenetic analyses of four wild K. striatum samples (31, 83, 98, and 105) suggest the existence of only two distinct varieties. Further examination of more samples based on both morphological and molecular analyses are needed to verify this finding. The cultivated Aring-aring varieties (Table 2), often with shades of pale brown or green, are slim with long, slender, and pointed branch apices. Morphological observations has also shown that the hemispheric and swollen cystocarps with dark, central patches of carposporangium exhibited by wild Kappaphycus sp. (Aring-aring) is different from the cone-shaped and somewhat pointed cystocarps of wild K. striatum samples (83, 98, and 105) of this study (fertile K. alvarezii was not observed). These suggest that Kappaphycus sp. (Aring-aring) is different from K. alvarezii and K. striatum which is supported by molecular data. Morphological observations (Doty and Norris 1985) and molecular data both confirmed Malaysian Spinosum variety as E. denticulatum.
Efforts are underway to find type K. alvarezii specimens around Karindingan Island. Wild and fertile K. alvarezii are rare in Malaysian waters, possibly due to the limiting spore dispersal mechanism (Conklin et al. 2009; Paula et al. 1999;Russell 1983); thus less invasive in nature. On the other hand, cystocarp-bearing K. striatum, and Kappaphycus sp. (Aring-aring) are relatively more abundant, especially around the proximity of Sabangkat although there are no reports of adverse ecological impacts of Kappaphycus and Eucheuma on the coral community at this time.
Molecular systematics
The usefulness of the mitochondrial-encoded cox2–3 spacer and plastid-encoded RuBisCO spacer for the phylogenetic analysis of Kappaphycus and Eucheuma is due to the relatively short length for simpler amplification and sequencing with minimal compromise on resolution power (Conklin et al. 2009; Zuccarello et al. 1999a, 1999b, 2006), although earlier studies employed much longer DNA sequences for analysis (Fredericq et al. 1999; Lluisma and Ragan 1995). The introduction of new primers, e.g., partial nuclear 28S rRNA, partial plastid 23S rRNA, and mitochondrial 5′ COI by Conklin et al. (2009) give further support to elucidating the phylogenetic relaitonships of Kappaphycus and Eucheuma. The cox2–3 spacer and RuBisCO spacer trees were not combined in the present study so as to demonstrate the better resolution power of the cox2–3 spacer which exhibits a better phylogenetic inference of Kappaphycus and Eucheuma; where the higher resolving capability could be attributed to the increased mutational rate and thus variability of nucleotides within the mitochondrial genome as compared to the plastid genome (Zuccarello et al. 1999a, b, 2006).
In terms of genetic distance (data not shown), the cox2–3 spacer showed a nucleotide variation range of 1.5 to 15.9 % among congeneric species, and <2.1 % within species. On the other hand, the RuBisCO spacer showed a genetic distance of 0.8 to 5 % in between species, and <0.8 % within species. Another study by Zhao and He (2011) on Kappaphycus and Eucheuma using the nuclear-encoded ITS marker has reported nucleotide variation rates ranging from 7.02 to 7.48 % among different species and less than 1.20 % among the species. The more consistent genetic distances indicate that the ITS can potentially be used as a molecular marker for strain identification and genetic studies of Eucheumatoid algae. However, further studies employing a larger number of taxa would be required in order to assess the actual feasibility.
Following the hypothesis of Zuccarello et al. (2006) on the paraphyletic nature of Eucheuma, a Solieria outgroup was used in this study on the cox2–3 spacer molecular marker, where the phylogenetic tree (Fig. 3) appeared congruent to that of Fredericq et al. (1999). This shows that E. isiforme samples are relatively distinct from other Eucheumoids with high support. Additionally, SH test results of the cox2–3 spacer DNA marker also rejected the monophyly of Eucheuma species in this study, again supported by the results of rbcL gene data (Fredericq et al. 1999). The paraphyletic nature of Eucheuma is now evident and further studies should be conducted accordingly in elucidating the taxonomic position of all the species in this genus. Taxonomic positions of K. cottonii and B. philippinensis in this study remain unresolved due to the arising polytomy; more sampling, higher resolution molecular markers as well as solid morphological studies are needed to address this issue. On a separate note, the addition of Solieria decreased the overall resolution of the RuBisCO spacer tree, probably due to less phylogenetically informative sites which is thus unsuitable for inter-generic comparisons and is hence omitted from this study.
Kappaphycus
Phylogenetic analysis in the present study was based on 25 Malaysian Kappaphycus samples in combination with 12 cox2–3 spacer and nine RuBisCO spacer sequences of Kappaphycus from the GenBank respectively. The cox2–3 spacer was able to differentiate samples down to a more specific level not achievable by the RuBiSCO spacer. Results (Fig. 3) showed that all Malaysian K. alvarezii varieties are more closely related to K. alvarezii from Venezuela, Vietnam, Columbia, Panama, and the Philippines (Zuccarello et al. 2006) as well as an unidentified Hawaiian Kappaphycus; as compared to K. alvarezii of other localities—K. alvarezii (E16 and E130) from Madagascar and Tanzania; and Hawaiian K. alvarezii (E57, E71, 919 and 3955)—which formed two distinctive evolutionary groups. Cox2–3 spacer results (Fig. 3) demonstrated that the Hawaiian K. alvarezii (E57, E71, 919, and 3955) is different from the main K. alvarezii clade (A1 and A2). However, the RuBisCO spacer (Fig. 4) showed that the Hawaiian K. alvarezii (E57) is a sister clade, and thus more closely related to K. striatum. The exact taxonomic status of these Hawaiian specimens remained unclear at this time. The occurrence of more than one species of Kappaphycus “alvarezii” in Hawaii might be due to: (1) the existence of an original local species; or (2) the introduction of more than one species from the Philippines during the 1970s (Doty 1985). The former is possible due to the strategic location of Hawaii within the tropical latitudes and also the lack of similar strains recorded in other parts of the globe as of now. More sampling and morphological studies would be required to support this theory. The inclusion of Kappaphycus from Pangkor into the Malaysian Kappaphycus clade supported its origin in Sabah. In time, this Pangkor population may develop into a distinct variety or species due to the different environmental conditions as well as mutation (Ridley 2000; Weiss and Buchanan 2004).
Morphological plasticity and color variations of Malaysian K. alvarezii congeners were not supported by molecular data, which is anticipated considering the tendency of Kappaphycus thalli structure and color to change indefinitely. No genotypic variations were observed between domesticated and wild specimens. Therefore all the local K. alvarezii varieties (Buaya, Tambalang Brown, Tambalang Green, Tambalang Giant, Tangan-tangan) refer to only one type of K. alvarezii.
K. striatum samples were grouped into two separate clades based on cox2–3 spacer sequences (Fig. 3), where Malaysian Flower samples, both wild and cultivated were grouped together with K. striatum (E89) from the Philippines. Three wild specimens from Sabangkat (83, 98, and 105) formed a clade along with two Indonesian K. striatum samples (E48 and E117), also collected from the wild. These results showed the existence of two K. striatum genotypes. However, it is still unclear which genotype is widely cultivated in Southeast Asia. Increased sample sizes and studies on wild and cultivated K. striatum may provide further insights to the taxonomy of these genotypes. K. striatum is generally less plastic morphologically compared to K. alvarezii and is thus easier to identify. Color variations again do not affect the genotype.
Based on the resulting phylogenetic trees (Figs. 3 and 4), Aring-aring (14 and 49) and wild sample 93 were conspecific, forming an individual monophyletic clade which grouped as a sister taxon to K. alvarezii and K. striatum. These samples (tentatively termed Aring-aring) were inferred with high support to have shared the same common ancestor with that of K. alvarezii and K. striatum. In Malaysia, Aring-aring has been processed by local carrageenan factories for decades and is believed to produce kappa-carrageenan as well. Aring-aring was once reported in the Philippines (Villanueva et al. 2011). However there is no indication whether Philippine and Malaysian “Aring-aring” are similar. There is also no affinity of Malaysian specimens to the gene sequences of other localities available in the GenBank and it might probably be a new species. Further studies, particularly in terms of macroscopic and microscopic morphology, currently in progress, are required to clarify this.
As noted by Zuccarello et al. (2006), the identity of the K. cottonii (E108) sample remains unresolved and requires further clarification. However, it is worth taking note that the K. cottonii (E108) from Zuccarello et al. (2006) was claded together with another Philippine K. cottonii (Lluisma et. al, GenBank sequence no.: AF489869) in the RuBisCO spacer tree. More specimens would be required, supposedly identified with several DNA markers to deepen the understanding of this particular Kappaphycus species.
Eucheuma
A total of six Malaysian Eucheuma samples were used in this phylogenetic study, along with 15 cox2–3 spacer sequences and 11 RuBisCO spacer sequences of Eucheuma from the GenBank. Eucheuma samples in this study were grouped into three main clades, consisting of E. denticulatum (E), E. platycladum (F), E. isiforme (G), and a couple of unidentified Eucheuma sp. The paucity of clear, distinctive identification traits and also the wide range of Eucheumoids have always posed a problem for the elucidation of the taxonomy of Eucheuma (Doty and Norris 1985). However, the cox2–3 spacer proved sufficient for the phylogenetic inference of Malaysian Eucheuma samples. E. denticulatum specimens were separated into two evolutionary groups, in which a lineage consisted of samples from Southeastern Africa (E3) whereas the other composed of Asian specimens (E1 and E2). These Eucheuma were suggested to have been domesticated several times by Zuccarello et al. (2006). Subclade E1 consisted both cultivated variants of Spinosum (44, 45, 46) as well as wild ones (56, 57, and 99) along with an Indonesian cultivated E. denticulatum (E13); whereas the other subclade E2 contained E. denticulatum specimens from Hawaii (888 and 3953) and Indonesia (E32). Phang et al. (2010) suggestion of Malaysian Spinosum variants being E. denticulatum is supported by molecular results in this study. Although no other Eucheuma species had been reported in Malaysia at this time, it is highly possible that other strains would also be available in Malaysian and Philippine waters because of the mutual sharing of the South China Sea with Indonesia. The separation of E. isiforme specimens (G) from the other Eucheuma suggests the need to relook into the taxonomy of this particular species along with its relationship with the rest of the Eucheumoids. As a whole, our knowledge on Eucheuma is unfortunately still very limited at this time, as more researches are being conducted to elucidate this puzzling taxon.
Results from this study have reinforced the molecular phylogenetics of Kappaphycus and Eucheuma. Further taxonomic studies should emphasize more on the integration of non-local Kappaphycus and Eucheuma, elucidation of taxonomically undetermined taxa, ecological impacts, and bioinvasive potential, as well as the characterization of type specimens. This study has elucidated the relationship between the Malaysian Kappaphycus and Eucheuma varieties. All the local varieties, namely Buaya, Tambalang Brown, Tambalang Green, Tambalang Giant, Tangan-tangan are shown to belong to K. alvarezii. K. striatum may possibly be separated into two groups, and the Kappaphycus sp. (Aring-aring) may be a new species. Currently, all the local Eucheuma varieties reported here belong to E. denticulatum. The information generated here can be used to improve the identification and selection of good varieties for improved production by the local farmers, which will help boost the local carrageenan industry in the long run.
References
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F (eds) Second International Symposium Information Theory. Akademia Kiado, Budapest, pp 267–281
Ask EI, Azanza RV (2002) Advances in cultivation technology of commercial eucheumatoid species:a review with suggestions for future research. Aquaculture 206:257–277
Azanza RV, Ask EI (2001) Kappaphycus alvarezii (Doty) Doty carposporeling growth and development in the laboratory. Paper presented at the 17th International Seaweed Symposium, Cape Town, South Africa,
Bindu MS (2010) Empowerment of coastal communities in cultivation and processing of Kappaphycus alvarezii—a case study at Vizhinjam village, Kerala, India. J Appl Phycol 23:157–163
Bindu MS, Levine IA (2010) The commercial red seaweed Kappaphycus alvarezii—an overview on farming and environment. J Appl Phycol 23:789–796
Bixler HJ, Porse H (2010) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 23:321–335
Bulboa C, de Paula EJ, Chow F (2008) Germination and survival of tetraspores of Kappaphycus alvarezii var. alvarezii (Solieriaceae, Rhodophyta) introduced in subtropical waters of Brazil. Phycol Res 56:39–45
Bulboa CR, Paula EJ, Chow F (2007) Laboratory germination and sea out-planting of tetraspore progeny from Kappaphycus striatum (Rhodophyta) in subtropical waters of Brazil. J Appl Phycol 19:357–363
Cheney D, Rudolph B, Wang LZ, Metz B, Watson K, Roberts K, Levine I (1998) Genetic manipulation and strain improvement in commercially valuable red seaweeds. In: Le Gal H, Halvorson O (eds) New developments in marine biotechnology. Plenum Press, New York, pp 101–104
Conklin KY, Kurihara A, Sherwood AR (2009) A molecular method for identification of the morphologically plastic invasive algal genera Eucheuma and Kappaphycus (Rhodophyta, Gigartinales) in Hawaii. J Appl Phycol 21:691–699
Dang DH, Hoang MH, Ngo HT, Hoang SN, Huynh QN, Tran MD (2008) Analysis of the genetic variation of Eucheuma and Kappaphycus strains in Vietnam using RAPD markers. In: Phang SM, Lewmanomont K, Lim PE (eds) Taxonomy of Southeast Asian seaweeds. Institute of Ocean and Earth Sciences University of Malaya, Kuala Lumpur
Doty MS (1985) Eucheuma alvarezii, sp. Nov. (Gigartinales, Rhodophyta) from Malaysia. In: Abbott IA, Norris JN (eds) Taxonomy of economic seaweeds: with reference to some Pacific and Caribbean spcies. California Sea Grant College Program, La Jolla, California, pp 37–45
Doty MS, Norris JN (1985) Eucheuma species (Solieriaceae, Rhodophyta) that are major sources of carrageenan. In: Abott IA, Norris JN (eds) Taxonomy of economic seaweeds: with reference to some Pacific and Caribbean species. California Sea Grant College Program, La Jolla, California, pp 47–61
Fredericq S, Freshwater DW, Hommersand MH (1999) Observations on the phylogenetic systematics and biogeography of the Solieriaceae (Gigartinales, Rhodophyta) inferred from rbcL sequences and morphological evidence. Hydrobiologia 398/399:25–38
Gerung GS, Ohno M (1997) Growth rates of Eucheuma denticulatum (Burman) Collins et Harvey and Kappaphycus striatum (Schmitz) Doty under different conditions in warm waters of Southern Japan. J Appl Phycol 9:413–415
Góes HG, Reis RP (2011) Temporal variation of the growth, carrageenan yield and quality of Kappaphycus alvarezii (Rhodophyta, Gigartinales) cultivated at Sepetiba Bay, southeastern Brazilian coast. J Appl Phycol. doi:10.1007/s10811-011-9665-4
Hayashi L, Oliveira EC, Bleicher-Lhonneur G, Boulenguer P, Pereira RTL, Seckendorff R, Shimoda VT, Leflamand A, Vallée P, Critchley AT (2007) The effects of selected cultivation conditions on the carrageenan characteristics of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) in Ubatuba Bay, São Paulo, Brazil. J Appl Phycol 19:505–511
Huelsenback JP, Ronquist F, Nielsen R, Bollback JP (2001) Bayesian inference of phylogeny and its impact on evolutionary biology. Science 294:2310–23114
Hurtado AQ, Agbayani RF, Chavoso EAJ (1996) Economics of cultivating Kappaphycus alvarezii using the fixed-bottom line and hanging-long line methods in Pangatan Cays, Caluya, Antique, Philippines. J Appl Phycol 105:105–109
Hurtado AQ, Agbayani RF, Sanares R, Castro-Mallare MTR (2001) The seasonality and economic feasibility of cultivating Kappaphycus alvarezii in Panagatan Cays, Caluya, Antique, Philippines. Aquaculture 199:295–310
Hurtado AQ, Critchley AT, Trespoey A, Bleicher-Lhonneur G (2008) Growth and carrageenan quality of Kappaphycus striatum var. sacol grown at different stocking densities, duration of culture and depth. J Appl Phycol 20:551–555
Jobb G, von Haeseler A, Strimmer K (2004) TREEFINDER: a powerful graphical analysis environment for molecular phylogenetics. BMC Evol Biol, 4:18–26
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) ClustalW and ClustalX version 2.0. Bioinformatics 23:2947–2948
Lim PE, Sakaguci M, Hayunda T, Kogame K, Phang SM, Kawai H (2007) Molecular phylogeny of crustose brown seaweeds (Ralfsiales, Phaeophyceae) inferred from rbcL sequences resulting in proposal for Neoralfsiaceae fam. nov. Phycologia 46:456–466
Lluisma AO, Ragan MA (1995) Relationships among Eucheuma denticulatum, Eucheuma isiforme and Kappaphycus alvarezii (Gigartinales, Rhodophyta) based on nuclear ssu-rRNA gene sequences. J Appl Phycol 7:471–477
Luhan MRJ, Sollesta H (2010) Growing the reproductive cells (carpospores) of the seaweed, Kappaphycus striatum, in the laboratory until outplanting in the field and maturation to tetrasporophyte. J Appl Phycol 22:579–585
Montes HRJ, Pobre KFR, Lluisma AO (2008) Phylogenetic affiliation of the ‘Endong’/‘Spaghetti’ variety of Eucheuma as revealed by molecular data. Philippine Agricultural Scientist 91:86–93
Munoz J, Freilepelegrin Y, Robledo D (2004) Mariculture of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) color strains in tropical waters of Yucatán, México. Aquaculture 239:161–177
Neish IC (2008) Good argonomy practices for Kappaphycus and Eucheuma: Including an overview of basic biology. SEAPlant.net Monograph HB2F 1008 V3 GAP. 72 pp
Page RDM (1996) TreeView: an application to display phylogenetic trees on personal computers. Computer Appl iBiosc 12:357–358
Paula EJ, Pereira R, Ohno M (1999) Strain selection in Kappaphycus alvarezii var. alvarezii (Solieriaceae, Rhodophyta) using tetraspore progeny. J Appl Phycol 11:111–121
Phang SM (2006) Seaweed resources in Malaysia: current status and future prospects. Aquat Ecosyst Health Management 9:185–202
Phang SM, Yeong HY, Lim PE, Adibi Rahiman MN, Gan KT (2010) Commercial varieties of Kappaphycus and Eucheuma in Malaysia. Malaysian J Sci 29:214–223
Pickering T (2006) Advances in seaweed aquaculture among Pacific Island countries. J Appl Phycol 18:227–234
Ridley M (2000) Species. In: Genome: the autobiography of a species in 23 chapters. Harper Collins, New York, pp 23–37
Russell DJ (1983) Ecology of imported red seaweed Kappaphycus alvarezii (E. striatum Schmitz) on Coconut Island, Oahu, Hawaii. Pac Sci 37:87–107
Santelices B (1999) A conceptual framework for marine argonomy. Hydrobiologia 398/399:15–23
Schwarz G (1978) Estimating the dimension of a model. Ann Stat 6:461–464
Shimodaira H, Hasegawa H (1999) Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol Biol Evol 16:1114–1116
Swofford DL (2003) PAUP*. Phylogenetic Analysis Using Parsimony (* and other methods). Version 4. Sinauer Associates, Sunderland, Massachusetts.
Tanabe AS (2007) Kakusan: a computer program to automate the selection of a nucleotide substitution model and the configuration of a mixed model on multilocus data. Mol Ecol 7:962–964
Thirumaran G, Anantharaman P (2009) Daily growth rate of field farming seaweed Kappaphycus alvarezii (Doty) Doty ex P. Silva in Vellar Estuary. World J Fish Mar Sci 1:144–153
Villanueva RD, Romero JB, Montaño MNE, de la Peña PO (2011) Harvest optimization of four Kappaphycus species from the Philippines. Biomass Bioenergy 35:1311–1316
Weiss KM, Buchanan A (2004) Understanding biological complexity: basic concepts and principles. In: Genetics and the logic of evolution. Wiley-LISS, New York, pp 1–43
Zhao S, He P (2011) Molecular identification based on ITS sequences for Kappaphycus and Eucheuma cultivated in China. Chinese Oceanol Limnol 29:1287–1296
Zuccarello GC, West JA (2006) Molecular phylogeny of the subfamily Bostrychioideae (Ceramiales, Rhodophyta): subsuming Stictosiphonia and highlighting polyphyly in species of Bostrychia. Phycologia 45:24–36
Zuccarello GC, Burger G, West JA, King RJ (1999a) A mitochondrial marker for red algal intraspecific relationships. Mol Ecol 8:1443–1447
Zuccarello GC, Critchley AT, Smith J, Sieber V, Lhonneur GB, West JA (2006) Systematics and genetic variation in commercial shape Kappaphycus and shape Eucheuma (Solieriaceae, Rhodophyta). J Appl Phycol 18:643–651
Zuccarello GC, West JA, Kamiya M, King RJ (1999b) A rapid method to score plastid haplotypes in red seaweeds and its use in determining parental inheritance of plastids in the red alga Bostrychia (Ceramiales). Hydrobiologia 401:207–214
Acknowledgments
We would like to express our sincerest gratitude to the Department of Fisheries Sabah, Associate Prof. Dr. Suhaimi Md. Yasir, En. Adibi Rahiman bin Md. Nor, Japson Wong, and Mr Beh Wong King for their extensive help in seaweed collection. We are grateful to the University of Malaya and the Institute of Earth and Ocean Sciences (IOES) for providing research facilities. Special thanks to IOES members Song Sze Looi, Fiona Keng Seh Lin, Yu Chew Hock, Ng Poh Kheng, and Poong Sze Wan for technical support and advice. This project is funded by the Department of Fisheries Malaysia (Grant Number: 53-02-03-1062) and University of Malaya, PPP (Phylogenetic analysis of Kappaphycus spp. and Eucheuma spp. with the application of various molecular markers; grant number: PV014/2011A).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, J., Lim, PE. & Phang, SM. Phylogenetic relationship of Kappaphycus Doty and Eucheuma J. Agardh (Solieriaceae, Rhodophyta) in Malaysia. J Appl Phycol 25, 13–29 (2013). https://doi.org/10.1007/s10811-012-9833-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9833-1