Abstract
Macroalgae represent 26 % of the global production of cultivated organisms, with Gracilaria spp. representing 12 % of that production; Eucheuma spp. and Kappaphycus alvarezii account for 34 % of world’s algae production. Despite the potential for cultivating seaweed in Brazil, and with its more than 8000 km of coastline, there is neither marine algaculture nor detailed knowledge even among aquaculture farmers concerning the utility of algae in agriculture, industry, and gastronomy, with the result that algaculture represents only the smallest fraction of national aquaculture production. The main cultivated species of seaweed sold in Brazil include the exotic K. alvarezii and native species of Gracilaria that are grown on small scales and do not meet national industrial demands, which must be supplemented by imports. We discuss Brazilian algaculture here, pointing out some of the problems that restrict commercial production of algae in that country and offer solutions that could be shared with other nations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2012, macroalgae accounted for 26 % of the world’s production of cultivated organisms, totalling 23.8 million tonnes (wet weight), valued at US $6.4 billion (FAO 2014). Knowledge concerning marine aquaculture and the economic uses of algae is quite scarce in Brazil. Few consumers or fishermen know that algae are used in various agricultural, industrial, and gastronomic sectors (as components of meats, cosmetics, etc.). Aquaculture is still a relatively new activity in Brazil and therefore undervalued. Algae cultivation is virtually unknown within the larger field of aquaculture, with only a few producers in southeastern and northeastern Brazil and some experimental cultivation in the southern region of that country. Although there are no official data available, even an optimistic approximation would not exceed a hundred farmers, and no more than 20 % of that number would be found in south/southeastern Brazil. Algaculture is the lowest producing aquaculture crop in Brazil, and there is not even official data available on its production in the Fisheries Global Information System of the Food and Agriculture Organization of the United Nations, FAO (FAO 2016).
The main commercial cultivated taxa of macroalgae sold in Brazil is the exotic species Kappaphycus alvarezii (cultivated in the southeastern region) and the small-scale cultivation of native species of Gracilaria in the northeast (Hayashi et al. 2014; Rebours et al. 2014; Castelar et al. 2015b). The biomass produced, however, does not meet national demands for colloids. In 2015, Brazil imported 1836 tonnes of carrageenan at a cost of over US $16 million (MDIC 2016). The species of Gracilaria (12 %) and Eucheuma, as well as K. alvarezii (34 %), represented almost half of total global algal production (FAO 2014). In addition to the potential for major increases in internal and international trade (FAO 2014), the tropical and subtropical climate and oceanographic conditions along the Brazilian coast are important factors arguing for the success of algaculture development in Brazil. These conditions (allied to nearly 8000 km of coastline, with various environments propitious for algaculture in many bays and estuaries) constitute fundamental factors for the successful consolidation of algae cultivation in Brazil (Oliveira 2006; Castelar et al. 2015a). Additionally, coastal human populations of that country need sustainable economic and environmental activities—making it difficult to understand why its algaculture has remained only incipient!
Our aim is to instigate a discussion regarding commercially cultivated algae production in Brazil, to look at the problems that have impeded algaculture from prospering, and examine various possible solutions. Some of these topics will be similar to situations in other countries, especially in Latin American, and this issue certainly merits international discussion.
Cultural issues
The infrequent practice of marine aquaculture (Oliveira 2006) in Brazil reflects the fact that there is no widespread customary consumption of seaweed, except in Japanese and highly contemporary cuisine. The consumption of seaweed as a food resource has increased in many countries in Europe and North America, following contemporary culinary trends and apparent health benefits (Patarra et al. 2014). Knowledge about the benefits of algae consumption to human health is still very limited among Brazilians, and even fewer know about its social and economic benefits; although in some northeastern states, where there have been initiatives promoting Gracilaria cultivation, information has become available concerning the benefits of algal production and use (Hayashi et al. 2014), but these have rarely spread to other regions. This lack of knowledge limits interest in harnessing algaculture as a source of income and hinders the expansion of this activity.
The importation of technological packages from other countries has been a common practice in Brazilian aquaculture. As such, most cultivated organisms there are exotic, such as the fresh water Nile tilapia (Oreochromis niloticus) and marine organisms such as the Pacific oyster Crassostrea gigas, the Pacific white shrimp Litopenaeus vannamei, and the seaweed K. alvarezii (Ostrensky et al. 2007). A technological package for producing K. alvarezii was adopted in the 1990s in Brazil and led to it becoming the main species for algal production in that country. Still, the amounts of seaweed produced in Brazil do not meet internal industrial demands for carrageenan—requiring the importation of this commodity (MDIC 2016).
According to the Brazilian Aquaculture Development Plan for the years 2015 to 2020 published by the Ministry of Fisheries and Aquaculture, the main aquatic organisms commercially cultivated in seawater are bivalve molluscs (especially oysters), scallops, and mussels. Seaweed and marine fish are not farmed on large commercial scales (only at artisanal/experimental levels), and their production technologies are not yet consolidated (MPA 2016a), so that the most productive sector is freshwater aquaculture. In 2011, freshwater fish aquaculture produced about 554,000 tonnes (87 %), while marine aquaculture contributed only about a quarter of that amount, 84,000 tonnes (MPA 2013). There is no official data available concerning algal production (MPA 2013; Rebours et al. 2014; IBGE 2016). In the table prepared by Rebours et al. (2014), regarding seaweed harvesting and commercial algaculture, some of the data was only estimated by the FAO.
The Brazilian state that has a tradition in marine shellfish farming is Santa Catarina in the southern region of that country (25° 57′ 41″ - 29° 23′ 55″ S × 48° 19′ 37″ - 53°50′00″ W), and interest has recently been shown for algae cultivation that was motivated by research conducted with K. alvarezii. These producers are interested in broadening their fish farm incomes with integrated aquaculture to produce algae (Santos et al. unpublished data). In general, aquaculture is still in its consolidation phase and suffers from bureaucratic problems in obtaining cultivation licenses. For algaculture to become a viable alternative source of income and effective employment, it will be necessary to establish public policies to disseminate its practice and promote legislation to facilitate professionalization in this activity.
Algae cultivation—its origin and factors limiting production
The commercialization of algae in Brazil began in 1960 with the harvesting of natural stocks of Hypnea musciformis (used to produce carrageenan) and species of Gracilaria (used to produce agar), which were soon overexploited to supply national industrial demands for colloids (Hayashi et al. 2014; Rebours et al. 2014, Castelar et al. 2015a, 2015b). Since then, stricter rules for harvesting these marine resources have been imposed, and Brazilian legislation regarding the capture/harvesting of marine organisms is now well established.
The Brazilian Institute of Environment and Renewable Natural Resources (IBAMA) issued Normative Instruction number 89 (February 2, 2006) that established standards for harvesting algae (IBAMA 2016). However, inspections of seaweed harvesting activities from natural beds have been very infrequent due to several factors, including a shortage of public employees for that purpose, and a lack of training of extension officers. Additionally, official plans for managing and harvesting natural beds are rare (and usually not obeyed) (Marinho-Soriano 2005).
Failure to adhere to these environmental laws has resulted in the overexploitation of natural stocks of Gracilaria spp. (Hayashi et al. 2014). Efforts have been made in the last two decades to minimize this overexploitation and to improve their algaculturing in the northeast, with the Brazilian government initiating an FAO Technical Cooperation Project (TCP/BRA/0065), together with its counterpart institution the Brazilian Cooperative Organization (OCB), in collaboration with the Ministry of Agriculture. Specialized professionals were engaged to professionalize poor coastal communities, and some of them have continued in those activities (Brennan 2013).
One of the main challenges of aquaculture has been to organize farmers into cooperative groups and create new fisherman associations to maintain constant production levels and to increase their harvests over time. Another similar project was established between 2006 and 2011 called “Coastal Communities Development (UTF/BRA/066/BRA)” with investments of US $5 million (Freddi and Aguilar-Manjarrez 2003; Ostrensky et al. 2007). In 2002, 11 families founded an organization to process and sell seaweed using sustainable harvesting practices and solar-powered drying techniques. Currently, only three families are still commercializing seaweed, and only one is still cultivating it. They now aggregate value to their seaweed products by producing gels, cosmetics, dried and packaged seaweed, decorative objects, etc. The lack of continuity of these farming efforts and the limited role of the government in these projects hinder successful growth (Brennan 2013), generating a general lack of faith among local communities in those activities.
According to the farmers who have continued to cultivate Gracilaria, cultivated seedlings obtained from natural stocks can only be used in three cultivation cycles. After the third cycle, the alga do not grow well (personal communication), resulting in the necessity of periodically harvesting new seedling from natural populations. This planting stock harvesting, coupled with commercial harvesting of Gracilaria birdiae for the colloid market, continues to negatively impact natural populations and deplete that resource (Hayashi et al. 2014).
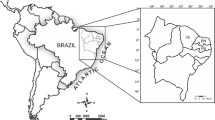
The introduction of the exotic alga K. alvarezii in 1995 was designed to meet industrial demands for carrageenan and to reduce pressure on the native northeastern beds of Hypnea musciformis (Castelar et al. 2009a, 2009b, 2015a; Hayashi et al. 2014). Exotic species production technologies are well established in Brazil, with the use of the tubular net technique on floating rafts in areas where commercial cultivation is permitted (Reis et al. 2015; Fig. 1); experimental cultivation in the south (Hayashi et al. 2011) is licensed by the Federal Government. Reis et al. (2015) compared two techniques (tubular net × tie-tie), and found that the tubular net technique resulted in 76 % higher daily growth rates (median daily growth rate = 3.48 % day−1) than the tie-tie technique (median daily growth rate = 1.97 % day−1); time management was also 125 % lower using the tubular net technique. This technique was found to be more efficient (in both the south and in the southeast) for holding the seedlings on the floating rafts in areas with strong water movement (Fig. 2), thus avoiding seedling losses (Marroig and Reis 2011, Santos 2014, Reis et al. 2015). Different techniques for planting seedlings using tubular nets on floating rafts can be used, depending on the cultivation site. In the south, the seedlings are closely placed (Fig. 2a) while, in the southeast, spaces are left (about 10 cm) between the seedlings (Fig. 2c).
a Map of Brazil, indicating the states of São Paulo and Rio de Janeiro (dark), and b map of the areas where the commercial cultivation of Kappaphycus alvarezii (gray) is permitted. Adapted from Góes and Reis (2011)
Production system of floating rafts with tubular netting used to cultivate Kappaphycus alvarezii in Brazil. a Seedling planting without spaces between them, as practiced in the southern region of that country; b the tubular netting at harvesting; c seedling planting as practiced in southeastern Brazil, with spaces between seedlings; and d the tubular netting at harvesting. Arrow indicates the tubular net
With regard to native algae, both the tie-tie technique (fixed on long lines) and the tubular net technique (fixed on floating rafts) can be used. There are currently no production data available to compare these techniques in the northeast, as has been done in the southeast. Producers in the south (Santa Catarina State) have reported that human resources represent one of the main limiting factors for the diversification of marine cultivation; production technologies using the tubular net technique facilitate the mechanization of the planting and harvesting processes and reduce the need for human resources and therefore production times and costs (Santos 2014).
With regard to actions needed to improve Brazilian algaculture, its mechanization falls within a national trend of substituting manual operations, and new materials should also be introduced to minimize production costs. Mechanization has been shown to reduce costs and increase aquaculture production and therefore represents a path to greater financial returns from marine farming. Positive results have been obtained with the mechanization of both freshwater fish and marine shellfish aquaculture (Capello et al. 2010, Novaes et al. 2011, Santos 2014). Novaes et al. (2011) noted that four workers working 8 h per day were needed to manually harvest 1 tonne of the mussel Perna perna in southern Brazil, while mechanized harvesting of that same tonne required only three people working for 30 min, indicating that manpower costs could be reduced by 30 %.
Many mistakenly believe that mechanization reduces jobs, when in fact, according to Dutra et al. (2011), mechanization will allow producers to increase production, productivity, reduce labor efforts, and prevent overuse (repetitive strain) injuries. This increasing production and productivity will strengthen the production chain, making more jobs available and attracting more labor to an economically responsible activity. To achieve this, however, fishing engineering professionals will need to be involved, and production costs and product sales should be managed by personnel specialized in calculating the profitability of production systems. Another problem observed in other aquaculture activities is the theft of cultivation materials (Santos 2014; INEA 2015). This likewise occurs in algaculture, increasing costs due to the necessity of contracting security services.
For algaculture to successfully enter the marketplace, a cooperative mind-set needs to be strengthened. The lack of data on Brazilian aquaculture activities means that the exact number of aquaculture cooperatives in the country is uncertain. Contrary to Brazilian agribusiness, cooperatives are still uncommon in aquaculture, mainly because of a lack of cohesion and organization among producers. Most aquaculture cooperatives are small regional operations (Ostrensky et al. 2007). Cooperatives can be a solution for low-income producers who do not have the capital to cover major expenses, but cooperative organizations among Brazilian algae producers is still very limited. Despite the fact that some northeastern algaculture farmers have expressed the desire to form cooperatives, most of them are organized into associations (Brennan 2013). In Santa Catarina State (the largest producer of aquaculture bivalves), cooperatives were crucial for the full development of mussel production (Santos 2014).
Inefficient business management is another factor that limits the prosperity of Brazilian algaculture. Most large commercial ventures producing K. alvarezii were not consolidated, which led to a consequent reduction in the credibility of this activity (personal observation). The Ondas Biomar Cultivo de Algas Ltda., for example, once employed more than 100 workers and had about 120 floating rafts. According to Góes and Reis (2012), each raft was 150 m long and 3 m wide, which was equivalent to 1.65 km of long line in each raft, with an average productivity (± standard deviation) of 39.3 ± 18.5 g DW−2 day−1 and daily growth rate of 3.76 ± 0.79 %.day−1—equivalent to nearly 2000 t year−1 of wet weight raw material.
Other entrepreneurs in the southeast (Rio de Janeiro State) did not officially register their companies, as the process of legalization of a new activity is very lengthy and confusing. Consequently, they did not have access to lines of credit and other benefits provided by the Federal Government, and their activities finally ended, with consequent unemployment—once again diminishing the credibility of algaculture (personal observation).
Human resources devoted to algaculture
The lack of qualified human resources is another obstacle to economic growth. Few academic researchers in Brazil dedicate time to this line of research, as most are trained in botany (ecology, physiology, and/or taxonomy) and few have links with farmers. The few researchers dedicated to algae production technologies, including integrated cultivation, are not encouraged to disseminate their work outside of the academic world and do not have continuous contacts with farmers. As such, the needs of the farmers are not passed on to the researchers and most research efforts are not directed towards problems of the productive sector. Producers are often unaware of the results of relevant research.
Another factor that contributes to the low levels of algaculture currently seen in Brazil is the discontinuity of training services (such as those that were implemented for Gracilaria cultivation). Some researchers from universities and research institutes have been involved in training but this has occurred only occasionally and without continuity. As an example of the importance of training, we can cite Santa Catarina State, which produces 95 % of the country’s molluscs. The consolidation of the commercial fish farms in this state was mainly the result of strong training services offered by the state government that provided practical technical assistance to producers for over 25 years (Santos 2014). If algaculture is negligible within Brazilian aquaculture, obviously few Brazilian training institutions will be dedicated to algaculture (although it is not even known how many professionals would be needed for that purpose).
Some researchers work on public policies to promote certain activities and generate information to help develop production and environmental standards, while others are consultants for institutions involved in production activities and inspections (personal observation). Thus, it is clear that the numbers of researchers and applied research projects are not compatible with the potential demand, and there is a need to unite the efforts of producer and researchers to strengthen training courses; researchers could use graduate programmes for training purposes.
Ilha Grande Bay (Rio de Janeiro State), which is located within the area designated for commercial cultivation of K. alvarezii (Fig. 1), stands out nationally as a producer of this species. Tourism is the main economic activity of the region, however, and this bay is the premier destination for leisure activities and nautical tourism (INEA 2015). That sector formally employs 15–50 % of the population, with the greatest concentration of boats and marinas in the country. Therefore, there is competition for labor between the two sectors, and in the summer, some of the farmers working in algaculture prefer to engage in more profitable activities, such as tourism (boat transportation) and selling beverages on the beaches, and often earning four times their usual salaries (personal observation).
Ministries responsible for incentives, financing, and aquaculture legislation
In 2009, the Ministry of Fisheries and Aquaculture (MPA in Portuguese) was created in Brazil to promote the planning and management of the country’s aquatic resources. The MPA, in conjunction with the Ministry of Environment (MMA), established rules, criteria, standards, and management measures for the sustainable use of fishery resources (MPA 2016b, Suplicy et al. 2015). In 2015, the MPA was extinguished and integrated into the Ministry of Agriculture, Livestock and Supply (MAPA) (Brasil 2016). Unfortunately, the ministerial budget that was formerly dedicated to aquaculture no longer exists, interrupting a decade of growing aquaculture activities. Even while the MPA did exist, however, algaculture received fewer resources than other aquaculture activities since it was a new production chain and lacked an efficient structure of production, transportation, processing, and marketing. Within the Development Plan for Brazilian Aquaculture for 2015–2020, for example, the development programme for new technologies for aquaculture, which included algaculture of micro- and macroalgae and three other activities, had less than 5 % of the total allocation of the former MPA, equivalent to approximately US $2 million (MPA 2016a).
Regarding aquaculture legislation, Normative Instruction number 6 of the MPA/MMA (31 May 2004) set standards for the use of bodies water in the country for aquaculture purposes (CRMV GO 2016). Normative Instruction number 17 (22 September 2005) created criteria and procedures for the formulation and establishment of Local Mariculture Development Plans that delimited aquaculture parks and aquaculture areas (Ostrensky et al. 2007, Viana and Novaes 2011). Very few areas were defined, however, as in Santa Catarina State.
Algaculture is possible in certain Brazilian Conservation Areas (UCs) if that activity is considered in their management plans. A number of UCs allow the cultivation of K. alvarezii, but many have not yet published management plans—which delays the implementation of cultivation efforts (INEA 2015).
A structured and organized productive chain is necessary to promote aquaculture, with economic agents being essential at all of the links of the chain, in addition to supporting organizations and efficient institutional frameworks. Successful cases have occurred with the close cooperation and coordination of farmers, the government, and society (MPA 2016a). Santa Catarina State is an example of cooperation among researchers, government services, with the participation of fisherman farmers themselves (Costa 1998). This arrangement helped make Santa Catarina the largest national shellfish producer (Crassostrea gigas and Perna perna), responsible for 95 % of the national production of bivalves (Santos and Costa 2015). There were, however, cases of failure, as reported previously for Gracilaria cultivation in the northeast.
The extinction of the MPA delayed the release of other areas for cultivation and hampered expansion to regions apt for cultivation. Compared to other production chains, algaculture is in its early development stages, requiring the construction of public policies to encourage this new productive activity and the involvement of related institutions, academic researchers, and the productive sector.
Algaculture legislation concerning the exotic species Kappaphycus alvarezii and its consequences
Brazilian laws that open areas to be used for the commercial cultivation of exotic seaweeds (Normative Instruction IBAMA number 185; 23 July 2008) (ICMBIO 2016) are very strict and only allow commercial cultivation in areas within the geographical coordinates—P1: 45° 27′ 55.56″ W × 23° 49′ 06.03″ S; P2: 45° 27′ 55.65″ W × 23° 59′ 09.10″ S; P3: 43° 33′ 50.1″ W × 23° 59′ 10.53″ S; P4: 43° 33′ 42.8″ W × 23° 04′ 30.88″ S (Góes and Reis 2011, Fig. 1). Several studies were carried out on K. alvarezii in this area by researchers from the University of São Paulo and the Rio de Janeiro Botanical Garden, including Paula and Pereira (1998); Paula et al. (1999, 2001, 2002), Oliveira and Paula (2003), Paula and Oliveira (2004), Bulboa and Paula (2005), Bulboa et al. (2007), Castelar (2006); Castelar et al. (2009a, 2009b), Creed et al. (2007), Hayashi et al. (2007), Marroig (2007), Reis et al. (2007), and Ghilardi et al. (2008); some of these researchers also participated in meetings and advisory services for the introduction of this seaweed.
In 2005, the Brazilian authorities requested the researcher who initiated macroalgae cultivation studies in Brazil, Dr. Eurico Cabral de Oliveira Filho, to offer his opinion concerning the introduction of this alga. He produced a document emphasizing that the introduction of any exotic organism must be carefully planned and preceded by detailed analyses to avoid deleterious consequences to the new environment, especially studies related to invasiveness. It was recommended that the economic and social impacts of its introduction be examined to determine whether they would meet the aspirations of those involved in production. After the decision of the feasibility of an introduction, it would be essential to establish quarantine and monitoring procedures to gauge algal production and related environmental risks. The responsibilities of the introducer/farmer and public agencies should be clearly established. Positive introduction scenarios were anticipated (reduction of overfishing, remediation of eutrophic environments, reduction of toxic seas, attractor fish, economic and social benefits, reduced importation) as well as possible negative effects [increased sedimentation, biomass accumulation, changes in marine fauna and microbiota under cultivation, decreased irradiance and phytoplankton, appearance of monoculture diseases, accumulations of cultivation wastes, algal seedlings in fishing nets, introduced pests, conflicts with other activities (such as tourism, fishing, and boating), and modifications of water circulation and erosion/sedimentation in neighboring areas].
In 2006, after commercial cultivations were in operation, this document was revised and amended by an ad hoc committee of experts coordinated by Dr. Oliveira Filho and indicated by the Directory of the Brazilian Society of Phycology. These scientists added recommendations to the original document concerning areas where cultivation should be avoided (in reef areas along the Brazilian coast, especially the Abrolhos Bank coral complex), and that farming in the northeast should be halted. They also reinforced the necessity of establishing quarantine and monitoring protocols and made additions to the licensing contracts between the farmers and the federal government.
The normative regulations (IN 185, 2008) that allowed commercial cultivation of K. alvarezii created the necessity of a monitoring plan (prepared by the farmer) to be submitted to the regulatory institution (IBAMA). Additionally, the introduction of new algae strains would require a certificate including the species name, its phytosanitary integrity, and the institution responsible for its quarantine. There are not yet any official quarantine protocols established for environmental risks, nor any designated institutions responsible for quarantine procedures.
After an area has been freed for commercial cultivation, additional investigations are carried out to ensure the government that there are no environmental risks associated with the cultivation of that species. Correct identification of the cultivated alga was a concern, but this was clarified through the use of molecular taxonomic techniques. Molecular studies indicated that the specimens introduced into Brazil to the states of Rio de Janeiro State and São Paulo State were in fact K. alvarezii (Doty) Doty ex P.C. Silva—considered a species presenting low environmental risks (Barros-Barreto et al. 2013).
Brazil has many priority areas for biodiversity conservation (Olson and Denerstein 2002) and its environmental regulations are quite restrictive (www.mma.org.br). From 2005 to 2008, professionals who elaborated the algaculture regulations were concerned about the introduction of K. alvarezii to certain regions of the country (personal observation), and classified potential environmental risks of cultivating that species along the Brazilian coast as low, medium, or high (Castelar et al. 2015b). These authors recommended the cultivation of only native species, such as Gracilaria spp. and Hypnea musciformis, as economic and environmentally sustainable alternatives in high-risk areas (Fig. 3).
Risk analysis map for the introduction of K. alvarezii into Brazil. Adapted from Castelar et al. (2015b)
These high-risk areas contain the world’s largest rhodolith beds (Amado-Filho and Pereira-Filho 2012), the majority of coral reefs from 0° to 18° S (Leão et al. 2003), and the largest populations of commercially harvested and/or cultivated native species (Marinho-Soriano et al. 2006). The necessity of preserving the environment and protecting other lucrative activities (such as tourism) must always be taken into account. The government should encourage research to improve production technologies using native species and stimulate native seaweed algaculture in sensitive areas.
In Chile, following the decrease in natural beds of Gracilaria chilensis and other commercial species the government encouraged several universities to study restocking and cultivation techniques (Buschmann et al. 2004; Hayashi et al. 2014). Chile is currently the world’s largest producer of agar (FAO 2014).
Eight years after the authorization to cultivate K. alvarezii in southeastern Brazil, no new areas with low environmental risks and high productive potentials, due to the qualified professionals working for this purpose, have been opened for commercial cultivation. In the south (Santa Catarina State), a research and agricultural training company (Epagri) expects to release experimental cultivations to commercial interests to expand cultivation activities in three municipalities. New techniques are being developed to adapt algaculture to the environmental conditions of that state, including mechanization. A census taken among bivalve farmers there revealed that 25 % (of 116) wished to incorporate K. alvarezii into their bivalve cultivation activities (Santos 2014). It is noteworthy that in certain areas where commercial cultivation of K. alvarezii is not allowed, as noted by Araújo et al. (2014) for Paraiba, Brazilian inspection make no provision for withdrawing their cultivation. This is a worrisome problem as a government authority stated that if any environmental damage was observed in northeastern Brazil that was caused by this species, all commercial cultivation in the country would be prohibited (personal observation).
In addition to the promising direct economic returns from algaculture, there is a potential benefit of bio-remediation of eutrophic environments. Brazil’s highest human population densities are concentrated along the coast, and sewage treatment facilities have failed to keep up with increasing population growth (Rodrigues and Silva 2011) resulting in those areas becoming severely polluted. Algaculture offers an efficient manner to remedy some of these environments problems, as marine macroalgae are known to remediate eutrophic environments (Neori 2008). Another example of bio-remediation of eutrophic environments is the integration of mussels with algaculture, which can benefit the phytosanitary requirements of the molluscs and improve the incomes of bivalve farmers (Santos 2014).
Conclusions
Despite the potential for algaculture along the Brazilian coast, the expansion of commercial markets for colloids, and huge potential consumer markets, algaculture remains incipient in Brazil and is barely encouraged. The government assigns less than 5 % of its fishery resource funds to promote algaculture activities, and there are no official data available concerning algaculture production. Many barriers exist within the algaculture production chain, and there has been no continuity to efforts directed towards involving and sustaining coastal community participation in this activity. Despite the designation of low environmental risk areas for commercial K. alvarezii cultivation, the government has not allowed new areas to be developed for farming. The cultivation of exotic algae species in high-risk areas has not been eliminated, which increases the risk of their invasion of reef environments and the outright prohibition of this algaculture in the country. There are no official protocols for environmental monitoring and quarantine, and the institutions responsible for quarantine processes have not been designated. Farmers are not enrolled in cooperatives which could increase production by decreasing costs and improving the mechanization and processing of their products (therefore increasing sales). The shortage of professionals working with applied research and aquaculture training, the lack of integration among researchers, worker training, and the scarcity of productive sector and public managers all delay the growth and consolidation of this activity.
Recommendations
Various factors will be essential to consolidate the productive chain of seaweeds, including (1) greater integration among researchers, worker training, and the recruitment of production sector and public managers; (2) greater integration among environmental experts of licensing agencies, academic professionals, and phycology experts who could provide important technical information for the development of regulations, while lending credibility to the process of issuing environmental permits; (3) the formulation of public policies for algaculture and for the establishment and management of aquaculture parks; (4) creating skilled human resources by federal and state universities and research institutes; (5) promoting research into the mechanization along the production chain; (6) investigating the use of algal biotechnology in Brazilian industries, supported by economic feasibility studies; (7) stimulating the Brazilian Association of State Technical Assistance and Rural Training Entities Association to restructure the national agricultural training programme; (8) promoting innovations in production technologies of native species and the greater involvement of related areas such as agronomy, aquaculture engineering, and gastronomy; and (9) encouraging the formation of cooperatives in marine aquaculture along the lines of freshwater fish culture cooperatives, as well as the evolution of the hallmark individualistic nature of marine farmers.
References
Amado-Filho G, Pereira-Filho GH (2012) Rhodolith beds in Brazil: a new potential habitat for marine bioprospection. Braz J Pharmacog 22:782–788
Araújo PG, Ribeiro ALNL, Yokoya NS, Fujii MT (2014) Temperature and salinity responses of drifting specimens of Kappaphycus alvarezii (Gigartinales, Rhodophyta) farmed on the Brazilian tropical coast. J Appl Phycol 26:1979–1988
Barros-Barreto MBB, Marinho LC, Reis RP, Mata CS, Ferreira PCG (2013) Kappaphycus alvarezii (Gigartinales, Rhodophyta) cultivated in Brazil: is it only one species? J Appl Phycol 25:1143–1149
Brasil (2016) Entenda a reforma ministerial e saiba como fica a Esplanada. http://www.brasil.gov.br/governo/2015/10/entenda-a-reforma-ministerial-e-saiba-como-fica-a-esplanada. Accessed 27 January.
Brennan W (2013) Cultivating change: women’s involvement in a Brazilian seaweed collective. Honors Projects. Paper 19. http://digitalcommons.macalester.edu/anth_honors/19
Bulboa CR, Paula EJ (2005) Introduction of non-native species of Kappaphycus (Rhodophyta, Gigartinales) in subtropical waters: comparative analysis of growth rates of Kappaphycus alvarezii and Kappaphycus striatum in vitro and in the sea in southeastern Brazil. Phycol Res 53:183–188
Bulboa CR, Paula EJ, Chow F (2007) Laboratory germination and sea out-planting of tetraspore progeny from Kappaphycus striatum (Rhodophyta) in subtropical waters of Brazil. J Appl Phycol 19:357–363
Buschmann AH, Varela D, Cifuentesa M, Hernández-González MC, Henríquez L, Westermeier R, Correa RA (2004) Experimental indoor cultivation of the carrageenophytic red alga Gigartina skottsbergii. Aquaculture 241:357–370
Capello FP, Menten MM, Manarim LK (2010) Mecanização racional. Brasil Hortifruti. CEPEA-ESALQ/USP 96:8–18
Castelar, B. (2006) estudo sobre a dispersão da macroalga exótica Kappaphycus alvarezii (Doty) Doty ex P. C. Silva, em cultivo comercial na Baía da Sepetiba, RJ, Brasil. Monograph. Universidade do Estado do Rio de Janeiro.
Castelar B, Reis RP, Bastos M (2009a) Contribuição ao Protocolo de Monitoramento Ambiental da Maricultura de Kappaphycus alvarezii (Doty) Doty ex P.C. Silva (Areschougiaceae - Rhodophyta) na Baía de Sepetiba, RJ, Brasil. Acta Botanica Brasilica. 23: 613-617
Castelar B, Reis RP, Moura AL, Kirk R (2009b) Invasive potential of Kappaphycus alvarezii off the south coast of Rio de Janeiro state, Brazil: a contribution to environmentally secure cultivation in the tropics. Bot Mar 52: 283–289.
Castelar B, Reis RP, Calheiros ACS (2015a) Ulva lactuca and U. flexuosa (Chlorophyta, Ulvophyceae) cultivation in Brazilian tropical waters: recruitment, growth, and ulvan yield. Aquacult Res 2015, 1-13.
Castelar B, Siqueira MF, Sanchez-Tapia A, Reis RP (2015b) Risk analysis using species distribution modeling to suport public policies for the alien alga Kappaphycus alvarezii aquaculture in Brazil. Aquaculture 446: 217–226.
Costa SW (1998) Cadeias produtivas do estado de Santa Catarina: aquicultura e pesca. EPAGRI Florianopolis, Brazil. Boletim Técnico n 97. 62p
Creed JC, Pires DO, Figueiredo MAO (2007) Biodiversidade Marinha da Baía da Ilha Grande. MMA/SBF, Brasília - DF
CRMV GO (2016) Instrução Normativa. Interministerial n° 6, de 31 de maio de 2004, http://www.crmvgo.org.br/legislacao/2_AQUICULTURA/040531_IN_inter_06.pdf.Accessed 15 January 2016
Dutra ARA, Garcia MA, Rossato IF, Barros Filho JRB (2011) A contribuição da ergonomia para a mecanização da produção catarinense de ostras. In: Proceedings ICIEOM-ABEPRO. pp 1-13. http://www.abepro.org.br/biblioteca/enegep2011_TN_STO_138_878_19175.pdf
FAO (2014) The state of world fisheries and aquaculture. Food and Agriculture Organization of the United Nations, Rome, p 243
FAO (2016). Commodity trade and production: quantity (t) http://www.fao.org/figis/servlet/SQServlet?file=/work/FIGIS/prod/webapps/figis/temp/hqp_8645407847772810809.xml&outtype=html. Accessed 27 January.
Freddi A, Aguilar-Manjarrez J (2003) Small-scale seaweed farming in North East Brazil. TCP/BRA/0065 35 FAO Aquaculture Newsletter 32
Ghilardi NP, Hayashi L, Berchez FAZ, Yokoya NS, Oliveira EC (2008) An alternative environmental monitoring approach for nonindigenous species introduced for maricultural purposes: the case of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) cultivation in Brazil. Oecologia Brasiliensis 12:270–274
Góes HG, Reis RP (2011) An initial comparison of tubular netting versus tie-tie methods of cultivation for Kappaphycus alvarezii (Rhodophyta, Solieriaceae) on the south coast of Rio de Janeiro State, Brazil. J Appl Phycol 23:607–613
Góes HG, Reis RP (2012) Temporal variation of the growth, carrageenan yield and quality of Kappaphycus alvarezii (Rhodophyta, Gigartinales) cultivated at Sepetiba bay, southern Brazilian coast. J Appl Phycol 24:173–180
Hayashi L, Bulboa C, Kradolfer P, Soriano G, Robledo D (2014) Cultivation of red seaweeds: a Latin American perspective. J Appl Phycol 26:719–727
Hayashi L, Oliveira EC, Bleicher-Lhonneur G, Boulenguer P, Pereira RTL, Seckendorff R, Shimoda VT, Leflamand A, Vallée P, Critchley AT (2007) The effects of selected cultivation conditions on the carrageenan characteristics of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) in Ubatuba Bay, São Paulo, Brazil. J Appl Phycol 19:505–511
Hayashi L, Santos AA, Faria GSM, Nunes BG, Souza MS, Fonseca ALD, Barreto PLM, Oliveira EC, Bouzon ZL (2011) Kappaphycus alvarezii (Rhodophyta, Areschougiaceae) cultivated in subtropical waters in Southern Brazil. J Appl Phycol 23:337–343
IBAMA (2016) Instrução normativa no 89, de 2 de fevereiro de 2006. http://www.ibama.gov.br/category/40?download=1265%3A89-2006-.p. Accessed 4 January 2016
IBGE (2016). Banco de dados Agregados. http://www.sidra.ibge.gov.br/bda/tabela/listabl.asp?c=3940&z=t&o=21. Accessed 15 January 2016.
ICMBIO (2016) Instrução Normativa IBAMA N° 185, de 22 de julho de 2008. http://www.icmbio.gov.br/cepsul/images/stories/legislacao/Instrucao_normativa/2008/in_ibama_185_2008_permitircultivokappaphycus_alvarezii_rs_sc_revoga_in_ibama_165_2007.pdf Accessed 4 January 2016.
INEA (2015) Diagnóstico do setor costeiro da Baía da Ilha Grande - Subsídios à elaboração do Zoneamento Ecológico-Econômico Costeiro. http://www.inea.rj.gov.br/cs/groups/public/documents/document/zwew/mdcz/~edisp/inea0073532.pdf Accessed 20 December 2015.
Leão ZMAN, Kikuchi RKP, Testa V (2003) Corals and coral reefs of Brazil. In: Cortés J (ed) Latin American Coral Reefs. Elsevier, Amsterdam, pp 1–52
Marinho-Soriano E (2005) Cultivo experimental de Gracilaria no Rio Grande do Norte,. In: Anais da X Reunião Brasileira de Ficologia Salvador 2004. Rio de Janeiro, Museu Nacional. Série Livros 10 pp 115-124
Marinho-Soriano E, Moreira WSC, Carneiro MAA (2006) Some aspects of the growth of Gracilaria birdiae (Gracilariales, Rhodophyta) in an estuary in Northeast, Brazil. Aquac Int 14:327–336
Marroig RG (2007) Epibiontes nas estruturas de cultivo de Kappaphycus alvarezii (Doty) Doty ex Silva na Baía de Sepetiba, RJ, Brasil. Universidade Santa Úrsula, Monograph
Marroig R, Reis RP (2011) Does biofouling influence Kappaphycus alvarezii (Doty) Doty ex Silva farming production in Brazil? J Appl Phycol 23:925–931
MDIC (2016). Dados oficiais do Governo Federal de importação de produtos muscilaginosos e espessantes de carragenina (Nomenclatura Comum do Mercosul – 13023910). http://aliceweb.mdic.gov.br//consulta-ncm/index/type/importacaoNcm. Accessed April 06 2016.
MPA (2013) Boletim Estatístico da Pesca e Aquicultura 2011, Ministério de Pesca e Aquicultura, MPA, Brasília
MPA (2016a) Plano de Desenvolvimento da Aquicultura - 2015/2020. http://www.mpa.gov.br/files/docs/Outros/2015/Plano_de_Desenvolvimento_da_Aquicultura-2015-2020.pdf. Accessed 2 January 2016.
MPA (2016b) Institucional. http://www.mpa.gov.br/institucional. Accessed 2 January 2016.
Neori A (2008) Essential role of seaweed cultivation in integrated multi-trophic aquaculture farms for global expansion of mariculture: an analysis. J Appl Phycol 20:567–570
Novaes ALT, Santos AA, Silva FM, Souza RV, Breda RR (2011) Colheita mecanizada de mexilhões (Perna perna) engordados a partir de coletores artificiais de sementes. Agropecuária Catarinense 24:38–41
Oliveira EC (2006) Seaweed resources of Brazil. In: Critchley AT, Ohno M, Largo DB (ed). World seaweed resources—an authoritative reference system. ETI BioInformatcs, Amsterdam. DVD-ROM
Oliveira EC, Paula EJ (2003) Exotic seaweeds: friends or foes? In Chapman, A.R.O.C., Anderson, R.J., Vreeland, V. & Davison, I.R. (eds). Proc. 17th Int. Seaweed Symp., Cape Town. Oxford Univ. Press, Oxford, Pp. 87-93
Olson DM, Denerstein E (2002) The Global 200: priority ecoregions for global conservation. Ann Mo Bot Gard 89:199–224
Ostrensky A, Borghetti, JR, Soto D (2007) Estudo setorial para a consolidação de uma aquicultura sustentável no Brasil, Curitiba
Paula EJ, Oliveira EC (2004) Macroalgas exóticas no Brasil com ênfase à introdução de espécies visando à maricultura. In: Silva JSV, Souza RCCL (eds) Água de Lastro e Bioinvasão. Editora Interciência, Rio de Janeiro, pp 99–112
Paula EJ, Erbert C, Pereira RTL (2001) Growth rate of the carrageenophyte Kappaphycus alvarezii (Rhodophyta, Gigartinales) in vitro. Phycol Res 49:155–161
Paula EJ, Pereira RTL (1998) Da “marinomia” a maricultura da alga exótica, Kappaphycus alvarezii (Doty) Doty ex. Silva (Rhodophyta) para produção de carragenanas no Brasil. Panorama da Aqüicultura 8:10–15
Paula EJ, Pereira RTL, Ohno M (1999) Strain selection in Kappaphycus alvarezii var. alvarezii (Solieriaceae, Rhodophyta) using tetraspore progeny. J Appl Phycol 11:111–121
Paula EJ, Pereira RTL, Ohno M (2002) Growth rate of the carragenophyte Kappaphycus alvarezii (Rhodophyta, Gigartinales) introduced in subtropical waters of São Paulo State, Brazil. Phycol Res 50:1–9
Patarra RF, Buschmann AH, Abreu MH, Neto AI (2014) Cultivo de macroalgas nos Açores. Oportunidades e desafios. Bol Soc Portuge Biotechnol série 2:16–18
Rebours C, Marinho-Soriano E, Zertuche-González JÁ, Hayashi L, Vásquez JÁ, Kradolfer P, Soriano G, Ugarte R, Abreu MH, Bay-Larsen I, Hovelsrud G, Rødven R, Robledo D (2014) Seaweeds: an opportunity for wealth and sustainable livelihood for coastal communities. J Appl Phycol 26:1939–1951
Reis RP, Bastos M, Góes HG (2007) Cultivo de Kappaphycus alvarezii no litoral do Rio de Janeiro. Panorama da Aqüicultura 17:42–47
Reis RP, Pereira RRC, Góes HG (2015) The efficiency of tubular netting method of cultivation for Kappaphycus alvarezii (Rhodophyta, Gigartinales) on the southeastern Brazilian coast. J Appl Phycol 27:421–426
Rodrigues IO, Silva MG (2011) Dinâmica populacional e rede coletora de esgoto In: IBGE (ed) Atlas de saneamento 2011, Brasilia, pp 249-252
Santos AA (2014) Potencial de cultivo da macroalga Kappaphycus alvarezii no litoral. Thesis, Univeridade de Santa Catarina
Santos AA, Costa SW (2015) Síntese informativa da maricultura 2014. EPAGRI. Santa Catarina State. Brazil.http://www.epagri.sc.gov.br/wpcontent/uploads/2013/08/Sintese_informativa_da_maricultura_2014.pdf. Accessed 15 Dec 2015
Suplicy FM, Vianna LFN, Rupp GS, Novaes ALT, Garbossa LHP, Souza RV, Guzenski J, Costa SW, Santos AA (2015) Planning and management for sustainable coastal aquaculture develompment in Santa Catarina State, south Brazil. Rev Aquacult. DOI: 10.1111/raq.12107
Viana LF, Novaes ALT (2011) Geocodificação de unidades de mapeamento aquícola para um sistema de controle de produção e rastreabilidade em Santa Catarina, Brasil. Geografia 36:163–178
Acknowledgments
The first author received a grant from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 303582/2014-6).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reis, R.P., Castelar, B. & Santos, A.A.d. Why is algaculture still incipient in Brazil?. J Appl Phycol 29, 673–682 (2017). https://doi.org/10.1007/s10811-016-0890-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0890-8