Abstract
Harvested by coastal populations for centuries, seaweeds have played an important role in the economy of a number of countries. In Brazil, they occur along the coastline, but are more diversified and abundant from the northeast to a portion of the southeast coast. Historically, the seaweed industry in Brazil is based on seaweed harvesting of natural beds. This practice continues to this day in a number of coastal communities in Northeastern Brazil. Since the 1960s, species of the genera Gracilaria and Hypnea have been collected in the intertidal zone for extraction of agar and carrageenan. Maximum production was achieved in 1973–1974, a period in which the country exported around 2000 t annually (dry weight) to Japan. Later (1977–1979), there was a sharp drop and annual exports fell to 250 t (dry weight). In 1981, Brazil exported only 150 t of dried seaweed for agar extraction. Between 1990 and 2000, overexploitation, decline in a number of agarophyte populations, poor quality, low price, and lack of a socioeconomic policy led to the almost total disappearance of this industry in Northeastern Brazil. Seaweed harvesting on natural beds is currently in decline, and the population that depended on this resource had to migrate or convert to other economic activities, such as fishing, aquaculture, and underwater tourism. However, the promising results obtained in pilot projects (Gracilaria and Kappaphycus) show that Brazil has significant potential as a seaweed biomass producer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Brief history of seaweed use as phycocolloids
The use of seaweeds as a source of phycocolloids dates from 1658, when the gelification properties of agar extracted from Gelidium amansii (J.V.Lamouroux) J.V.Lamouroux were discovered in Japan. The worldwide seaweed industry in Western countries was founded around 1690 with the production of soda ash. Alginate and carrageenan extraction began only in 1930 and 1940, respectively (Craigie 2011). Japan, the first country to use seaweeds, remained the main supplier to Europe and North America until the late 1930s. During the Second World War, the production and sale of seaweeds in Japan fell drastically and Western countries intensified studies on native species aimed at producing a product with similar characteristics. As a result, colloids started to be extracted from hitherto unused species, such as Gelidium cartilagineum (=Plocamium cartilagineum (Linnaeus) P.S.Dixon), Pterocladia capillacea (=Pterocladiella capillacea (S.G.Gmelin) Santelices & Hommersand), Chondrus crispus Stackhouse, and Hypnea musciformis (Wulfen) J.V.Lamouroux.
Although industrial use of seaweed extracts increased steadily after the Second World War, demand exceeded the supply of natural stocks and the availability of raw material became limited at certain times of the year, due to seasonality of the species. An important step for the expansion of the agar industry was the use of alkaline pretreatment in the 1950s, which improved the quality of gel from species of Gracilaria, until then considered unsuitable for agar production due to its low quality. This discovery enabled the expansion of agarophyte harvesting on natural beds as well as a variety of native species of Gracilaria in countries such as Chile, Argentina, Indonesia, Namibia, and Brazil (McHugh 2003). However, harvesting increased over the years, decreasing the biomass of natural beds.
In Brazil, intensive, uncontrolled harvesting caused a significant drop in native populations, primarily agarophytes (Bezerra and Marinho-Soriano 2010). Problems related to the overexploitation of seaweeds have also been described for other South American countries (Rebours et al. 2014). However, Brazil has great seaweed farming potential, as evidenced in a number of experimental studies (Marinho-Soriano et al. 2006, 2009; Bezerra and Marinho-Soriano 2010; Pellizzari and Reis 2011).
Environmental factors and species with economic potential
Brazil has an extensive 8000-km coastline between the mouths of the Oiapoque (04° 52′ 45″ N) and Chui rivers (33° 45′ 10″ S). The Brazilian coastline features warm waters on the northern and northeastern coasts and cold waters on the southeastern and southern coasts, harboring a wide variety of ecosystems including reefs, sandy beaches, rocky shores, estuaries, and mangrove swamps, which contain numerous species of flora and fauna (MMA 2008). In the north, the material discharged at the mouth of the Amazon River and the interaction between ocean waves and currents produce complex oceanographic processes that exert a strong influence on the distribution of live organisms from the region. On the northeast coast, the absence of large rivers and predominance of warm waters from the equatorial current provide an adequate environment for the formation of reefs with considerable biological diversity. These reef areas are highly diversified environments of significant ecological, economic, and social importance. Fish stocks in these settings contribute to the subsistence of a number of traditional coastal communities (Prates 2007). In the south-southeastern coast, the presence of colder Southern Atlantic waters on the continental shelf favors the resurgence in certain areas along the coast, contributing to the increase in productivity (MMA 2008).
In Brazil, seaweeds occur along the whole coast, but are more diversified and abundant from the northeastern to part of the southeastern coast. The exuberance of seaweeds in this area is related primarily to favorable temperature, salinity, and light conditions, in addition to rocky substrates and reefs, adequate for seaweed establishment and growth (Oliveira 1998; Marinho-Soriano 1999). The Brazilian coast has more than 800 species of seaweeds, 62 % of which are Rhodophyta, 13 % Ochrophyta, and 25 % Chlorophyta (Oliveira et al. 2002). These values are certainly underestimated, since there are still areas that have never been inventoried and owing to the need for a taxonomic review of a number of groups.
The northeastern coast of Brazil contains a fringe of beach rocks along the coast that emerge at low tide, offering ideal substrate for a large number of seaweeds. Given their tropical location, the environmental parameters are relatively constant, without large changes over the course of the year, as is the photoperiod (12:12 h). The amplitude of the tide ranges from 0.0 to 2.9 m. There are two distinct seasons in this region: the rainy season from March to July and the dry season during the rest of the year.
A number of commercially valuable agarophyte species were recorded on the Brazilian coast (Durairatnam 1980; Oliveira 1998). However, despite the large number of economically important species, only Gracilaria and Hypnea are commercially exploited in Brazil (Marinho-Soriano et al. 2001, 2006). The species P. capillacea was also exploited for a time in the southeast as raw material for agar production. However, overexploitation of the species resulted in the depletion of natural beds (Oliveira 1998).
Marine algae found on the northeastern coast form multispecific beds, with the presence of commercially valuable agarophytes, especially those belonging to the genus Gracilaria. Species of Gelidium and Gelidiella are also found, but the small amount of biomass precluded commercial exploitation. These seaweed beds provide shelter, food, and nursery grounds for many fish species commercialized by the local community.
Seaweed exploitation in Brazil
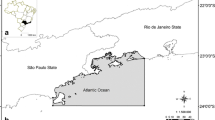
Historically, the seaweed industry in Brazil has been based on harvesting these organisms from natural beds. This continues in some coastal communities in the northeast of the country (CE, RN, and PB) (Fig. 1). Since the 1960s, species of the genus Gracilaria, Gracilariopsis, and Hypnea have been collected in the intertidal zone for agar and carrageenan extraction (Oliveira 1998; Marinho-Soriano et al. 2006; Rebours et al. 2014). For many years, seaweed harvesting has been a relatively important source of income for small-scale fishing families in this region. Furthermore, its positive social effects have been accentuated by the significant participation of women in this activity. Women in these communities have a long history of seaweed harvesting, based on their knowledge of the coastal environment, including tides, currents, and marine life. This set of skills is passed on from generation to generation in the form of practical knowledge that starts in childhood and traces its origins to ancient fishing traditions.
In 1970, with the increase in demand for Gracilaria and fish stocks showing signs of decline, men hitherto involved in traditional fishing and lobster harvesting decided to spend more time harvesting seaweed. Gracilaria production in Brazil reached its maximum in 1973–1974, when the country exported around 2000 t annually (dw) to Japan (UNCTAD/GATT 1981). At that time, seaweeds were abundant, the business thrived, and many fishermen began to work full time at this activity. With so many fishermen involved in seaweed harvesting, hundreds of families became totally dependent on it for their source of income.
Between 1977 and 1979, there was a sharp drop in seaweed production and exports fell to 250 t (dw) per year. This decline in exports, instead of being accompanied by an increase in domestic industrialization, was followed by a decrease in agar production, which clearly indicated the limited availability of the raw material. This decline was attributed to predatory harvesting, poor management of natural beds, the increase in sea urchin populations, and major flooding that occurred in the region during this period (Ferreira et al. 1981).
In 1979 and 1980, due to the lack of scientific information on natural seaweed beds in Northeastern Brazil, the Superintendence for Development of the Northeast (SUDENE) financed a project entitled “Seaweed Project” with the aim of quantifying seaweed populations with economic potential for current or eventual exploitation. This survey was conducted in two stages, at depths of 0–10 and 10–45 m. The study showed that the richest beds were in reef areas near the coastline that did not exceed 5 m in depth. The study also demonstrated that among the species with economic potential, only Gracilaria and Hypnea were viable for economic exploitation. The estimated amount of biomass at the moment of prospection was more than 1500 t of fresh weight for each of the aforementioned genera (SUDENE 1981). In 1980, the annual production of carrageenan from H. musciformis was 100 t.
According to FAO (1991), in 1981 Brazil exported 150 t of dry seaweed for agar extraction. In 1983, local companies extracted approximately 60 t of agar from the different species of Gracilaria. Agarophyte production rose slightly in 1984, resulting in exports of 607 and 20 t of Gracilaria and Gelidium spp., respectively, to Japan. The word “Gelidium” is likely an erroneous term, due to the confusion with the species P. capillacea, found in Southeastern Brazil and harvested on natural beds since the mid 1980s.
The major seaweed species exploited from natural beds were Gracilaria verrucosa (= G. birdiae), G. caudata, G. cornea, G. cervicornis, and G. domingensis, in addition to the carrageenophyte H. musciformis for kappa-carrageenan. Studies conducted with Gracilaria showed marked differences in gel yield and hardness (Durairatnam et al. 1990; Marinho-Soriano et al. 2001; Bezerra and Marinho-Soriano 2010). However, at the time of harvest and drying, the seaweeds were mixed and sent to the processing plants without prior separation of the species. This contributed to the low prices obtained by the harvesters and had a significant effect on agar quality. In recent decades, seaweed production has declined markedly. Current extraction on natural beds is falling drastically. In some areas, activity was abandoned due to the complete destruction of resources. Most of the population that depended on this resource was forced to migrate or convert to other economic activities, such as fishing, aquaculture, and underwater tourism.
The lack of raw material led national companies to close or reduce seaweed purchases. According to reports of residents, seaweed harvesting during the production “boom” sustained the local economy and for a time was the main economic activity in coastal communities. However, the drop in production caused men to increasingly migrate to high seas fishing, leaving only women to harvest seaweeds.
The depletion of Gracilaria beds has been described for different areas in the Northeast region, but the reason for this phenomenon has never been satisfactorily explained. However, overexploitation, low prices, international competition, and lack of a socioeconomic policy for the activity have certainly contributed to the decline in activity. In 1990, some of the fishermen returned to more lucrative lobster fishing, while others began to work on shrimp farms (Litopenaeus vannamei), an economically important activity for the region and significantly encouraged by Brazilian public policies. In the early part of the decade, shrimp farming became one of most important economic activities in Northeastern Brazil, contributing to 93 % of shrimp production in the country (Marinho-Soriano 2007).
With the men returning to their original activity—fishing—seaweed harvesting became the sole responsibility of women and their children and the elderly, representing an important family income supplement. Despite the decreased number of harvesters, natural stocks did not recover and no young plant recruitment was observed. Seaweed harvest, formerly in the intertidal zone, began to take place several hundred meters from shore with the help of small artisanal boats.
In the 2000s, overexploitation, the decline or disappearance of a number of agarophyte populations (i.e., G. cornea), poor raw material quality, and unfavorable international market led to the near disappearance of this industry in Northeastern Brazil, with only one company, Agar Brasileiro Indústria e Comércio Ltda, still in operation. This medium-sized company currently works with only 60 t of dried seaweed per year (personal communication) from natural beds. This represents only 10 % of the company’s capacity, the remaining 90 % being imported from Indonesia and the Philippines. Concerned with the depletion of natural beds, in 2006 the Brazilian government implemented regulations on seaweed harvesting along the coast (Rebours et al. 2014). This law allows only duly registered natural persons and professional fishermen to exploit and sell the product.
Brazil is currently an importer of seaweed and its derivatives. Figure 2 shows the evolution of seaweed exports and imports between 1989 and 2014 (MDIC 2015). Brazilian exports of dry seaweeds between 1989 and 1999 were unstable, varying significantly from year to year. The highest values were recorded in 1993 (9945 t dw) and 1996 (10,783 t dw). All the raw material is classified as dry seaweeds, without discriminating the volume or value of production by species. According to information provided by the company Agar Brasileiro, only a small amount of the red algae Meristiella is exported wet to Japan in refrigerated containers, where it is consumed as salad. From 1997 onward, there has been a gradual increase in imports, especially Kappaphycus, for the production of carrageenan and a small percentage for human consumption (nori, kombu, wakame). The decline in production in Brazil has resulted in an increase in seaweed imports, creating a continuous deficit (Fig. 3).
Profile of harvesters
Most seaweed harvesters are women, and the few men involved are usually elderly retired individuals. Although there is no official data on the number of men and women in seaweed harvesting, it is estimated that 80 % of the harvesters are women (Brennan 2013). The predominance of women is due to the fact that this activity demands less time than fishing, since it takes place only at low tide and near their residences, making it compatible with their domestic responsibilities including caring for children.
Seaweed harvesting is considered a secondary activity performed without the participation of the head of the household, generally a fisherman. Harvesters are normally assisted by their children (Fig. 4a, b) during school breaks and weekends. In times past, all the family took part in this activity, which represented the main source of income. Due to the decline in natural stocks, few people are currently involved in harvesting. For women, although seaweed extraction is conducted part time, it is still considered the main occupation. Low schooling levels and the lack of professional qualification in this population did not enable other employment opportunities.
Gracilaria resources
Today, the type of Gracilaria that predominates in the region exhibits a firm, cylindrical reddish-brown thallus. It is popularly known as “cisco” (trash), because when it washes up on the beach it is considered worthless. Harvesters remove the seaweeds from natural beds and classify them according to thallus thickness. The species G. caudata (“thin cisco”) is collected from the middle and upper parts of the intertidal zone, whereas G. birdiae (“thick cisco”) is found on the lower part of the intertidal zone, near the infralittoral. These species are predominantly found on beach rocks that are exposed at low tide. Harvesting on offshore reefs is done in small sailboats (Fig. 4c), where extraction is accomplished by free diving.
Seaweed harvesting depends on the tidal cycle. At falling, tide collectors use net bags to harvest in the intertidal zone and return to the beach with the rising tide. Extraction is performed manually throughout the year, but only at low tide (spring and neap). The harvest is done manually using a knife or by twisting the tuft of the seaweed near the base of the plant. The seaweeds are then placed in bags, taken to the shore, spread out on the sand, and dried in the sun (Fig. 4d). The average drying time is 2 days. During this stage, the seaweeds are turned over at least once a day to ensure uniform drying. In the rainy season, this period may be longer and the plants are protected by a plastic tarp until the rain stops. After drying, the seaweeds are placed in bags and stored until the moment of sale.
The seaweeds are sold directly to the purchasing agent, who then resells them to the processing plant. Mixing the various species with different gel hardness, in addition to mixing them with many impurities in the form of epiphytes, sand, and small stones, contributes to the loss of quality and low price paid per kilogram of dry seaweed. The Agar Brasileiro company pays R$2.00 (US$ 0.5) per kilogram of dry weight to collectors in the states of Paraiba (PB), Rio Grande do Norte (RN), and Ceará (CE). This value varies as a function of species and degree of purity.
Farming experiments
Farming experiments with seaweeds have been conducted in the Northeast, Southeast, and South of Brazil. Despite the promising perspectives, this activity is still in the initial stages. In 2001, the Brazilian government, together with the FAO, introduced a technical cooperation project (TCP/BRA/0065) aimed at implementing seaweed (G. birdiae) farming in coastal communities of three Northeastern states (CE, RN, and PB). In addition to testing the technical and economic feasibility, the project sought to promote cooperation among producers in order to establish a new production sector (Freddi and Aguilar 2003). This project demonstrated that fishermen were able to learn farming techniques easily and quickly and that seaweed cultivation could become an important source of income for this population.
After this first phase was validated, a second project was approved between FAO and the Brazilian government in 2006 to conclude training. The new project entitled “Development for Coastal Communities” (UTF/BRA/066/BRA), with a budget of 5 million dollars (US), aimed at consolidating and expanding Gracilaria farming, diversifying mariculture production (i.e., fish, oysters), and developing co-management activities (i.e., ecotourism). Promising G. birdiae growth rates (7.45 % per day) were obtained in different communities (Bezerra and Marinho-Soriano 2010). In 2009, a group of women founded the Association of Seaweed Farming (AMAR), whose objective is to promote activities that generate income and a better life for women engaged in seaweed farming (Fig.4e, f).
On the other hand, given that the Northeast is the largest shrimp producer in Brazil (93 % of national production), studies have focused on integrated farming of seaweed and shrimp (Marinho-Soriano 2007). The latter produces algal biomass, which minimizes the eutrophication effects caused by aquaculture waste products (Marinho-Soriano et al. 2002, 2009; Oliveira et al. 2012). Selection of seaweed species for this type of study is based on three main aspects: (1) adaptation of seaweeds to the eutrophic conditions of ponds and effluents, (2) absorption efficiency and nutrient storage, and (3) economic potential of the seaweeds.
In the South and Southeast of Brazil, commercial initiatives involving the carrageenophyte Kappaphycus have been implemented. Present in several tropical countries, this exotic seaweed was intentionally introduced into the country in 1995 to reduce carrageenan imports. Experiments carried out in São Paulo state in the Ubatuba region showed that the species has a high growth rate (Paula et al. 2002). Follow-up studies demonstrated that the species poses no risk to the local flora and fauna, considering that no invasive aspect was observed in areas surrounding the farm. In 1998, Kappaphycus alvarezii was introduced into Ilha Grande, Rio de Janeiro state, and into bay of Sepetiba in 2004, the start of production on a commercial scale. Private companies established in the region have commercially farmed the species for carrageenan production (Reis et al. 2007). In the Southern state of Santa Catarina, the results obtained in experimental studies have shown that K. alvarezii displays good farming perspectives and considerable potential for integrating shellfish farms (Hayashi et al. 2011).
Finally, a number of factors demonstrate the significant potential of Brazil becoming an algae biomass producer through mariculture. These include the climate, topography, favorable environmental conditions (temperature, salinity, light), during most of the year, native species with good farming potential, possibility of expanding allochthonous species farming, a large consumer market, and the possibility of the activity becoming a source of employment and income. However, a number of considerations are necessary, such as respecting regional peculiarities; regulating activities; and qualifying human resources, producer associations, and sales and marketing strategies.
References
Bezerra AF, Marinho-Soriano E (2010) Cultivation of the red seaweed Gracilaria birdiae (Gracilariales, Rhodophyta) in tropical waters of northeast Brazil. Biomass Bioenergy 34:1813–1817
Brennan, W (2013) Cultivating Change: Women’s Involvement in a Brazilian Seaweed Collective . Honors projects. Paper 19. Available in: http://digitalcommons.macalester.edu/anth_honors/19 (Accessed: April 15 2016)
Craigie JS (2011) Seaweed extract stimuli in plant science and agriculture. J Appl Phycol 23:371–393
Durairatnam M (1980) Studies on the agar producing seaweeds and their distribution in Northeast Brazil. Ciência e Cultura 32:1358–1372
Durairatnam M, Medeiros TMB, Sena AM (1990) Studies on the yield and gel strength of Agar from Gracilaria domingensis Sonder ex Kuetzing (Gracilariales, Rhodophyta) following the addition of calcium. Hydrobiologia 204/205:551–553
FAO (1991) Yearbook of fishery statistics: catches and landings, 1989, vol 68. Food and Agricultural Organisation of the United Nations, Rome
Ferreira MT, Câmara-Neto OEC, Morais SB, Vasconcelos MDT (1981) Prospecção dos bancos de algas marinhas do Estado do Rio Grande do Norte, 1ª Parte—Profundidade 0-10m. SUDENE 2:27–81
Freddi A, Aguilar-Manjarrez J (2003) Small-scale seaweed farming in North East Brazil. TCP/BRA/0065 35 FAO Aquaculture Newsletter 32
Hayashi L, Santos AA, Faria GSM, Nunes BG, Souza MS et al (2011) Kappaphycus alvarezii (Rhodophyta, Areschougiaceae) cultivated in subtropical waters in Southern Brazil. J Appl Phycol 23:337–343
Marinho-Soriano E (1999) Species composition of seaweeds in Buzios Beach, Rio Grande do Norte, Brazil. Seaweed Res Utiln 21:9–13
Marinho-Soriano E (2007) Seaweeds biofilters: an environmentally friendly solution. World Aquacult 38:31–33
Marinho-Soriano E, Silva TSF, Moreira WSC (2001) Seasonal variation in the biomass and agar yield from Gracilaria cervicornis and Hydropuntia cornea from Brasil. Bioresour Technol 77:115–120
Marinho-Soriano E, Morales C, Moreira WSC (2002) Cultivation of Gracilaria (Rhodophyta) in shrimp pond effluents in Brazil. Aquacult Res 33:1081–1086
Marinho-Soriano E, Moreira WSC, Carneiro MAA (2006) Some aspects of the growth of Gracilaria birdiae (Gracilariales, Rhodophyta) in an estuary in northeast Brazil. Aquacult Int 14:327–336
Marinho-Soriano E, Panucci RA, Carneiro MAA, Pereira DC (2009) Evaluation of Gracilaria caudata J. Agardh for bioremediation of nutrients from shrimp farming wastewater. Bioresour Technol 100:6192–6198
McHugh DJ (2003) A guide to the seaweed industry. FAO Fisheries Technical paper, No 441: 105 p.
MDIC – Ministério do Desenvolvimento, Indústria e Comércio Exterior. Dados de Exportação. Available in: http//aliceweb.mdic.gov.br/(Accessed: Nov 20 2015)
MMA – Ministério do Meio Ambiente (2008) Áreas prioritárias para conservação, uso sustentável e repartição de benefícios da biodiversidade brasileira. 328 pp.
Oliveira EC (1998) The seaweed resources of Brazil. In: Critchley AT, Ohno M (eds) Seaweed resources of the world. Japan International Cooperation Agency, Yokosuka, pp 367–371
Oliveira EC, Horta PA, Amancio CE, Santanna CL (2002) Algas e angiospermas marinhas bênticas do litoral brasileiro: diversidade, explotação e conservação. In Avaliação e ações prioritárias para a conservação da biodiversidade das zonas costeira e marinha. (Ministério do Meio Ambiente, ed.). Brasília CDRom
Oliveira VP, Freire FAM, Marinho-Soriano E (2012) Influence of depth on the growth of the seaweed Gracilaria birdiae (Rhodophyta) in a shrimp pond. Braz J Aquat Sci Technol 16:33–39
Paula EJ, Pereira RTL, Ohno M (2002) Growth rate of the carrageenophyte Kappaphycus alvarezii (Rhodophyta, Gigartinales) introduced in subtropical waters of São Paulo State, Brazil. Phycol Res 50:1–9
Pellizzari F, Reis RP (2011) Seaweed cultivation on the southern and southeastern Brazilian coast. Braz J Pharmacog 21:305–312
Prates APL (2007) National Plan for Protected AreasMarine and CoastalAreas Context. In: MMA/SBF. 2007. Aquatic Protected Areas as Fisheries Management tools. 18–24 p
Rebours C, Marinho-Soriano E, Zertuche-González JA, Hayashi L, Vásquez JA, Kradolfer P, Soriano G, Ugarte R, Abreu MH, Bay-Larsen I, Rødven R, Robledo D (2014) Seaweeds: an opportunity for wealth and sustainable livelihood for coastal communities. J Appl Phycol 26:1939–1951
Reis RP, Bastos M, Góes HG (2007) Cultivo de Kappaphycus alvarezii no litoral do Rio de Janeiro. Panorama Aqüicultura 17(89):42–47
SUDENE (1981) Projeto Algas—Estado do Rio Grande do Norte. Estudos de Pesca 9:1–120
UNCTAD/GATT (1981) Estudio Piloto sobre la Industria y el Comercio Mundiales de Algas. Center for International Commerce, Geneva, 99 pp
Acknowledgments
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). I would like to thank the editor and two anonymous reviewers for their suggestions and comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marinho-Soriano, E. Historical context of commercial exploitation of seaweeds in Brazil. J Appl Phycol 29, 665–671 (2017). https://doi.org/10.1007/s10811-016-0866-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0866-8