Abstract
This study investigated the optimal N and P requirements for the growth of Isochrysis galbana under phototrophic and mixotrophic conditions. Algae were cultured in the f/2 basal medium modified with N and P concentrations. In the phototrophic condition, three N forms: sodium nitrate, ammonium sulphate and urea were tested at six N levels (0, 12.5, 25, 50, 100 and 200 mg N L−1) and P was tested at five levels (0, 1.3, 2.6, 5.2 and 10.4 mg P L−1). In the mixotrophic condition, the N or P modified f/2 basal medium was supplemented with 50 mM glycerol as the source of organic carbon. Growth was significantly influenced by the trophic conditions and N sources. The optimal N was 12.5–200 mg NO3-N L−1, 12.5–25 mg NH3-N L−1 or 12.5–50 mg urea-N L−1. The optimal P was 1.3–5.2 mg PO4-P L−1 for growth. In all treatments, the algal production in mixotrophy was over twofold higher than that in phototrophy. The maximal algal dry weight (235.7 mg L−1) and nutrient to algal biomass conversion efficiency (21.7 mg mg−1) were obtained in the cultures with urea as the N source under mixotrophy. The requirements of N and P were not different in phototrophy and mixotrophy, but dry weight production and nutrient conversion efficiency in mixotrophy were greater than in phototrophy, indicating that mixotrophic culture is more effective in the production of I. galbana.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most marine microalgae are photosynthetic organisms that are the essential food for marine grazers including mollusc, crustacean larvae and zooplankton due to the proper nutritional value and digestibility of microalgae (Gladue and Maxey 1994). With the increasing demand of seafood from aquaculture, hatcheries require a large quantity of live algae to feed marine larvae in their early developmental stage (Borowitzka 1997). To a certain extent, live microalgae are indispensable as a diet for major aquatic animals in aquaculture as alternative feed to live algae usually gives poor growth and survival for marine larvae (Hemaiswarya et al. 2011). Thus, the supply of adequate microalgae with high nutrition quality is a challenge for a marine hatchery (Azma et al. 2011). Currently, the cultivation of microalgae in a phototrophic system is the dominant protocol to supply live algae in aquaculture, but algal productivity in such a system is low due to self-shading and light limitation (Wang et al. 2014). A high algal growth rate and high cell density can be achieved by the manipulation of algal growing conditions (Li et al. 2014). Under a heterotrophic or mixotrophic conditions, organic carbon substrates such as sugar and alcohol play an important role to supply energy and carbon for algal growth (Chen and Chen 2006). Heterotrophic and mixotrophic growth have been reported as a useful approach to boost production for some microalgae species with no light or low light supply (Andrade and Costa 2007; Andruleviciute et al. 2013; Cheirsilp and Torpee 2012). Consequently, the addition of organic carbon to the culture medium has been used to increase algal production and improve algal nutrition as live food for marine larvae. For example, in the culture of Nannochloropsis sp., the addition of glycerol to the media can increase dry weight production by 40 % and the lipid content by 30 % compared with the solely phototrophic culture (Das et al. 2011).
Besides carbon and light supply, microalgae also require other nutrients for growth and cell division. Nitrogen and phosphorus are the two fundamental elements required in algal culture media. Microalgae can utilize nitrogen in different forms such as nitrate, ammonium and urea, and the preferred nitrogen source is alga species specific (Perez-Garcia et al. 2011). While most microalgae prefer nitrate and urea, ammonium is considered an inconvenient source of nitrogen since the assimilation of ammonium causes pH to drop and the acidic condition may lead to the decline of algal growth (Kim et al. 2013a; Wen and Chen 2001; Yongmanitchai and Ward 1991). The effect of nitrogen source on the uptake rate depends on culture conditions. For instance, the growth rate of Phaeodactylum tricornutum was lower in ammonium than in nitrate or urea in a phototrophic condition (Yongmanitchai and Ward 1991). However, Cerón García et al. (2000) found that the maximal growth of P. tricornutum occurred in ammonium chloride when the culture medium was supplemented with organic carbon as glycerol. In contrast, Combres et al. (1994) found that the uptake rate of ammonium by Scenedesmus obliquus was lower in a mixotrophic condition than in a phototrophic condition. Moreover, the requirement of phosphorus differs between trophic conditions. For instance, Chlorella pyrenoidosa require less phosphate in the presence of glucose than without glucose (Qu et al. 2008). Therefore, there is a need to further explore the difference of nutrient requirements in algae between phototrophic and mixotrophic conditions.
The golden brown flagellate Isochrysis galbana is a common species used as a live food in aquaculture because of its rapid growth in mass culture (Liu et al. 2013). This species is preferred by most marine larvae due to cell size, nutrient content and digestibility (Wikfors and Patterson 1994). In addition, its proximate composition contains a high level of polyunsaturated fatty acids, particularly docosahexaenoic acid (DHA) (Liu et al. 2013). This species is able to grow mixotrophically with high growth rate and biomass production (Alkhamis and Qin 2013). The requirements of nitrogen and phosphorus for the growth of I. galbana have been studied only under phototrophic conditions (Fidalgo et al. 1998; Liu et al. 2013). However, the requirements of nitrogen and phosphorus by I. galbana under a mixotrophic condition are unknown. This study aimed to compare the responses of I. galbana to different nutrient sources, nitrogen concentrations and phosphorus concentrations in phototrophic and mixotrophic conditions. The optimization of nutrient requirements of I. galbana in mixotrophic conditions will contribute to the improvement of algal growth efficiency and the mass production of this commonly used species in aquaculture and other industrial uses.
Materials and methods
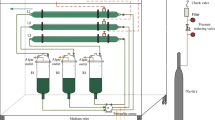
The marine microalga I. galbana (CS-22) was obtained from the Australian National Algae Culture Collection (Hobart, Tasmania). Algal cultures were carried out in natural seawater (35 ‰) enriched with the basal f/2 nutrients (Guillard and Ryther 1962) with variations of N and P concentrations. Prior to the experiment, the culture media were autoclaved at 121 °C for 15 min. Glycerol as a source of organic carbon was sterilized in an autoclave at 115 °C for 10 min and was only supplemented to the mixotrophic cultures. All cultures were carried out in 250 mL sterilized flasks containing 100 mL medium and 10 % (v/v) algal inoculum. The flasks were placed on an orbital shaker at 100 rpm at 24 °C under a daily illumination of 12 h light at 50 μmol photons m−2 s−1 measured at the surface of the media using a Light ProbeMeter (Extech Instruments Corp, USA). Illumination was provided with white cool fluorescent lamps.
Experimental design
Three nitrogen sources: nitrate as NaNO3, ammonium as (NH4)2SO4 and urea (CH4N2O) at six concentrations 0,12.5, 25, 50, 100 and 200 mg N L−1 were tested in the phototrophic condition with three replicates. Except for nitrogen, other nutrients were added as the same as in the f/2 medium. The environmental and nutrient conditions for the mixotrophic algal culture were identical to the phototrophic conditions except that 50 mM glycerol was supplemented to each treatment as organic carbon. The dissolved organic carbon in the original seawater was not measured, but the zero glycerol addition in the phototrophic medium was used as the control to the mixotrophic medium added with 50 mM glycerol. All experiments with different nitrogen sources lasted 10 days when the stationary phase of growth was reached.
Based on the results of the nitrogen experiments, urea was identified as the optimal source of nitrogen, and 12.5 mg urea-N L−1 was the optimal level of nitrogen for I. galbana growth in the trial for testing phosphorus requirement. Five levels of P as sodium di-hydrogen orthophosphate (NaH2PO4) at 0, 1.3, 2.6, 5.2 and 10.4 mg P L−1 were used in the media contained 12.5 mg urea-N L−1. In the mixotrophic condition, 50 mM glycerol was supplemented to each P treatment as the source of organic carbon. Three replicates were used in both phototrophic and mixotrophic cultures, and each experiment lasted 10 days when the stationary phase of algal growth was reached.
Determination of algal growth
Algal cell density was quantified by taking 2 mL sample from each flask every 2 days during the experimental period. The absorbance was measured at 680 nm. The regression between algal cell densities and optical densities was assessed by measuring the optical density of a series of diluted algal samples with known cell densities counted on a microscope with a haemocytometer. The optical densities (OD) at 680 nm were plotted versus known cell densities to determine the linear relation (Fig. 1). The algal cell density (Y) was calculated according to the linear Eq. (1) with the optical density (x) at 680 nm.
Algal production was determined by measuring the total dry weight as the algal production at the end of each experiment. A volume of 100 mL culture was centrifuged at 5000 × g for 10 min, and the algal pellets were washed with distilled water and dried in an oven at 65 °C until the constant weight. The algal weight was measured to the nearest 0.001 mg. The nutrient conversion efficiency (Y X/N ) was calculated using the amount of biomass production and nutrient reduction (Eq. 2) (Doran 1995).
where dx is the change of biomass and ds is the change of nutrient concentration in the substrate concentrations during time t.
Determining nutrient concentrations
The utilization rate of each nutrient was determined from the samples which were cultured in the phototrophic and mixotrophic conditions. The initial nutrient concentrations were the optimal N 12.5 mg N L−1 and optimal P concentration (2.6 mg P L−1) determined from the previous trials. A sufficient volume of the culture media was taken every 2 days to measure residuals of N and P, and the samples were filtered through GF/C filters and kept in a −20 °C freezer until analysis. The residual of N as nitrate and ammonia and P as phosphate was measured photometrically using nutrient test kits (Aquaspex Water testing products, Blackwood, SA, Australia). The residuals of urea were analyzed by the decomposition of total nitrogen in the sample into nitrogen monoxide, then the total nitrogen concentration was detected using a Shimadzu Total Organic Carbon and Total Nitrogen Analyzer (TOC-VCSH/CSN + TNM-1, Shimadzu, Japan) by a chemiluminescence gas analysis.
Statistical analysis
The experimental design to test the effect of nitrogen on algal growth included three factors (nitrogen sources, concentrations and trophic conditions). Significant differences between means of each variable were tested by three-way ANOVA. To detect the treatment effects, this test was followed by MANOVA unless the interaction effects were found significant (P < 0.05). In the phosphorus trial, the differences between the means of the algal density, algal production and nutrient conversion efficiency were tested by two-way ANOVA with P concentration and trophic conditions as two fixed factors. Multiple comparisons were tested by Tukey post hoc analysis when the main treatment effect was significant at P < 0.05. Data were analyzed using SPSS (version 18).
Results
Effect of nitrogen sources and nitrogen concentrations
The growth of I. galbana was tested in six N concentrations (0, 12.5, 25, 50, 100 and 200 mg N L−1) of each nitrate, ammonium and urea under both phototrophic and mixotrophic conditions. Each of the three treatment factors nitrogen sources, nitrogen concentration and trophic conditions had significant (P < 0.05) impact on the cell density of I. galbana (Table 1). The interaction effect between these factors was also significantly different (P < 0.05). The effect of N concentration and trophic conditions on algal growth with N source is presented in Fig. 2. As algal growth was not detectable in the phototrophic or mixotrophic treatments without N, these growth rates were removed from the analysis. The effect of N concentrations on growth was not significantly different between trophic conditions when nitrate was the N source (P > 0.05). The alga was able to grow in a wide range of nitrate concentrations, but there were no increases in cell densities in both mixotrophic and phototrophic cultures at nitrate concentrations of 12.5 to 200 mg NO3-N L−1. The impact of N concentration on cell density in the phototrophic and mixotrophic conditions was significant when N was supplied as ammonium or urea (P < 0.05). The algal cell density in mixotrophy was enhanced when the N concentration was 12.5 to 25 mg NH4-N L−1 (P < 0.05, Fig. 2) compared with phototrophy, though algal densities were not significantly different between the N concentrations of 12.5 and 25 mg NH4-N L−1 (P > 0.05). When ammonium was increased to 50 mg NH4-N L−1, algal densities sharply decreased and algal growth was much inhibited when the N concentration was >100 mg NH4-N L−1 in both photo and mixotrophic conditions. Algal cell density in mixotrophy was higher than that in phototrophy when urea was the N source (P < 0.05). However, the effect of urea concentration on cell density was not significant at N concentrations between 12.5 and 50 mg urea-N L−1 in both trophic conditions (P > 0.05). Cell density significantly decreased when the urea concentration increased from 100 to 200 mg urea-N L−1 (P < 0.05). Among all N treatments, the maximal algal density occurred when nitrogen was in the range of 12.5–50 mg urea-N L−1 in both phototrophic and mixotrophic conditions.
Effect of phosphorus concentrations
The final cell densities at the end of the trial were significantly affected by phosphorus concentrations and trophic conditions (P < 0.05, Fig. 3). However, P concentrations did not significantly affect growth in phototrophic cultures. The final algal densities in the culture of 0–5.2 mg P L−1 were not significantly different in the phototrophic condition (P > 0.05). However, P enrichments increased algal density in the mixotrophic culture compared with the control (P < 0.05), but there were no significant differences in density when phosphorus was >1.3 mg P L−1 (P > 0.05). The maximum cell density reached 5.8 × 106 cells mL−1 in the mixotrophic culture with P addition, while the minimum cell density was 3.8 × 106 cells mL−1 in cultures without P addition. Despite the same P additions in both trophic conditions, the final cell densities in mixotrophic conditions were significantly greater than those in phototrophic conditions (P < 0.05).
Algal production and nutrient conversion efficiency
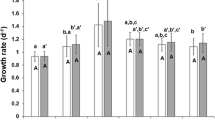
Algal production and nutrient conversion efficiency are presented in Fig. 4. These parameters were significantly affected (P < 0.05) by N sources and trophic conditions. Among the three N sources, the algal production in the culture with urea was significantly higher than that with ammonium or nitrate as the N source. In addition, production was promoted in the mixotrophic condition, and the maximum algal dry weight in mixotrophy (235.7 mg L−1) was two times higher than that in phototrophy (115.3 mg L−1). The values of nutrient conversion efficiency to algal biomass based between N sources were significantly different (P < 0.05) in both trophic conditions. The nutrient to biomass conversion efficiency (Fig. 4) in mixotrophy with urea and ammonium was 21.7 and 18.8 mg mg−1, respectively, which was significantly higher than that with nitrate (P < 0.05). While in phototrophy, the nutrient conversion efficiency in the urea treatment was 14.85 mg mg−1, which was significantly higher than that in the nitrate (11.97 mg mg−1) or ammonium (9.92 mg mg−1) treatments (P < 0.05).
Nutrient depletion
The reduction of nitrate, ammonium and urea concentration in the culture media over time is shown in Fig. 5. The reduction rates of the three nitrogen sources in the mixotrophic condition were faster than that in the phototrophic condition. At the end of trial, 56 % nitrate, 58 % ammonium and 62 % urea were removed from the substrates in phototrophic culture, but 93 % nitrate, 90 % ammonium and 87 % urea were depleted from the substrates in the mixotrophic culture. Phosphorus depletion rate was also higher in the mixotrophic condition than that in the phototrophic condition.
Discussion
This study compared the N and P requirements of I. galbana in phototrophic and mixotrophic conditions with three N sources over a broad range of N concentrations. Algal cell abundance in all mixotrophic cultures was higher than in the phototrophic cultures regardless of N source. Although the dissolved organic matter was not measured in the original seawater, the significantly higher algal abundance in the mixotrophic medium compared with that in the phototrophic medium without organic carbon addition suggests that the added organic carbon has enhanced algal growth. Similarly, enhanced growth in mixotrophic culture compared to phototrophic culture has been reported in other marine species such as Nannochloropsis sp. (Xu et al. 2004), P. tricornutum (Cerón Garcı́a et al. 2006), Dunaliella salina (Wan et al. 2011) and freshwater species such as Chlorella vulgaris (Heredia-Arroyo et al. 2011) and Scenedesmus sp. (Andruleviciute et al. 2013). The fast growth in mixotrophy is possibly due to the supply of both light and organic carbon as energy sources (Wang et al. 2014).
Soluble nitrogen is an essential nutrient for the growth of I. galbana as well as for other phytoplankton species (Grobbelaar 2004). In this study, the growth of I. galbana depended on N concentrations, but the algae were able to utilize nitrate, ammonium and urea as the sole of nitrogen source to support growth in both phototrophic and mixotrophic conditions. The effects of these nitrogen compounds were also studied on the phototrophic growth of I. galbana in phototrophic conditions (Feng et al. 2011; Liu et al. 2013) and on the heterotrophic growth of Nitzschia laevis and Tetraselmis suecica where organic carbon was supplied in the substrate (Azma et al. 2011; Cao et al. 2008). In these studies, nitrate and urea were identified as the most appropriate nitrogen sources for algal growth, but ammonium was least effective. Urea as a source of organic N plays a dual role in algal nutrition as it is metabolized into ammonia and carbon dioxide through hydrolysis. In the present study, the impacts of these three N sources on growth were comparable between phototrophic and mixotrophic conditions, but growth was N concentration dependent, except for the nitrate nitrogen. Similarly, comparable growth rates were also found in Cyclotella cryptica in heterotrophic culture when different N sources were used (Pahl et al. 2012).
In this study, although the growth of I. galbana in the mixotrophic cultures was faster than that in the phototrophic cultures, the cell densities were not different between nitrate concentrations from 12.5 to 200 mg N L−1 in both trophic conditions. Nitrate can stimulate the growth of Chlorella protothecoides and N. laevis at a broad range of concentrations from 14 to 560 mg NO3-N L−1 (Shi et al. 2000; Wen and Chen 2001). Liu et al. (2013) found that the cell density of I. galbana was enhanced when the culture medium was enriched with nitrate from 6.5 to 200 mg N L −1. However, we found that the impact of the trophic condition on growth depended on nitrogen concentrations. The growth of I. galbana was enhanced more in the mixotrophic condition than in the phototrophic condition when the N concentration was <50 mg N-NH4 or <100 mg N-urea L−1. However, the advantage of fast growth disappeared in the mixotrophic condition when the ammonium and urea concentrations exceeded these threshold values. This phenomenon was previously observed in the heterotrophic growth of C. cryptica when the ammonium or urea concentrations were 25–300 mg N L−1, but the growth advantage in heterotrophic conditions disappeared when the N concentration exceeded 25 mg NH4-N or 150 mg urea-N L−1 (Pahl et al. 2012). The negative impact of ammonia on cell density at high concentrations is possibly due to its toxic effect on growth (Källqvist and Svenson 2003).
The growth of I. galbana was affected by phosphorus, but the impact of P concentration was much less than nitrogen. In this study, I. galbana could grow phototrophically and mixotrophically in medium without P addition. The algal abundance was not significantly affected by P concentrations from 0 to 10.4 mg P L−1 in phototrophy. The P requirement in microalgae is species dependent in phototrophic culture. For instance, Yongmanitchai and Ward (1991) and Kim et al. (2012) found that P. tricornutum and D. salina showed the same growth pattern at P concentrations of 8.9–88.9 and 0.77–12.40 mg P L−1, respectively. In addition, we found that the P concentration in the range of 1.3–10.4 mg P L−1 had little effect on mixotrophic growth of I. galbana. Interestingly, when phosphate was not added to the mixotrophic culture medium, the cell density was significantly lower than that in the cultures with P additions. However, this P-dependent growth did not happen in the phototrophic culture. According to Martínez et al. (1997), S. obliquus could grow in a P-free medium depending on the internal reserve P content such as polyphosphate. In our study, it is likely that algae depleted P reserves faster in mixotrophy than in phototrophy, and the cell density significantly declined in the mixotrophic culture without P addition.
Although the optimal N and P concentrations for the growth of I. galbana were not different between phototrophic and mixotrophic conditions, algae in the mixotrophic culture utilized nutrient faster than in the phototrophic culture. The depletion rates of N and P in mixotrophy were two times faster than in phototrophy, which is similar to the growth of Chlorella sorokiniana where nutrients are depleted two times faster in mixotrophy than in phototrophy (Kim et al. 2013b). Moreover, we found that the nutrient conversion efficiency to biomass production was higher in mixotrophy than in phototrophy. In other studies, the conversion efficiency of nutrients in the substrate into the biomass of N. laevis and Spirulina sp. was also increased when algae were cultured mixotrophically (Chojnacka and Zielińska 2012; Wen and Chen 2000). In this study, the maximal value of nutrient conversion efficiency and biomass production were achieved when the alga was cultured mixotrophically with urea as the N source. Urea is a source of organic nitrogen and supports fast growth either in phototrophic and mixotrophic conditions (Perez-Garcia et al. 2011). Feng et al. (2011) demonstrated that urea is a superior N source to produce maximal cell density and dry weight of Isochrysis zhangjiangensis. Although the required nitrogen concentration for algal growth was not different between trophic conditions in the present study, the change of N source and trophic conditions could improve growth suggesting that the mixotrophic mode is a feasible process to grow I. galbana with urea as the recommended N source.
In conclusion, the optimal N and P requirements for the growth of I. galbana was studied under phototrophic and mixotrophic conditions. Growth was enhanced in mixotrophy compared with that in phototrophy, but the growth advantage disappeared when the N concentrations exceeded 50 mg NH4-N or 100 mg urea-N L−1. The P requirements for the growth of I. galbana were similar between phototrophic and mixotrophic conditions. Algal production and the efficiency of nutrient conversion to biomass were enhanced when the algae were cultivated mixotrophically. This study shows that the algae grow faster mixotrophically than phototrophically, while the requirements for N and P concentrations are similar between the two trophic conditions. Urea is recommended as the N source for I. galbana at 12.5–50 mg urea-N L−1.
References
Alkhamis Y, Qin JG (2013) Cultivation of Isochrysis galbana in phototrophic, heterotrophic, and mixotrophic conditions. BioMed Res Int. doi:10.1155/2013/983465
Andrade M, Costa J (2007) Mixotrophic cultivation of microalga Spirulina platensis using molasses as organic substrate. Aquaculture 264:130–134
Andruleviciute V, Makareviciene V, Skorupskaite V, Gumbyte M (2013) Biomass and oil content of Chlorella sp., Haematococcus sp., Nannochloris sp. and Scenedesmus sp. under mixotrophic growth conditions in the presence of technical glycerol. J Appl Phycol 26:83–90
Azma M, Mohamed MS, Mohamad R, Rahim RA, Ariff AB (2011) Improvement of medium composition for heterotrophic cultivation of green microalgae, Tetraselmis suecica, using response surface methodology. Biochem Eng J 53:187–195
Borowitzka MA (1997) Algae for aquaculture: opportunities and constraints. J Appl Phycol 9:393–401
Cao X, Li S, Wang C, Lu M (2008) Effects of nutritional factors on the growth and heterotrophic eicosapentaenoic acid production of diatom Nitzschia laevis. J Ocean Univ China 7:333–338
Cerón García MC, Fernández Sevilla JM, Acién Fernández FG, Molina Grima E, García Camacho F (2000) Mixotrophic growth of Phaeodactylum tricornutum on glycerol: growth rate and fatty acid profile. J Appl Phycol 12:239–248
Cerón Garcı́a MC, Garcı́a Camacho F, Sánchez Mirón A, Fernández Sevilla JM, Chisti Y, Molina Grima E (2006) Mixotrophic production of marine microalga Phaeodactylum tricornutum on various carbon sources. J Microbiol Biotechnol 16:689–694
Cheirsilp B, Torpee S (2012) Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour Technol 110:510–516
Chen GQ, Chen F (2006) Growing phototrophic cells without light. Biotechnol Lett 28:607–616
Chojnacka K, Zielińska A (2012) Evaluation of growth yield of Spirulina (Arthrospira) sp. in photoautotrophic, heterotrophic and mixotrophic cultures. World J Microbiol Biotechnol 28:437–445
Combres C, Laliberté G, Reyssac JS, de la Noüe J (1994) Effect of acetate on growth and ammonium uptake in the microalga Scenedesmus obliquus. Physiol Plant 91:729–734
Das P, Aziz SS, Obbard JP (2011) Two phase microalgae growth in the open system for enhanced lipid productivity. Renew Energy 36:2524–2528
Doran PM (1995) Bioprocess engineering principles. Academic Press Ltd, San Diego
Feng D, Chen Z, Xue S, Zhang W (2011) Increased lipid production of the marine oleaginous microalgae Isochrysis zhangjiangensis (Chrysophyta) by nitrogen supplement. Bioresour Technol 102:6710–6716
Fidalgo JP, Cid A, Torres E, Sukenik A, Herrero C (1998) Effects of nitrogen source and growth phase on proximate biochemical composition, lipid classes and fatty acid profile of the marine microalga Isochrysis galbana. Aquaculture 166:105–116
Gladue RM, Maxey JE (1994) Microalgal feeds for aquaculture. J Appl Phycol 6:131–141
Grobbelaar JU (2004) Algal nutrition—mineral nutrition. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell Publishing Ltd, Oxford, pp 95–115
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can J Microbiol 8:229–239
Hemaiswarya S, Raja R, Ravi Kumar R, Ganesan V, Anbazhagan C (2011) Microalgae: a sustainable feed source for aquaculture. World J Microbiol Biotechnol 27:1737–1746
Heredia-Arroyo T, Wei W, Ruan R, Hu B (2011) Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenergy 35:2245–2253
Källqvist T, Svenson A (2003) Assessment of ammonia toxicity in tests with the microalga, Nephroselmis pyriformis, Chlorophyta. Water Res 37:477–484
Kim W, Park J, Gim G, Jeong S-H, Kang C, Kim D-J, Kim S (2012) Optimization of culture conditions and comparison of biomass productivity of three green algae. Bioprocess Biosyst Eng 35:19–27
Kim S, Lee Y, Hwang S-J (2013a) Removal of nitrogen and phosphorus by Chlorella sorokiniana cultured heterotrophically in ammonia and nitrate. Int Biodeterior Biodegrad 85:511–516
Kim S, J-e P, Cho Y-B, Hwang S-J (2013b) Growth rate, organic carbon and nutrient removal rates of Chlorella sorokiniana in autotrophic, heterotrophic and mixotrophic conditions. Bioresour Technol 144:8–13
Li TT, Zheng YB, Yu L, Chen SL (2014) Mixotrophic cultivation of a Chlorella sorokiniana strain for enhanced biomass and lipid production. Biomass Bioenergy 66:204–213
Liu J, Sommerfeld M, Hu Q (2013) Screening and characterization of Isochrysis strains and optimization of culture conditions for docosahexaenoic acid production. Appl Microbiol Biotechnol 97:4785–4798
Martínez ME, Camacho F, Jiménez JM, Espínola JB (1997) Influence of light intensity on the kinetic and yield parameters of Chlorella pyrenoidosa mixotrophic growth. Process Biochem 32:93–98
Pahl SL, Lewis DM, King KD, Chen F (2012) Heterotrophic growth and nutritional aspects of the diatom Cyclotella cryptica (Bacillariophyceae): effect of nitrogen source and concentration. J Appl Phycol 24:301–307
Perez-Garcia O, Escalante FME, de Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45:11–36
Qu CB, Wu ZY, Shi XM (2008) Phosphate assimilation by Chlorella and adjustment of phosphate concentration in basal medium for its cultivation. Biotechnol Lett 30:1735–1740
Shi XM, Zhang XW, Chen F (2000) Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzyme Microb Technol 27:312–318
Wan MX, Liu P, Xia JL, Rosenberg JN, Oyler GA, Betenbaugh MJ, Nie ZY, Qiu GZ (2011) The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl Microbiol Biotechnol 91:835–844
Wang J, Yang H, Wang F (2014) Mixotrophic cultivation of microalgae for biodiesel production: status and prospects. Appl Biochem Biotechnol 172:3307–3329
Wen ZY, Chen F (2000) Production potential of eicosapentaenoic acid by the diatom Nitzschia laevis. Biotechnol Lett 22:727–733
Wen Z-Y, Chen F (2001) Optimization of nitrogen sources for heterotrophic production of eicosapentaenoic acid by the diatom Nitzschia laevis. Enzyme Microb Technol 29:341–347
Wikfors GH, Patterson GW (1994) Differences in strains of Isochrysis of importance to mariculture. Aquaculture 123:127–135
Xu F, Hu HH, Cong W, Cai ZL, Ouyang F (2004) Growth characteristics and eicosapentaenoic acid production by Nannochloropsis sp in mixotrophic conditions. Biotechnol Lett 26:51–53
Yongmanitchai W, Ward OP (1991) Growth of and omega-3 fatty acid production by Phaeodactylum tricornutum under different culture conditions. Appl Environ Microbiol 57:419–425
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alkhamis, Y., Qin, J.G. Comparison of N and P requirements of Isochrysis galbana under phototrophic and mixotrophic conditions. J Appl Phycol 27, 2231–2238 (2015). https://doi.org/10.1007/s10811-014-0501-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0501-5