Abstract
The understanding of how nitrogen (N) to phosphorus (P) ratios regulate growth and chemical composition of algae is important to control the nutritional value of microalgae for industrial application. This study compared the impacts of N:P ratio manipulations on the growth, elements, lipid, fatty acids and protein contents of Tisochrysis lutea and Nannochloropsis oculata. F/2 medium was used as the basal formula to obtained six N:P ratios of 5:1, 10:1, 20:1, 30:1, 60:1 and 120:1 and tested on the algae species in triplicate. Growth rate was similar in both algal species across all N:P ratios, and the carbon content in T. lutea was higher than in N. oculata. However, the carbon contents were high in the N:P ratios of 5:1 and 120:1 and low from 10:1 to 60:1 N:P ratios for both T. lutea and N. oculata. There were no significant differences in cellular N and P, but the protein contents depended on algae species and were significantly affected by N:P ratios. The N:P ratio of 20:1 favoured algal growth and protein content, while the N:P ratio of 120:1 reduced algal growth and protein synthesis but increased lipid in both algae. The 20:1 N:P ratio favoured eicosapentaenoic acid (EPA) production in N. oculata and the 30:1 N:P ratio favours docosahexaenoic acid (DHA) production in T. lutea. This study indicates that N:P ratio manipulation is an effective strategy to change biochemical composition in algae and N or P limitation tends to lower polyunsaturated fatty acids (PUFA) contents in algae.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are photosynthetic organisms that constitute the first primary link of an aquatic food chain (Marchetti et al. 2012). The products of microalgae have various applications, particularly in pharmaceuticals, cosmetics, biofuel production, and aquaculture (Pulz and Gross 2004; Rosenberg et al. 2008; Borowitzka 2013). In nature, microalgae are the primary food for mollusks, zooplankton and crustacean larvae (Borowitzka 1997; Brown and Hohmann 2002; Duerr et al. 1998). Among various forms of alternative diets, such as algal paste (McCausland et al. 1999; Robert et al. 2001), yeast (Nell 2002), bacteria (Douillet and Langdon 1994) or even lipid compounds such as oil emulsions (Coutteau et al. 1996; Knauer and Southgate 1997), live microalgae remain essential to the production of larval fish and juvenile bivalves in captivity (Robert and Trintignac 1997).
As live food, the nutritional compositions, particularly polyunsaturated fatty acids 20:5(n-3) (eicosapentaenoic acid, EPA) and 22:6(n-3) (docosahexaenoic acid, DHA) in algae are critically important because they can affect the growth and reproduction of aquatic animals (Jonasdottir et al. 2009; Mueller-Navarra et al. 2000). However, the amount of EPA and DHA in algae differs greatly among algae species and environmental conditions. For instance, the EPA content is 4.8 mg g−1 in Tetraselmis sp. but is 23.4 mg g−1 in Nannochlopsis sp., while the DHA content is 0.2 mg g−1 in Tetraselmis sp. but is 15.8 mg g−1 in Isochrysis sp. (Patil et al. 2007). Marine microalgae such as Isochrysis sp. and Nannochloropsis sp. have received increasing attention as live food for aquatic animals because of their high contents of DHA and EPA (C-Pa and Lin 2001; Patil et al. 2007) which are the essential fatty acids in the diet of marine animals for growth, health and reproduction.
Nutrient availability in the environment can regulate the growth and biochemical composition of algae (Qin and Culver 1996; Johansson and Graneli 1999). Phosphorus (P) and nitrogen (N) are the two major elements limiting primary productivity (Villar-Argaiz and Sterner 2002), and their availability can alter the biochemical composition particularly the lipid content of algae (Ahlgren and Hyenstrand 2003; Sharma et al. 2012). A typical effect of nutrient limitation is a reduction of growth and changes of cellular composition in algae (Beardall et al. 2001b). N and P limitations usually increase the cellular carbon (C) content (Cembella et al. 1984; Berdalet et al. 1994), and C accumulation in algal cells may reduce nutrient utilisation efficiency in luxurious supply and increase the efficiency of nutrients in short supply (Sterner and Hessen 1994). Furthermore, the nutrient availability in the environment can affect macromolecular composition (e.g. protein, carbohydrate and lipid) in algae (Geider and La Roche 2002). Molecular N:P ratios thereby can provide an integrated measure of the ability of nutrient uptake for algal cells (Beardall et al. 2001a; Fresnedo and Serra 1992; Qin et al. 2012). Thus, N limitation usually results in low protein content and high carbohydrate or lipid storage (Shifrin and Chisholm 1981; Ganf et al. 1986) while P limitation can also shift the relative contents of protein, lipid and carbohydrate in algal cells (Theodorou et al. 1991; Reitan et al. 1994).
Many studies on nutrient limitation have emphasised the capacity of total lipid change through nutrient manipulation (Liang et al. 2013; Sharma et al. 2012; Reitan et al. 1994), but little research has focused specifically on the changes of PUFA and elemental contents of algae under different N:P ratios. N: P ratios have been manipulated to simulate situations from nitrogen deficiency to phosphorus deficiency as reported in other studies (Hessen et al. 2002; Breteler et al. 2005; Rodolfi et al. 2009) on a variety of green and golden algae species. The N and P levels were manipulated under different proportions of N or P reduction in the F/2 media to reach the target N:P ratios. Since most previous studies have focused on manipulating nutrients to evaluate the overall lipid composition of algae (Kunikane et al. 1984; Fong et al. 2004; Anderson and Pond 2000; Lai et al. 2011; Ahlgren and Hyenstrand 2003), this study fills the knowledge gap by evaluating the effects of different N:P ratios on the biochemical composition particularly the major components of fatty acids such as eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), α-linolenic acid (ALA) and arachidonic acid (ARA) as well as on some elemental compositions (N, P and C), protein and lipid in two algae species with intrinsic distinctions in biochemical compositions.
The supply of a single nutrient can affect physiological, biochemical and molecular adaptation of algae (Lai et al. 2011; Breteler et al. 2005; Liu et al. 2013), but our knowledge on the role of N:P ratio in regulating the contents of EPA, DHA and other fatty acids in algae is limited. Therefore, in this study, we selected two algal species Tisochrysis lutea (previously known as the Tahitian strain of Isochrysis, T-Iso, Bendif et al. 2013) and Nannochloropsis oculata that have been widely used as live food for zooplankton. The former contains high DHA and the latter has high EPA, which allow us to assess how N:P ratios affect algae on their biochemical compositions. Specifically, we aimed to assess the impact of N:P ratio on algal growth, elemental composition, fatty acid profile and the contents of protein and lipid in algal cells. The understanding of these key issues will enable us to identify the optimal nutrient regimes for algal culture to produce high nutritional algae as live food for zooplankton. This study extends our knowledge on using N:P ratios as a tool to manipulate the value of algal composition for their use as zooplankton food or in commercial industries.
Materials and methods
We used six different N:P ratios to examine the changes in growth and chemical composition of the algae including C, N, P and biochemical contents, particularly the fatty acid profile of the two algae, N. oculata and T. lutea. These two species were used to compare whether the effects of N:P ratios on algal growth and cellular contents were species-specific. The algae used in this study were obtained from South Australian Research and Development Institute Aquatic Science Centre, Adelaide. The F/2 media was used as the basal formula for nutrient ratio manipulations at N:P = 5:1 (72 μM N; 14.37 μM P), 10:1 (144 μM N; 14.37 μM P), 20:1 (287 μM N; 14.37 μM P), 30:1 (287 μM N; 9.6 μM P), 60:1 (287 μM N; 4.8 μM P) and 120:1 (287 μM N; 2.4 μM P). N and P levels were manipulated under different proportions of N or P reduction in the F/2 medium to reach the appropriate N:P ratios. The amount of N and P in the seawater and the medium from algal inoculation were considered in the final nutrient manipulation at different N:P ratios. All cultures were carried out in triplicate. The C:N molar ratios for each treatment were 4.5 (N:P 5:1), 4.7 (N:P 10:1), 5.2 (N:P 20:1), 5.5 (N:P 30:1), 6.1 (N:P 60:1) and 6.5 (N:P 120:1). Two litres of culture were harvested for biochemical analysis when the stationary growth phase was reached.
Algal growth at different N:P ratios
Starter algal cultures of 50–100 mL in the mid exponential growth phase were used as inoculum giving initial cell densities of The 2.0 × 105 cells mL−1 for T. lutea and 2.5 × 105 cells mL−1 for N. oculata. Each experiment was carried out in a 2-L aerated glass Schott bottles exposed to an irradiance of 115 μmol photons m−2 s−1 with fluorescent tubes on a 12:12-h light-dark cycle. Mean temperature was 23 ± 1 °C in the light period and 20 ± 1 °C in the dark period. All cultures were aerated with a 0.22-μm-filtered air enriched with 1 % CO2 at approximately 500 mL−1 min−1. Aliquots (1 mL) of each culture were collected for cell density counts. Algal cell counts (six replicates per culture) were undertaken using a Neubauer haemocytometer. The specific growth rate (μ) was calculated from the exponential phase obtained through the two points, N 1 and N 2, at the two ends of this linear phase (exponential) using the equation: μ = ln (N 2 − N 1)/(t 2 − t 1) where N 2 = number of cells mL−1 at time of harvest t 2 and N 1 = cells mL−1 at time t 1 from day 2 until day 6 prior to the stationary growth phase in both species.
Biochemical analysis
In order to optimise the fatty acid content, algae were harvested during the stationary phase (Mansour et al. 2005). Samples for measuring protein, total lipid and fatty acid contents were centrifuged at 7000×g at 15 °C for 15 min to obtain concentrated algal pellets and were freeze-dried prior to analysis. Lipids were extracted with the chloroform-methanol method (Bligh and Dyer 1959; Martínez-Fernández et al. 2006). The fatty acid content was measured and then the samples were methylated in 5 mL of 1 % H2SO4 in methanol at 70 °C for 3 h. The fatty acid methyl esters were extracted by adding 750 μL of distilled water and 2 mL of n-heptane. The heptane layer was transferred to a 2 mL vial for analysis using gas chromatography (GC, PerkinElmer Gas Chromatograph Clarus 500). Fatty acid methyl esters (FAMEs) were separated and measured on the GC equipped with a 30 m capillary column (0.32 mm internal diameter, Zebron ZB-FFAP). Helium was the carrier gas (1.5 mL min−1). The injector temperature was set at 250 °C and the detector temperature at 300 °C. The initial oven temperature was 140 °C for 3 min, then ramped at 10.0 °C min−1 from 1 to 160 °C for 5 min followed by 10.0 °C min−1 from 1 to 230 °C for 10 min. FAMEs were identified on the GC using software of TotalChrom Navigator (version 6.3.2 0646, PerkinElmer). The level of the internal standard 17:0 was used to calculate the FAME concentration in each sample. Protein contents were calculated from total Kjeldahl nitrogen (N × 6.25) by the combustion technique using an Elementar RapidN analyser where N values were obtained as a percent of dry mass for further calculations (Barbarino and Lourenço 2005; Martínez-Fernández et al. 2006.)
Nutrient concentrations
Cell N and C were analysed using a C/H/N elemental analyser (Carlo-Herba) (Fidalgo et al. 1998). Cellular P was determined using the nitric acid/hydrogen peroxide digestion method with inductively coupled plasma optical emission spectrometry (ICP-OES) (Wheal et al. 2011; Fong et al. 2004).
Data analyses
The data in this study are expressed as mean ± SD, and the results of growth, protein, total lipid and fatty acid composition were analysed by two-way analysis of variance (ANOVA) to test the interaction between the N:P ratio and algae species. This was followed where applicable with post hoc Tukey’s multiple comparison test to determine the significant differences of means between treatments for each independent factor (Ferrão-Filho et al. 2003). All data were tested for normality (Shapiro-Wilk Test), homogeneity and independence (Levene’s test) to satisfy the assumptions for ANOVA. The level of significant difference was set at P < 0.05.
Results
Algal growth
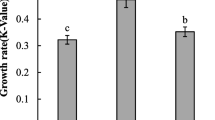
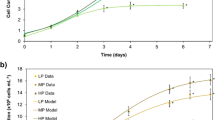
The specific growth rate between T. lutea and N. oculata was not different in all treatments of N:P ratios (P > 0.05, Fig. 1). As the N:P ratio increased from 5:1 to 20:1, the specific growth rate of T. lutea reached the maximum (1.42 d−1, P < 0.05, Fig. 1) with no significant changes with further increase of the N:P ratio, but declined when the N:P ratio reached 120:1. Similarly, when the N:P ratio increased from 5:1 to 20:1, the specific growth rate of N. oculata reached the maximum (1.49 d−1, P < 0.05, Fig. 1) but decreased significantly with the increase of N:P ratios from 20:1 to 120:1 (P = 0.004). Growth curves of T. lutea and N. oculata under different N:P ratios are shown in Fig. 2. The exponential growth phase ended within the first 6 days under all test conditions in both species. At the end of the stationary phase, cell densities for both T. lutea and N. oculata at the N:P ratio of 20:1 were significantly higher than those at other N:P ratios (P < 0.05, Fig. 2).
Growth rate of Tisochrysis lutea and Nannochloropsis oculata under different N:P ratios (n = 3). Different capital letters inside the same bar type represent significant N:P ratio effects (P < 0.05) between the algal species, while different small letters on the top indicate a significant difference in each species (P < 0.05) at different levels of N:P ratio. Error bars represent the standard deviations. White and grey bars represent T. lutea and N. oculata, respectively
Carbon, nitrogen and phosphorus in T. lutea and N. oculata cells
The carbon content in T. lutea was significantly higher than in N. oculata in all N:P ratio treatments (P = 0.001, Fig. 3). The impact of N:P ratios on the cellular C content depended on algae species (P = 0.001). The highest C content was observed at the N:P ratios of 5:1 and 120:1 for both T. lutea and N. oculata (P < 0.05, Fig. 3). The lowest C content (19.65 pg cell−1) in N. oculata was found in the N:P ratio of 10:1 (P = 0.001), but C contents in T. lutea and N. oculata were not significantly different as the N:P ratio increased from 10:1 to 60:1 (P = 0.436). The carbon contents in both species depended on the N:P ratio (P < 0.05). Cellular N contents were not different between species in all N:P ratios (P > 0.05, Fig. 3), but as the N:P levels increased from 5:1 to 120:1, the cellular N increased in both T. lutea and N. oculata (P < 0.05). Similarly, cellular P contents were not different between species in all N:P ratios (P > 0.05, Fig. 3), but a significant declining trend of P contents was observed with the increase of N:P ratio in both T. lutea and N. oculata with highest at N:P = 5:1 and lowest at N:P = 120:1 (P < 0.05, Fig. 3).
Changes of cellular C, N, and P in Tisochrysis lutea and Nannochloropsis oculata under different N:P ratios. Different capital letters inside each bar type represent significant N:P ratio effects (P < 0.05) between the algal species, while different small letters on the top indicate significant species effects (P < 0.05) at different levels of N:P ratio. White and grey bars represent T. lutea and N. oculata, respectively
Protein content
The protein content increased with the increase of N:P ratio from 5:1 to 30:1 in both T. lutea and N. oculata (P < 0.05, Fig. 4). The protein content in both species depended on N:P ratio (P = 0.024). Both species yielded lower protein content when cultured under high (120:1) and low (5:1) N:P ratios (P < 0.05). The protein contents in T. lutea and N. oculata were higher at the N:P ratios of 20:1 and 30:1 than at other N:P ratios (P < 0.05), but there was no difference between these two ratios (P > 0.05). The protein content in N. oculata was 60.67 % when grown at the N:P ratio of 20:1, but it was reduced when the ratio of N:P increased to 120:1 (P < 0.05, Fig. 4).
Protein and lipid content (% dry matter) in Tisochrysis lutea and Nannochloropsis oculata corresponding to different N:P ratios. Different capital letters inside the same bar type represent significant N:P ratio effects (P < 0.05) between the algal species, while different small letters on top indicate significant difference in each species (P < 0.05) at different levels of N:P ratio. White and grey bars represent T. lutea and N. oculata, respectively
Lipid content
The impact of the N:P ratio on cellular lipid depended on algal species (P = 0.001). The lipid content in T. lutea and N. oculata gradually increased with the increase of N:P ratio from 5:1 to 120:1 (P = 0.001, Fig. 4) and was not significantly different from the N:P ratio of 30:1 to 60:1 (P > 0.05) . However, the lipid content was significantly higher in the N:P ratio of 120:1 compared to that at lower N:P ratios. N:P ratios significantly affected the lipid content in both algal species (P = 0.001).
Fatty acid compositions in T. lutea and N. oculata under different N:P ratios are shown in Tables 1 and 2. The fatty acid contents were significantly affected by the N:P ratio supplied to the culture (P < 0.05). The highest level of fatty acids in T. lutea was the saturated fatty acids, and the content was significantly higher in the treatment of the highest N:P ratio (43.38 %, N:P 120:1, Table 1). The amounts of myristic acid (C14:0) in T. lutea were higher in the N:P ratio of 120:1 (28.53 ± 0.32 %) than those at lower N:P ratios (P < 0.05). The increase of N:P ratios from 5:1 (11.27 %) to 120:1 (23.93 %) significantly augmented the C18:1 monosaturated fatty acids in T. lutea (P < 0.05, Table 1).
The contents of polyunsaturated fatty acids (PUFA) were significantly higher in the N:P ratio of 20:1 (37.94 %) than at other higher N:P ratios in T. lutea (P < 0.05). The highest alpha-linolenic acid (ALA) content in T. lutea was found in the N:P ratios of 20:1 and 30:1 (7.53 ± 0.15 % and 6.44 ± 0.10 %, respectively, P < 0.05, Table 1). The amount of DHA in T. lutea decreased (6.73 ± 0.21 %) as N:P ratios increased from 30:1 to 120:1 (P < 0.05, Table 1). The content of C18:4 polyunsaturated fatty acids was higher in the N:P ratio of 20:1 compared to other N:P ratios (P < 0.05).
Saturated fatty acids were highest among other fatty acid groups in N. oculata, and its content was higher in algae at the N:P ratio of 120:1 than at 5:1 (P < 0.05, Table 2). The C16:0 saturated fatty acid was higher at the N:P ratio of 120:1 (38.29 ± 0.19) than that at other N:P ratios (P < 0.05, Table 2). The highest amount of monosaturated fatty acids was C16:1 under the N:P ratio of 120:1 (30.28 ± 0.12 %, P < 0.05). The PUFA content significantly increased when the N:P ratio increased from 5:1 to 20:1 and decreased as the N:P ratio increased to 120:1 (P < 0.05). Linoleic acid (C18:2) in N. oculata was higher (3.41 ± 0.09) at the N:P ratio of 20:1 than at other N:P ratios. The amount of EPA (38.67 ± 0.06 %) in N. oculata was significantly higher at the N:P ratio of 20:1 than at the 120:1 ratio (10.27 ± 0.06 %; P < 0.05). The amount of EPA in N. oculata was regulated by the N:P ratio in the culture media (P < 0.05). The ARA in N. oculata was significantly higher at the N:P ratio of 20:1 than at other ratios (P < 0.05).
The interactive effects of algae species and N:P ratios were detected on major species of polyunsaturated fatty acids (P < 0.05). The EPA content was not affected by N:P ratios in T. lutea, but it was highest at the N:P ratio of 20:1 and lowest at the N:P ratio of 120:1 (P < 0.05). The DHA content was significantly higher in T. lutea compared to N. oculata regardless of N:P ratios (P < 0.05). The ALA content was not affected by N:P ratios in N. oculata, but it was highest at the 20:1 or 30:1 N:P ratio and lowest at the 60:1 or 120:1 N:P ratio (P < 0.05) in T. lutea. In contrast, the ARA content was not affected by N:P ratios in T. lutea, but it was highest at the N:P ratio of 20:1 and lowest at 60:1 or 120:1 N:P ratio in N. oculata (P < 0.05).
Discussion
In this study, algal growth was enhanced when the N:P ratio increased from 5:1 to 20:1, and then there was a gradual decline from 20:1 to 120:1 N:P ratios. This finding is similar to a previous study by Vanucci et al. (2012) where final cell yields of the dinoflagellate Ostreopsis ovata was increased from a low N:P ratio of 5:1 to a high N:P ratio of 16:1, which is consistent with another study using similar N:P ratios on the same dinoflagellate O. ovata (Vidyarathna and Granéli 2013). Similarly, Mayers et al. (2014) have found that the maximum cell density was obtained when the Nannochloropsis sp. was cultured under the N:P ratios of 16:1 and 32:1 compared to 64:1 and 80:1. Our results demonstrated that the N:P ratio of 20:1 increased the final cell densities in T. lutea and N. oculata compared to those at the N:P ratio of 5:1. Furthermore, the current results showed that within each species, algal growth was affected by the range of N:P ratios. Geider and La Roche (2002) stated that phytoplankton is N-limited at N:P <16 and that it is P-limited at N:P >16 based on the Redfield ratio (Redfield, 1934). However, in the present study, the highest growth rate was obtained for both algae species under the N:P ratio of 20:1 compared to the rest of the N:P ratios. This finding is consistent with a previous study that the N:P ratio of 20:1 favoured the growth of Thalassiosira sp. and Chroomonas salina (Zhang and Hu 2011). In contrast, another study on other algal species such as Cylindrotheca closterium and Platymonas helgolandica var. tsingtaoensis (Sun et al. 2004) showed a different pattern, where the algal growth rate is higher at the N:P ratios of 160:1 and 80:1 than at 16:1, and this suggests that algal preference for optimal N:P ratios varies among species as reported in previous studies (Clark et al. 2002; Flynn et al. 2002). The response of algal growth to N:P ratios depends on the physiological requirement for nutrients and varies among species (Lagus et al. 2004). Our results demonstrate that the higher growth rates of T. lutea and N. oculata occurred close to the N:P ratio of 20:1, which is slightly higher than the Redfield ratio of N:P = 16:1.
The increase of N:P ratio from 5:1 to 120:1 increased the cellular N content of T. lutea and N. oculata in the current study. Similarly, in a previous study, the increase of N:P ratio from 22:1 to 3000:1 also increased the cellular N content in Calcidiscus leptoporus (Langer et al. 2012). In the treatments of N:P ratios at 20:1 and 30:1 where phosphorus was limited, the excess N may be easily incorporated into protein as shown in Scenedesmus sp. when N was in excessive supply relative to P in the medium (Rhee 1978). Our results demonstrated that the increase of the N:P ratios from 5:1 to 120:1 (ascending N supply) increased the cellular N contents, but phosphorus limitation might lead to the accumulation of excess nitrogen stored as protein in the cells of T. lutea and N. oculata. The increase of N:P ratio from 20:1 to 120:1 leads to P limitation and a tendency of PUFA reduction in both algal species, which is consistent with the report on Dunaliella parva where the N:P ratio of 118:1 decreased the cellular P content compared with the N:P ratios of 30:1 and 6:1, though the PUFA contents were not reported by Suzuki et al. (1997).
Cellular C contents of algae at the N:P ratios of 5:1 and 120:1 were high in T. lutea and N. oculata in the present study, in line with a previous result on C. leptoporus where lower N:P ratios of 0.17:1 and higher N:P ratios of 3000:1 increased C content compared with the N:P ratio of 22:1 (Langer et al. 2012). Similarly, N:P ratios of 0.57:1 and 115:1 also increased the carbon content in T. lutea (Marchetti et al. 2012). Under the optimum range of N:P ratios (20:1–30:1), carbon contents were lower than the high (120:1) and low N:P ratios (5:1) in T. lutea and N. oculata. Langer et al. (2012) also found that in C. leptoporus, the N:P ratio of 22:1 resulted in low carbon contents compared to the N:P ratios of 0.17:1 and 3000:1. At a high or low N:P ratio, algae can produce excess C that may be stored internally as starch and lipids for metabolism and reproduction (Sterner and Hessen 1994; Claquin et al. 2002). This suggests that carbohydrates may be the preferential storage at low N:P ratios, while lipids might be the preferential storage at high N:P ratios. However, in this study, we did not measure the conversion of cellular C to carbon compounds and further investigation is needed to identify whether a high C in the low N:P ratio can be converted to lipids or starch for physiological functions in algae.
In this study, protein contents in T. lutea and N. oculata were affected by N:P ratios. The protein contents of algae at the N:P ratios of 20:1 to 30:1 were higher than other N:P ratios. In another study, the high protein yield in Prorocentrum donghaiense also happened to fall into the range of the N:P ratios of 8:1 to 64:1 (Lai et al. 2011). Besides, Berdalet et al. (1994) and Kilham et al. (1997) reported that algae at high N:P ratios of 200:1 to 486:1 resulted in a lower protein concentration than at a medium N:P ratio of 20:1 to 24:1 due to phosphorus deficiency, which coincides with the low protein content at the high N:P ratio in this study. The amount of N in the N:P ratios from 20:1 to 30:1 was 287 μM which coincides with high protein contents in both algae species. This finding is consistent with a previous study that the protein level is high in Microcystis aeruginosa at medium N:P ratios from 18:1 to 50:1, and the reason is that these N:P ratios with high ambient N promote protein synthesis (Downing et al. 2005). Similarly, the N content was higher in the N:P ratios of 20:1 to 30:1 (287 μM) than that in 5:1 (72 μM) and 10:1 (144 μM) in both species, suggesting that a relatively low N:P ratio with absolutely high N concentration favours a high constituent of protein in algae (Flores and Herrero 2004). Thus, a lack of N would result in a decrease in the rate of protein synthesis (Berdalet et al. 1994), as shown in the low protein content under the N:P ratios of 5:1 and 10:1 in the current study. Protein is the major macromolecular pool of intracellular N, and thus N availability in the medium can influence protein synthesis (Zhao et al. 2009). Hence, the N:P ratios from 20:1 to 30:1 are recommended to increase the protein content of algae as long as the N concentration in the medium is sufficient for protein synthesis.
Nitrogen and phosphorus are essential elements for algal metabolism, since their deficiency can affect biochemical synthesis in algae (Kilham et al. 1997). A high N:P ratio above 22:1 indicates a P-limiting condition (Healey and Hendzel 1979; Nalewajko et al. 1981). For T. lutea and N. oculata, at the N:P ratio of 120:1, the lipid accumulation was higher than at low N:P ratios, indicating that T. lutea and N. oculata can accumulate lipids at a high N:P ratio. In accordance with our results, Chlorella sp. can accumulate a high content of lipids under high N:P ratios of 55:1 and 110:1 (P limitations) compared to the lower N:P ratios of 11:1 and 7:1 (N limitations) (Liang et al. 2013). A few algal species, including Chlorella spp. (Reitan et al. 1994; Liang et al. 2013), Dunaliella spp. (Gordillo et al. 1998; Takagi et al. 2006), Parietochloris incisa (Bigogno et al. 2002), Neochloris oleoabundans (Tornabene et al. 1983) and Botryococcus braunii (Li and Qin 2005), have also been reported to have the capacity of accumulating large quantities of lipids in cells at high N:P ratios. A high N:P ratio usually indicates low P availability and may cause P deficiency for algal growth and alter their lipid biosynthetic pathways towards the formation and accumulation of lipids (Hu et al. 2008). When there is a lack of P source in the medium, cell division will be reduced and C source is absorbed continuously by algal cells, leading to high lipid synthesis in the Krebs cycle (Ratledge and Wynn 2002), suggesting that the N:P ratio of 120:1 in this study has a lower P content and thus may have increased lipid synthesis by stimulating the lipid metabolic pathways. Unfortunately, we did not measure the mechanism of lipid metabolic pathways in algae under a low P concentration. Further research is required to examine the lipid metabolic pathways at high N:P ratios to gain more understanding on the process of lipid synthesis at low P concentrations. The N:P ratios can be manipulated based on the need of cultivation. For instance, if the aim of cultivation is for lipid production, then higher N:P ratios should be used and vice versa (Mayers et al. 2014).
In this study, the impact of N:P ratio on the contents of ARA, ALA, EPA and DHA depended on algal species. To our best knowledge, this is the first study reporting the impact of N:P ratios on biochemical properties between algae species that possess different EPA and DHA profiles. The increase of N:P ratio from 20:1 to 120:1 decreased the amount of EPA and DHA in N. oculata and T. lutea, which is consistent with a previous study where a higher N:P ratio of 555:1 (P limitation) decreased the amounts of EPA and DHA in the diatoms Thalassiosira pseudonana and Chaetoceros calcitrans compared to a lower N:P ratio of 14:1 (Harrison et al. 1990). Similarly, the EPA and ARA contents in Phaeodactylum tricornutum and Porphyridium cruentum were higher at the 20:1 N:P ratio than at the 7:1 N:P ratio, while DHA in T. lutea and ALA in Chlorella vulgaris and Dunaliella tertiolecta were increased at the N:P ratio of 20:1 compared with at the N:P ratio of 7:1 (Breuer et al. 2012). In comparison, we found that cellular DHA and ALA in T. lutea and cellular ARA and EPA in N. oculata were low at low N: P ratios (5:1–10:1) and high at N:P ratios of 20:1 to 30:1. Under an unfavourable condition such as low N and low P that inhibits biochemical synthesis, algae are unable to increase the PUFA content by de novo synthesis, but algae in a medium with sufficient nitrogen and phosphorus can more efficiently produce energy-rich PUFA (Solovchenko et al. 2008).
In conclusion, the N:P ratio significantly affected the growth, cellular elements and biochemical composition in both T. lutea and N. oculata. N:P ratios of 20:1 and 30:1 favoured algal growth and protein content, while the high N:P ratio (120:1) reduced algal growth and protein content but increased lipid in both algae. The N:P ratio of 20:1 increased the EPA content in N. oculata while the N:P ratio of 30:1 increased the DHA content in T. lutea. This study indicates that N:P ratio manipulation is an effective strategy to change the nutritional content in algae. Further research is needed to understand the physiological mechanisms of the biochemical changes in T. lutea and N. oculata under different N:P ratios and to enhance the production of valuable biochemical compounds such as lipid and PUFA in algae.
References
Ahlgren G, Hyenstrand P (2003) Nitrogen limitation effects of different nitrogen sources on nutritional quality of two freshwater organisms, Scenedesmus quadricauda (Chlorophyceae) and Synechococcus sp. (Cyanophyceae). J Phycol 39:906–917
Anderson TR, Pond DW (2000) Stoichiometric theory extended to micronutrients: comparison of the roles of essential fatty acids, carbon, and nitrogen in the nutrition of marine copepods. Limnol Oceanogr 45:1162–1167
Barbarino E, Lourenço S (2005) An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J Appl Phycol 17:447–460
Beardall J, Berman T, Heraud P, Omo Kadiri M, Light BR, Patterson G, Roberts S, Sulzberger B, Sahan E, Uehlinger U, Wood B (2001a) A comparison of methods for detection of phosphate limitation in microalgae. Aquat Sci 63:107–121
Beardall J, Young E, Roberts S (2001b) Approaches for determining phytoplankton nutrient limitation. Aquat Sci 63:44–69
Berdalet E, Latasa M, Estrada M (1994) Effects of nitrogen and phosphorus starvation on nucleic acid and protein content of Heterocapsa sp. J Plankton Res 16:303–316
Bendif EM, Probert I, Schroeder DC, de Vargas C (2013) On the description of Tisochrysis lutea gen. nov. sp. nov. and Isochrysis nuda sp. nov. in the Isochrysidales, and the transfer of Dicrateria to the Prymnesiales (Haptophyta). J Appl Phycol 25:1763–1776
Bigogno C, Khozin-Goldberg I, Boussiba S, Vonshak A, Cohen Z (2002) Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 60:497–503
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borowitzka MA (1997) Microalgae for aquaculture: opportunities and constraints. J Appl Phycol 9:393–401
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Breteler WCMK, Schogt N, Rampen S (2005) Effect of diatom nutrient limitation on copepod development: role of essential lipids. Mar Ecol Prog Ser 291:125–133
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2012) The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresource Technol 124:217–226
Brown MR, Hohmann S (2002) Effects of irradiance and growth phase on the ascorbic acid content of Isochrysis sp. T.ISO (Prymnesiophyta). J Appl Phycol 14:211–214
Cembella AD, Antia NJ, Harrison PJ (1984) The utilization of inorganic and organic phosphorus compounds as nutrients by eukaryotic microalgae—a multidisciplinary perspective. Crit Rev Microbiol 11:13–81
Claquin P, Martin-Jézéquel V, Kromkamp JC, Veldhuis MJW, Kraay GW (2002) Uncoupling of silicon compared with carbon and nitrogen metabolisms and the role of the cell cycle in continuous cultures of Thalassiosira pseudonana (Bacillariophyceae) under light, nitrogen, and phosphorus control. J Phycol 38:922–930
Clark DR, Flynn KJ, Owens NJP (2002) The large capacity for dark nitrate-assimilation in diatoms may overcome nitrate limitation of growth. New Phytol 155:101–108
Coutteau P, Castell JD, Ackman RG, Sorgeloos P (1996) The use of lipid emulsions as carriers for essential fatty acids in bivalves: a test case with juvenile Placopecten magellanicus. J Shellfish Res 15:259–264
Douillet PA, Langdon CJ (1994) Use of a probiotic for the culture of larvae of the pacific oyster (Crassostrea gigas). Aquaculture 119:25–40
Downing TG, Sember CS, Gehringer MM, Leukes W (2005) Medium N:P ratios and specific growth rate comodulate microcystin and protein content in Microcystis aeruginosa PCC7806 and M. aeruginosa UV027. Microb Ecol 49:468–473
Duerr EO, Molnar A, Sato V (1998) Cultured microalgae as aquaculture feeds. J Mar Biotechnol 6:65–70
Ferrão-Filho AS, Fileto C, Lopes NP, Arcifa MS (2003) Effects of essential fatty acids and N and P-limited algae on the growth rate of tropical cladocerans. Freshwater Biol 48:759–767
Fidalgo JP, Cid A, Torres E, Sukenik A, Herrero C (1998) Effects of nitrogen source and growth phase on proximate biochemical composition, lipid classes and fatty acid profile of the marine microalga Isochrysis galbana. Aquaculture 166:105–116
Flores E, Herrero A (2004) Assimilatory nitrogen metabolism and its regulation. In: Bryant D (ed) The Molecular Biology of Cyanobacteria, vol 1, Advances in Photosynthesis and Respiration. Springer, Dordrecht, pp 487–517
Flynn T, Ghirardi ML, Seibert M (2002) Accumulation of O2-tolerant phenotypes in H2-producing strains of Chlamydomonas reinhardtii by sequential applications of chemical mutagenesis and selection. Int J Hydrogen Ener 27:1421–1430
Fong P, Fong JJ, Fong CR (2004) Growth, nutrient storage, and release of dissolved organic nitrogen by Enteromorpha intestinalis in response to pulses of nitrogen and phosphorus. Aquat Bot 78:83–95
Fresnedo O, Serra JL (1992) Effect of nitrogen starvation on the biochemistry of Phormidium laminosum (Cyanophyceae). J Phycol 28:786–793
Ganf GG, Stone SJL, Oliver RL (1986) Use of protein to carbohydrate ratios to analyze for nutrient deficiency in phytoplankton. Aust J Mar Fresh Res 37:183–197
Geider R, La Roche J (2002) Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. Eur J Phycol 37:1–17
Gordillo FJL, Goutx M, Figueroa FL, Niell FX (1998) Effects of light intensity, CO2 and nitrogen supply on lipid class composition of Dunaliella viridis. J Appl Phycol 10:135–144
Harrison PJ, Thompson PA, Calderwood GS (1990) Effects of nutrient and light limitation on the biochemical composition of phytoplankton. J Appl Phycol 2:45–56
Healey FP, Hendzel LL (1979) Indicators of phosphorus and nitrogen deficiency in 5 algae in culture. J Fish Res Board Can 36:1364–1369
Hessen DO, Færøvig PJ, Andersen T (2002) Light, nutrients, and P:C ratios in algae: grazer performance related to food quality and quantity. Ecology 83:1886–1898
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacyglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Johansson N, Graneli E (1999) Cell density, chemical composition and toxicity of Chrysochromulina polylepis (Haptophyta) in relation to different N : P supply ratios. Mar Biol 135:209–217
Jonasdottir SH, Visser A, Jespersen C (2009) Assessing the role of food quality in the production and hatching of Temora longicornis eggs. Mar Ecol Prog Ser 382:139–150
Kilham S, Kreeger D, Goulden C, Lynn S (1997) Effects of nutrient limitation on biochemical constituents of Ankistrodesmus falcatus. Freshwater Biol 38:591–596
Knauer J, Southgate PC (1997) Growth and fatty acid composition of Pacific oyster (Crassostrea gigas) spat fed a microalga and microcapsules containing varying amounts of eicosapentaenoic and docosahexaenoic acid. J Shellfish Res 16:447–453
Kunikane S, Kaneko M, Maehara R (1984) Growth and nutrient uptake of green alga, Scenedesmus dimorphus, under a wide range of nitrogen/phosphorus ratio—I. Experimental study. Water Res 18:1299–1311
Lai J, Yu Z, Song X, Cao X, Han X (2011) Responses of the growth and biochemical composition of Prorocentrum donghaiense to different nitrogen and phosphorus concentrations. J Exp Mar Biol Ecol 405:6–17
Lagus A, Suomela J, Weithoff G, Heikkilä K, Helminen H, Sipura J (2004) Species-specific differences in phytoplankton responses to N and P enrichments and the N: P ratio in the Archipelago Sea, northern Baltic Sea. J Plankton Res 26:779–798
Langer G, Oetjen K, Brenneis T (2012) Calcification of Calcidiscus leptoporus under nitrogen and phosphorus limitation. J Exp Mar Biol Ecol 413:131–137
Li Y, Qin JG (2005) Comparison of growth and lipid content in three Botryococcus braunii strains. J Appl Phycol 17:551–556
Liang K, Zhang Q, Gu M, Cong W (2013) Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. J Appl Phycol 25:311–318
C-Pa L, Lin L-P (2001) Ultrastructural study and lipid formation of Isochrysis sp. Bot Bull Acad Sinica 42:207–214
Liu Y, Song X, Cao X, Yu Z (2013) Responses of photosynthetic characters of Skeletonema costatum to different nutrient conditions. J Plankton Res 35:165–176
Mayers JJ, Flynn KJ, Shields RJ (2014) Influence of the N:P supply ratio on biomass productivity and time-resolved changes in elemental and bulk biochemical composition of Nannochloropsis sp. Bioresource Technol 169:588–595
Mansour M, Frampton DF, Nichols P, Volkman J, Blackburn S (2005) Lipid and fatty acid yield of nine stationary-phase microalgae: applications and unusual C24–C28 polyunsaturated fatty acids. J Appl Phycol 17:287–300
Marchetti J, Bougaran G, Le Dean L, Mégrier C, Lukomska E, Kaas R, Olivo E, Baron R, Robert R, Cadoret JP (2012) Optimizing conditions for the continuous culture of Isochrysis affinis galbana relevant to commercial hatcheries. Aquaculture 326–329:106–115
Martínez-Fernández E, Acosta-Salmón H, Southgate PC (2006) The nutritional value of seven species of tropical microalgae for black-lip pearl oyster (Pinctada margaritifera, L.) larvae. Aquaculture 257:491–503
McCausland MA, Brown MR, Barrett SM, Diemar JA, Heasman MP (1999) Evaluation of live microalgae and microalgal pastes as supplementary food for juvenile Pacific oysters (Crassostrea gigas). Aquaculture 174:323–342
Mueller-Navarra DC, Brett MT, Liston AM, Goldman CR (2000) A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 403:74–77
Nalewajko C, Lee K, Shear H (1981) Phosphorus kinetics in lake superior—light intensity and phosphate uptake in algae. Can J Fish Aquat Sci 38:224–232
Nell JA (2002) Farming triploid oysters. Aquaculture 210:69–88
Patil V, Källqvist T, Olsen E, Vogt G, Gislerød H (2007) Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquacult Int 15:1–9
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Qin JG, Culver DA (1996) Effect of larval fish and nutrient enrichment on plankton dynamics in experimental ponds. Hydrobiologia 321:109–118
Qin S, Lin HZ, Jiang P (2012) Advances in genetic engineering of marine algae. Biotechnol Adv 30:1602–1613
Ratledge C, Wynn JP (2002) The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol 51:1–51
Redfield AC (1934) On the proportions of organic derivatives in sea water and their relation to the composition of plankton. In: Daniel RJ (ed) James Johnstone Memorial Volume. Liverpool University Press, Liverpool, pp 176–192
Reitan KI, Rainuzzo JR, Olsen Y (1994) Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J Phycol 30:972–979
Rhee GY (1978) Effects of N:P atomic ratios and nitrate limitation on algal growth, cell composition, and nitrate uptake. Limnol Oceanogr 23:10–25
Robert R, Parisi G, Rodolfi L, Poli BM, Tredici MR (2001) Use of fresh and preserved Tetraselmis suecica for feeding Crassostrea gigas larvae. Aquaculture 192:333–346
Robert R, Trintignac P (1997) Substitutes for live microalgae in mariculture: a review. Aquat Liv Res 10:315–327
Rodolfi GC, Bassi ZN, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Rosenberg JN, Oyler GA, Wilkinson L, Betenbaugh MJ (2008) A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr Opin Biotechnol 19:430–436
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5:1532–1553
Shifrin NS, Chisholm SW (1981) Phytoplankton lipids—interspecific differences and effects of nitrate, silicate and light-dark cycles. J Phycol 17:374–384
Solovchenko AE, Khozin-Goldberg I, Didi-Cohen S, Cohen Z, Merzlyak MN (2008) Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J Appl Phycol 20:245–251
Sterner RW, Hessen DO (1994) Algal nutrient limitation and the nutrition of aquatic herbivores. Ann Rev Ecol System 25:1–29
Sun J, Liu D, Chan Z, Wei T (2004) Growth of Platymonas helgolandica var. tsingtaoensis, Cylindrotheca closterium and Karenia mikimotoi and their survival strategies under different N/P ratios. J Appl Ecol 15:2122–2126
Suzuki T, Imai M, Ishida M (1997) Influence of environmental factors on phosphorous content in the marine microalga Dunaliella. Environ Toxicol Chem 64:173–181
Takagi M, Karseno YT (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101:223–226
Theodorou ME, Elrifi IR, Turpin DH, Plaxton WC (1991) Effects of phosphorus limitation on respiratory metabolism in the green alga Selenastrum minutum. Plant Physiol 95:1089–1095
Tornabene TG, Holzer G, Lien S, Burris N (1983) Lipid-composition of the nitrogen starved green alga Neochloris oleoabundans. Enzyme Microb Tech 5:435–440
Vanucci S, Pezzolesi L, Pistocchi R, Ciminiello P, Dell’Aversano C, Iacovo ED, Fattorusso E, Tartaglione L, Guerrini F (2012) Nitrogen and phosphorus limitation effects on cell growth, biovolume, and toxin production in Ostreopsis cf. ovata. Harmful Algae 15:78–90
Vidyarathna NK, Granéli E (2013) Physiological responses of Ostreopsis ovata to changes in N and P availability and temperature increase. Harmful Algae 21–22:54–63
Villar-Argaiz M, Sterner RW (2002) Life history bottlenecks in Diaptomus clavipes induced by phosphorus-limited algae. Limnol Oceanog 47:1229–1233
Wheal MS, Fowles TO, Palmer LT (2011) A cost-effective acid digestion method using closed polypropylene tubes for inductively coupled plasma optical emission spectrometry (ICP-OES) analysis of plant essential elements. Anal Methods-UK 3:2854–2863
Zhang Q, Hu G (2011) Effect of nitrogen to phosphorus ratios on cell proliferation in marine micro algae. Chin J Oceanol Limn 29:739–745
Zhao XW, Tang XX, Wang Y (2009) Interactions between two species of marine bloom microalgae under controlled laboratory conditions: Heterosigma akashiwo and Karenia mikimotoi. Chinese J Plant Ecol 33:958–965
Acknowledgments
We wish to thank Leslie Morrison, Dominic Reppucci, Julie Voyer, Ralphael Livingstone and Brett Norsworthy for their kind advice and facilities support. This study was supported by a PhD scholarship to Nadiah Rasdi by the Malaysian Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rasdi, N.W., Qin, J.G. Effect of N:P ratio on growth and chemical composition of Nannochloropsis oculata and Tisochrysis lutea . J Appl Phycol 27, 2221–2230 (2015). https://doi.org/10.1007/s10811-014-0495-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0495-z