Abstract
The influence of specific autism spectrum disorder (ASD) deficits in Intelligence Quotients (IQ), Indexes and subtests from the Wechsler Intelligence Scale for Children-III was investigated in 445 school-aged children: ASD (N = 224) and other neurodevelopmental disorders (N = 221), matched by Full-Scale IQ and chronological age. ASD have lower scores in the VIQ than PIQ. The core distinctive scores between groups are Processing Speed Index and “Comprehension” and “Coding” subtests with lower results in ASD. ASD group with normal/high IQ showed highest score on “Similarities” subtest whereas the lower IQ group performed better on “Object Assembly”. The results replicated our previous work on adaptive behaviour, showing that adaptive functioning is positively correlated with intellectual profile, especially with the Communication domain in ASD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is an early-onset, life-long severe neurodevelopmental disorder with a high worldwide prevalence and a distribution of four males (M) to one female (F) (Centers for Disease Control and Prevention 2009; Oliveira et al. 2007; Fombonne 2003). Deficits in social interaction and communication, as well as repetitive patterns of behaviour and interests, are the core characteristics of ASD (American Psychiatric Association 2013).
Although comorbidity with intellectual disability (ID) is decreasing, as it is shown in recent studies, it remains very common, being that about one third to half of ASD subjects have co-occurring ID (Centers for Disease Control and Prevention 2009, 2012, 2014). However, ID as measured by Intelligence Quotient (IQ) scores can vary, depending on the test used. Many children with ASD are described as having low intelligence quotients which is partially due to the use of various editions of the Wechsler Intelligence Scale for Children (WISC) (Wechsler 1949). WISC is undoubtedly the most widely used test to estimate intelligence among ASD subjects (Joseph 2011; Mottron 2004; Goldstein et al. 2008b). An important issue is whether the tasks included in the Wechsler scales are sensitive to unique characteristics of children with ASD, which might affect test performance (Carothers and Taylor 2013) and differ in gender (Ryland et al. 2014; Rivet and Matson 2011). Even though non-verbal children garner low scores on verbal IQ tests, they can, at times, obtain scores appropriate to their age level on tests of spatial intelligence, for example. The Wechsler Intelligence Scale for Children—third edition (WISC-III) (Wechsler 1991) overcomes this difficulty by separately scoring both verbal and non-verbal, performance, IQ, which can then be further broken down into more discrete categories such as Indexes.
WISC-III (Wechsler 1991, 2003b), the most recently normed measure for our country’s population, is an individually administered intelligence test intended for children that can be completed without reading or writing and was designed to measure human intelligence as reflected in both verbal and nonverbal (performance) abilities. WISC-III (Wechsler 1991, 2003b) include questions of general knowledge, traditional arithmetic problems, vocabulary, completion of mazes, and arrangement of blocks and pictures (for a detailed description, see “Methods” section).
There is a host of research on intellectual functioning among patients with ASD. Although IQ measures are not used as diagnostic tool for ASD, one primary use of it in this population is the differentiation between high- and low-functioning individuals. The knowledge of intellectual profiles allows technicians to assist parents of children with neurodevelopmental disorders in making decisions and academic curricula adaptations to further stages of education (Oliveras-Rentas et al. 2012), as well as to predict future achievements of their offspring. The monitoring of progresses in the therapeutic process (Koegel et al. 1997) and the acquisition of additional information required for the purposes of differential diagnosis and outcome prediction are also motivations to continue to use Wechsler scales (Koyama et al. 2006; Mayes and Calhoun 2008).
Most of the studies focusing on the intellectual functioning of individuals with ASD, in which Wechsler scales were used, concluded that when examining subtest performance at the group level, subjects with ASD obtain the lowest scores in “Comprehension” (Siegel et al. 1996; Freeman et al. 1985; Asarnow et al. 1987; Narita and Koga 1987; Ohta 1987; Rumsey and Hamburger 1988; Lincoln et al. 1988; Allen et al. 1991; Venter et al. 1992; Happe 1994; Bailey et al. 1996; Dennis et al. 1999; Koyama et al. 2007; Mayes and Calhoun 2003), and the highest in “Digit span” (Allen et al. 1991; Lincoln et al. 1988; Narita and Koga 1987; Ohta 1987; Rumsey and Hamburger 1988; Siegel et al. 1996; Bailey et al. 1996; Szatmari et al. 1990; Dennis et al. 1999) among verbal scales. On the other hand, in the performance scales, the lowest scores are obtained in “Picture arrangement”(Allen et al. 1991; Lincoln et al. 1988; Ohta 1987; Rumsey and Hamburger 1988; Venter et al. 1992; Szatmari et al. 1990; Shah and Frith 1993) and “Coding”(Asarnow et al. 1987; Freeman et al. 1985) and the highest scores in “Block design” (Allen et al. 1991; Asarnow et al. 1987; Freeman et al. 1985; Happe 1994; Lincoln et al. 1988; Rumsey and Hamburger 1988; Siegel et al. 1996; Venter et al. 1992; Bailey et al. 1996; Shah and Frith 1993; Szatmari et al. 1990; Lockyer and Rutter 1970; Bowler 1992; Dennis et al. 1999; Koyama et al. 2007; Mayes and Calhoun 2003).
Despite the differences in individual studies, all of them point to two common elements in intellectual profile of autistic population: “Block design” is the subtest with highest results and “Comprehension” the one with the lowest. In contrast, there is no conclusive data regarding the relationship between verbal and non-verbal intelligence quotients. A number of studies also reported that individuals with ASD are characterized by higher scores on PIQ, rather than on VIQ (Asarnow et al. 1987; Freeman et al. 1985; Narita and Koga 1987; Ohta 1987; Siegel et al. 1996; Allen et al. 1991; Lincoln et al. 1988; Venter et al. 1992; Schneider and Asarnow 1987). Contrarily, others have documented higher scores on verbal scales than on PIQ (Minshew et al. 1992; Szatmari et al. 1990). More recent studies using WISC-III (Wechsler 1991) found no differences between the level of verbal and non-verbal intelligence (Ghaziuddin and Mountain-Kimchi 2004; Goldstein et al. 2008a).
A specific Wechsler profile, commonly reported among school age children with ASD when taking into consideration the factor analysis, includes higher scores on Verbal Comprehension Index (VCI) and on the Perceptual Organization Index (POI), when compared to the Freedom from Distractibility Index (FDI) and the Processing Speed Index (PSI) (Mayes and Calhoun 2003, 2008; Nyden et al. 2001; Wechsler 2003a). Mayes and Calhoun (2004) were able to identify children with high-functioning autism, with 73 % accuracy, that had obtained lower results in FDI and PSI indexes and Comprehension subtest scores on the WISC-III. This profile has been consistently found across various age groups and functioning levels, but it is not used as a diagnostic tool (Siegel et al. 1996).
The pursuit of a result that can be a tool with diagnostic utility for autism versus other neurodevelopmental problems led to more empirically oriented classification systems for WISC-III results, such as Bannatyne’s categories (Bannatyne 1974) and Kaufman’s factors (Kaufman 1975, 1994). Bannatyne (1974) proposed four categories that were composed of a group of subtests: Spatial Ability (includes Picture Completion, Block Design, Object Assembly), Verbal Conceptualisation Ability (Comprehension, Similarities, Vocabulary), Sequencing Ability (Digit Span, Arithmetic, Coding), and Acquired Knowledge (Information, Arithmetic, Vocabulary). These had a more interpretative meaning of the subjects’ capabilities than the Verbal and Performance Scales. Kaufman (1975, 1994) analysed the WISC-R standardisation data and proposed various factors, namely: Arithmetic, Coding, Information and Digit Span subtests—ACID; Symbol Search, Coding, Arithmetic and Digit Span subtests—SCAD; and Freedom from Distractibility Index (Arithmetic, Digit Span subtests)—FDI. Ottem (1999) argued that the Bannatyne’s categories and Kaufman’s factors did not explained the differences in the profiles of two populations: ASD and reading impaired subjects. In fact, more studies with different approaches to Wechsler scales are needed, as well as intellectual profile comparisons with groups with other neurodevelopmental disorders.
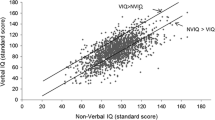
Besides global intellectual level, specific cognitive deficits are linked to ASD, so it should be expected that children with ASD would show weaknesses in some subtests of the Wechsler scales and different patterns in Verbal IQ (VIQ) or Performance IQ (PIQ) (Baron-Cohen 2001; F. Happe and Frith 2006; Pennington and Ozonoff 1996; Pisula 2010). For instance, discrepancies between verbal and nonverbal IQ are frequently found in ASD children (Kaufman and Lichtenberger 2000) and have been related to ASD features. Black et al. (2009) showed that both discrepantly higher PIQ and VIQ, as measured by the WISC-III (Wechsler 1991), WISC-IV (Wechsler 2003a) or WASI (Wechsler 1999), were associated with higher (i.e. more abnormal) social symptoms scores as assessed by the Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 1989) and the Autism Diagnostic Interview (ADI) (Le Couteur et al. 1989) in a sample of 78 high-functioning children with autism aged 6–17 years. However, two studies report otherwise. One of the studies including 156 children aged 10–14 years with ASD could not establish any relation between such a discrepant score and symptom presentation of ASD (Charman et al. 2011b). The other study, including 325 children, found that VIQ–PIQ discrepancies were, to some extent, unrelated to ASD symptoms (Ryland et al. 2014).
Despite the fact that much progress has been made in determining the cognitive profile of strengths and weaknesses of subjects with ASD, a number of outstanding questions remain to be answered: (1) if the strengths and deficits are the same in high and low-functioning ASD; (2) whether cognitive subgroups exist; (3) and how cognition is associated with core ASD features and adaptive behaviour, as well as associated psychopathology. Small sample sizes, a focus on single domains of cognition and the absence of comprehensive behavioural phenotypic information are methodological factors that have contributed to these limitations in the scientific knowledge (Charman et al. 2011a).
The present study involves participants with the principal diagnosis of ASD with and with no ID (ASD_ID/ASD_NID) and participants with other neurodevelopmental disorders (OND), such as intellectual disability or learning disabilities, with and with no ID (OND_ID/OND_NID). It is important to know whether the performance on a standard cognitive test can be used to clearly separate ASD from other neurodevelopmental disorders, aiding in the diagnosis of ASD but also in the interpretation of its pathogenicity. Therefore, the purpose of this study is to examine the influence of the primary diagnosis of ASD versus OND, matched for IQ and chronological age (CA), on cognitive ability; its intellectual profile; and study whether performance on a standard cognitive test can be used to clearly separate or as clinical aid, in order to discriminate autism spectrum disorder from other neurodevelopmental disorders.
Methods
Participants
Participants included 445 school-aged children and adolescents, ranging in age from 6 to 16 years and 11 months. They were divided into two clinical main groups: ASD (N = 224; mean age = 117 ± 21 months, 202 Male/22 Female) versus OND (N = 221; mean age = 113 ± 26 months, 147 Male/74 Female). Participants were seen as part of an outpatient clinic between 2004 and 2015.
To be included in this study, all participants had to be given an individually administered IQ test [Portuguese version of Wechsler Intelligence Scale for Children—Third Edition (WISC-III) (Wechsler 2003b)] and the participants’ primary caregiver had been administered the Vineland Adaptive Behavior Scale (VABS)-Survey form (Sparrow et al. 1984). Another requirement was to have between 6 and 16 years old at the moment of evaluation. ASD diagnosis was assigned on the basis of the gold standard instruments: parental or caregiver interview [Autism Diagnostic Interview—Revised, ADI-R (Lord et al. 1994)], direct structured proband assessment [Autism Diagnostic Observation Schedule, ADOS (Lord et al. 1989)] and clinical examination performed by an experienced neurodevelopmental Paediatrician. The current diagnostic criteria for autism were revised according to the Diagnostic and Statistical Manual of Mental Disorders 5, DSM-5 (American Psychiatric Association 2013). All ASD patients had positive results in the ADI-R and ADOS for autism or ASD, and met the criteria for ASD from the DSM-5. A comprehensive medical observation excluded associated medical conditions such as epilepsy, neurocutaneous or other genetic syndromes, or other usual comorbidity in ASD samples. All population in this study is routinely followed by this team in a clinical set at least two times per year.
The gold standard diagnostic assessment scales ADI-R (Lord et al. 1994) and ADOS (Lord et al. 1989) are also used to characterize the ASD symptomatology and to correlate with the WISC-III results. ADI-R (Lord et al. 1994) is a structured interview used for diagnosing autism, planning treatment, and distinguishing autism from other developmental disorders. It can be used for diagnostic purposes in subjects with a mental age of at least 18 months and provides quantitative measures of behaviour in the areas of (1) reciprocal social interactions, (2) language and communication, and (3) repetitive behaviours/interests. ADOS (Lord et al. 1989) is used for assessing and diagnosing autism and pervasive developmental disorder (PDD) across ages, developmental levels, and language skills. It consists of a series of structured and semi-structured tasks that involve social interaction between the examiner and the subject. The examiner observes and identifies segments of the subject’s behaviour and assigns these to predetermined observational categories, which are combined to a score. The score is assessed through cut-offs in (1) reciprocal social interaction and (2) communication and language that identify the potential diagnosis of autism spectrum disorder.
In the OND group were included subjects diagnosed and followed in our clinic with ID (full-scale IQ–FSIQ ≤ 70) or learning disabilities (FSIQ > 70). The parents of participants included in OND group completed the Social Communication Questionnaire (Rutter et al. 2003) to exclude comorbidity with ASD. Associated medical conditions were also excluded as in the ASD group.
Measures
All measures (even the ones referred to in the previous point for clinical characterization) were administered by experienced psychologists and neurodevelopmental paediatricians for diagnostic or treatment planning, during routine clinical multidisciplinary assessments in a neurodevelopmental Unity that it is a National reference for ASD and other neurodevelopmental disorders in a Terciary Pediatric Hospital.
Wechsler Intelligence Scale for Children—Third Edition (WISC-III)
The Wechsler Intelligence Scale for Children—Third Edition—WISC-III (Wechsler 1991) is an individually administered cognitive assessment tool used to evaluate the intelligence of subjects aged between 6 and 16 years and 11 months. It has been adapted and standardized for the Portuguese population by Simões and colleagues in 2003 (Wechsler 2003b).
The WISC-III is a reference in the assessment of intelligence and is used to establish a pattern of intra and inter-comparison, which identifies a global level of cognitive ability (or an estimate of intellectual potential). Its analysis also allows for the verification of the performance in a specific subtest and whether it suggests the presence of a specific cognitive deficit or, on the contrary, is widespread evidence of global intellectual deficit (Hynd et al. 1988).
This scale assumes that intelligence has a composite nature, that is, that the intellectual capacity of the subject is based on a potential arising from the integration and balancing of diverse skills and cognitive functions (Wechsler 1991). It thus explores the intellectual functioning in its varied aspects through both the division into diverse subtests and the plurality of tasks that individuals have the possibility of evidencing their abilities in (Wechsler 2003b).
This evaluation instrument consists of thirteen subtests (M = 10; SD = 3) spread over two subscales: Verbal and Performance, each one evaluating a different aspect of intelligence (Wechsler 2003b). The performance of the subjects in the various subtests is clustered in three composite results: a general intelligence measure (FSIQ) and two ratios divided by the nature of its subtests: the VIQ, measurement of verbal intelligence, and the PIQ, a nonverbal intelligence measure (Wechsler 2003b).
The subtests that compose the WISC-III enable a first distinction between skills or psychological functions, providing a reference point for the examination of higher cortical functions (Kaufman 1994).
The WISC-III yields three composite IQs scores (M = 100; SD = 15): VIQ, PIQ and FSIQ, and four index scores: VCI, POI, PSI and FDI resulting from groupings of the subtests (Wechsler 1991, 2003a, 2003b).
The various composite scores correspond to different levels of interpretation (Wechsler 1991). The first level of interpretation is the FSIQ, determined by the sum of the standardized results of subtests of the subscales Verbal and Performance. The analysis of VIQ and PIQ defined, respectively, by the sums of standardized results in verbal and performance subtests, refers to the second level of interpretation. In this level, the comparison of results between VIQ and PIQ is valued. The interpretation of the difference between VIQ and PIQ must be carried out carefully considering a number of factors, like the presence of language, hearing or motor problems, motivational questions or cultural and language differences. Thus, even though the VIQ–PIQ discrepancy could be the basis for formulating hypotheses, their presence or absence cannot be regarded as conclusive evidence of an inability (Hynd et al. 1988). The VIQ–PIQ dichotomy is useful to know if the child has a deficit that only harms the language skills (VIQ) or the perceptual space capabilities (PIQ) too. Through the dominance of the analysis concerning these skills, it can be known if the weak areas of the child’s intellectual capacity match their language skills (VIQ < PIQ) or the perceptual spatial skills (PIQ < VIQ) (Hynd et al. 1988). The third level of interpretation concerns to the indexes identified by factor analysis, providing more detail in the search for strong and weak areas of the cognitive function of the subject. Thus, the VCI is composed of four verbal subtests (Information, Similarities, Vocabulary and Comprehension), the POI of four subtests (Picture Completion, Picture Arrangement, Block Design and Object Assembly), and the PSI of two subtests (Code and Symbol Search).
The fourth level of interpretation is the analysis of each subtest: Information, Similarities, Arithmetic, Vocabulary, Comprehension, Digit Span, Picture Completion, Coding, Picture Arrangement, Block Design, Object Assembly, Symbol Search and Mazes (Hynd et al. 1988). Further information about each subtest is in the “Discussion” section, when considered necessary.
The factor structure of the Portuguese version of WISC-III yields a three-factor model (VCI, POI and PSI), however in this study, the FDI was analysed as a profile (sum of the scaled scores of Arithmetic and Digit Span) rather than as an index score. The Mazes subtest was not administered.
All participants were tested with the Portuguese version of WISC-III (Wechsler 2003b).
Procedure
Data was collected from a database according to the National policy on archival research of the Pediatric Hospital. The group of participants included in this study represents a subset of patients, which information is usually collected for clinical and research characterization of the outpatient clinic. A total of 445 records meeting the inclusion criteria were included in this study.
The two clinical main groups: ASD and OND were each further subdivided into two, totalizing four subgroups, taking into account the FSIQ. The classification of ID of the International Classification of Diseases, 10th Revision (Bramer 1988) was applied. According to this classification, a subject has ID when the FSIQ is equal to or below 70 and has no ID when the FSIQ is above 70. The four subgroups were: [ASD with no ID (ASD_NID, N = 166); ASD with ID (ASD_ID; N = 58); OND with no ID (OND_NID; N = 166); OND with ID (OND_ID; N = 55])]. They were matched by chronological age and FSIQ score (T test, p > .05).
In the two main groups and in the four clinical subgroups we compared the intellectual profile of WISC-III analysing the standard scores (SS) of IQs, index scores, subscales, Kaufman’s factors (Kaufman 1975, 1994; Reynolds and Kaufman 1990) and Bannatyne’s categories (Bannatyne 1968, 1974).
Data Analysis
Data was analysed using the version for Microsoft Windows® of the Statistical Package for Social Sciences software (SPSS ®, Chicago, IL, USA).
Paired sample t tests were calculated to investigate the significance of differences between quantitative variables, VIQ and PIQ, in the different groups and subgroups. Independent samples t tests with Bonferroni correction were calculated to investigate the significance of differences in WISC-III IQs, index scores, subtests, Kaufman’s factors and Bannatyne’s categories between groups. Cohen’s d was additionally calculated to determine the effect sizes of these differences.
Additionally, we performed Pearson-correlation analysis with Bonferroni correction to determine the linear correlation between each result of WISC-III and VABS scores and CA in the two main groups and ASD symptomatology (Language/Communication, Reciprocal Social Interactions, and Repetitive Behaviours/Interests results from ADI-R and ADOS) in the ASD group.
We considered the significance level (α) = 0.05 (p < .05).
Ethics Statement
This study and all the procedures were reviewed and approved by the Ethics Commission of our Pediatric Hospital and was conducted in accordance with the declaration of Helsinki. Informed consent was obtained from the parents/guardians of all younger participants. Children and adolescents also gave oral informed consent.
Results
Initial analysis was conducted to ensure that participants were matched with respect to chronological age and FSIQ in both two main clinical groups and four subgroups (T test, p > .05).
The average SS of IQs, index scores, subtests from WISC-III evaluation, Kaufman’s factors and Bannatyne’s categories in the two main clinical groups and four subgroups, as well as group comparisons, significance levels and effect sizes are reported in Tables 1, 2 and 3.
IQs and Index Scores
VIQ–PIQ Differences
A paired sample t test showed a statistically significant difference between VIQ and PIQ for both clinical main groups: ASD t(223) = −2.615, p = .010, d = −0,16 and OND t(220) = −2.302, p = .022, d = −0,12 with PIQ > VIQ in both ASD and OND. For the subgroups there was a pattern related with ID. In the subgroups with ID, there was a significant difference between with PIQ > VIQ in both, although it was higher in ASD: ASD_ID t(57) = −4.192, p = .000, d = −0,11 and OND_ID t(54) = −2.280, p = .027, d = −0,40. In the subgroups with no ID there was no significant difference: ASD_NID t(165) = −1.113, p = .267, d = −0,78 and OND_NID t(165) = −1.593, p = .113, d = −0,12.
ASD Versus OND
When we analyse the two main groups (ASD vs. OND), not taking into account the level of IQ, there are no significant statistical differences in FSIQ, VIQ, PIQ (t test, p > .05, see Table 1 for details on exact p values, specific comparisons and effect sizes).
Relative to the WISC-III index scores, statistically significant differences (Table 2) were found between children with ASD and OND for PSI (p < .000), with the ASD group having lower results than the OND group in PSI. For VCI and POI, no significant difference was found.
ASD_ID Versus OND_ID
Regarding the subgroups with ID (ASD_ID and OND_ID), there was also a significant effect for diagnosis, with the ASD_ID subgroup having lower scores for VIQ [t(111) = −2895, p = .015]. However, no significant differences were found in the FSIQ and PIQ (t test, p > .05). In what concerns the WISC-III index scores, t tests indicated that these subgroups differ in VCI [t(111) = −4227, p = .006] and PSI [t(111) = −3157, p = .000]. In the indexes where the groups differ, the OND_ID had better results (see Table 2 for details on exact p values and specific comparisons).
ASD_NID Versus OND_NID
When comparing the subgroups with no ID (ASD_NID and OND_NID), t test did not show significant differences in IQ’s. In respect to Index scores, there was a significant effect for diagnosis, with ASD_NID having higher scores in POI [t(330) = 2520, p = .036] and lower scores in PSI [t(330) = −2.973, p = .009] (Table 3).
Subtests Scores
ASD Versus OND
As shown in Table 1, the two main clinical groups, ASD and OND, differ in three subtests scores: Comprehension [t(443) = −4444, p = .000], Coding [t(443) = −4173, p = .000] and Block Design [t(443) = 4549, p = .000]. The ASD group had higher results in Block Design, while the OND group had higher results in Comprehension and Coding. The highest score for the ASD group was in Block Design (M = 10.11) and the lowest was on Comprehension (M = 6.43). In the OND group the subtests with highest and lowest scores were Similarities (M = 9.30) and Arithmetic (M = 7.52), respectively. ASD showed a more heterogeneous profile than the OND, which is homogeneous, as it is shown in Fig. 1.
ASD_ID Versus OND_ID
When we analyse the subgroups with ID, statistically significant differences were found between the ASD_ID and OND_ID subgroups in four subtests: Vocabulary [t(111) = −2943, p = .048], Comprehension [t(111) = −4816, p = .000], Coding [t(111) = −3397, p = .012] and Symbol Search [t(111) = −3454, p = .012], with the OND_ID subgroup having higher scores (Table 2). The highest score for ASD_ID subgroup was in Object Assembly (M = 6.74) and the lowest was on Comprehension (M = 3.10). In the OND_ID subgroup, the subtests with highest and lowest scores were Similarities (M = 6.18) and Arithmetic (M = 4.38), respectively. ASD_ID showed a more heterogeneous profile than the OND_ID, as shown in Fig. 2.
ASD_NID Versus OND_NID
The two subgroups with no ID, ASD_NID and OND_NID, differ in five subtests scores: Information [t(330) = 3137, p = .024], Similarities [t(330) = 3278, p = .012], Comprehension [t(330) = −3715, p = .000], Coding [t(330) = −3416, p = .012] and Block Design [t(330) = 5386, p = .000]. ASD_NID subgroup had higher results in Information, Similarities and Block Design, while the OND_NID subgroup had higher results in Comprehension and Coding. The highest score for both groups was in Similarities (ASD_NID: M = 11.44; OND_NID; M = 10.33) and the lowest was on Coding (ASD_NID: M = 7.38; OND_NID; M = 7.52). ASD showed a more heterogeneous profile than the OND, as it is shown in Fig. 3.
Profiles Scores
ASD Versus OND
Statistically significant differences between the ASD and OND groups were found in one of the seven analysed WISC-III profiles (SPA [t(443) = 2531, p = .048]) (Table 1). In the Bannatyne’s spatial abilities ASD group scored lower than the OND.
ASD_ID Versus OND_ID
T tests showed statistically significant differences between the ASD_ID and OND_ID subgroups in SCAD [t(111) = −2864, p = .015], VCA [t(111) = −3252, p = .008] and ACK [t(111) = −2628, p = .040], with OND obtaining higher scores (Table 2).
ASD_NID Versus OND_NID
In the subgroups without ID, the ASD_NID subgroup had higher scores than the OND_NID in the profiles where statistically significant differences were found: SPA [t(330) = 2909, p = .016] and ACK [t(330) = 2734, p = .028] (Table 3).
Correlations
We performed Pearson correlation analysis between the SS of WISC-III (IQ’s, Indexes and Subtests), Kaufman’s factors, Bannatyne’s categories and the domains from VABS, as well as chronological age at the two main groups of diagnosis: ASD versus OND (see Tables 4 and 5 for details on exact p values and specific correlations).
The VABS (Sparrow et al. 1984) is a recognized, semi-structured interview designed to assess global adaptive functioning in three main domains: Communication (COM), Daily Living Skills (DLS), and Socialization (SOC), attributing a total score, the Adaptive Behaviour Composite (ABC). In previous work (Mouga et al. 2015), was found that there was a significant effect for diagnosis, with ASD, ASD_NID and ASD_ID groups having lower scores than OND, ASD_NID, OND_ID groups in most areas of adaptive behaviour, and with the domain of socialization skills remaining as a distinctive factor of ASD versus OND. In this study, we replicated these results, with ASD and ASD_NID having lower results in DLS and SOC (p < .05) than the OND and ASD_NID group and subgroup, respectively. In the subgroups with ID, the ASD_ID had lower results in DLS, SOC and ABC (p < .05).
We observed that, in the ASD group, the SS of WISC-III (IQ’s, Indexes and Subtests), Kaufman’s factors and Bannatyne’s categories were all statistically significant (p < .05) and positively correlated with all VABS SS domains, being the strongest association between the VABS Communication and FSIQ (r = .512), VIQ (r = .627), VCI (r = .605), ACID (r = .669), SCAD (r = .552), FDI (r = .585), VCA (r = .555), SQA (r = .580), ACK (r = .650), Information (r = .650), Similarities (r = .522), Arithmetic (r = .520), Vocabulary (r = .536).
In the OND group, most of the SS of WISC-III (IQ’s, Indexes and Subtests), Kaufman’s factors and Bannatyne’s categories were statistically significant (p < .05) and positively correlated with all VABS SS domains, being the strongest association the one between the VABS Communication and FSIQ (r = .610), VIQ (r = .604), VCI (r = .593), ACID (r = .630), SCAD (r = .570), FDI (r = .522), VCA (r = .564), SQA (r = .576), ACK (r = .611), Information (r = .574), Arithmetic (r = .514), Vocabulary (r = .562); and between the ABC and PIQ (r = .564), VSI (r = .505), POI (r = .529) and SPA (r = .507).
In what concerns CA, the only WISC-III SS that were statistically significant (p < .05) and moderately (r between ± .300 and ± .390) correlated were: VCA (r = −.344), Similarities (r = −.361) and Comprehension (r = −.302), in the ASD group, and the Comprehension (r = −.310) and VCA (r = −.320), in the OND group. In both groups these associations between chronological age and WISC-III SS were negative.
Concerning ASD symptomatology, the WISC-III SS were not correlated with the data from Language/Communication, Reciprocal Social Interactions, and Repetitive Behaviours/Interests from ADI-R and ADOS (p > .05).
Discussion
In the current work, we have studied the influence of specific neurodevelopmental ASD deficits on intellectual profiles of children. For that purpose, we compared the cognitive profile measured by one of the most studied tools for this, WISC-III, between two groups, one with ASD and another without ASD (other neurodevelopmental disorders), controlled for chronological age and global intellectual level.
The population with neurodevelopmental disorders, including the ones with ASD, was characterized by significantly lower scores in the VIQ than PIQ, which became even more evident whenever ID was present. These results corroborate, in part, the typical VIQ–PIQ discrepancies of individuals with ASD (Charman et al. 2011b; Ryland et al. 2014; Minshew et al. 1992; Szatmari et al. 1990), although in our study they were not correlated with ASD symptomatology from the scores from ADI-R and ADOS, as in many of the previous studies (Kaufman and Lichtenberger 2000; Black et al. 2009). Possibly the lack of subgrouping in our study may explain the absence of correlation between the ASD symptomatology and the intellectual measures.
The distinctive profile of ASD, when compared to a sample with neurodevelopmental disorders without autism was more evident when the WISC-III results were analysed in a further complex view of their indexes and subtests. In fact, FSIQ, VIQ and PIQ were unable to discriminate accurately the ASD subjects when we looked at the main groups and at individuals with no ID. Nevertheless, the verbal abilities of ID groups were significantly lower in the ASD sample and it was possible to conclude that this phenotypic marker will help signalize autism.
Regarding the WISC-III index scores, a commonly reported Wechsler profile among school age children with ASD includes higher scores on VCI and the POI, when compared with the PSI (Mayes and Calhoun 2003, 2008; Nyden et al. 2001; Wechsler 2003a), which was partially corroborated in the present study, with the exception of the ASD_ID subgroup, that had lower VCI. The index scores were also able to differentiate between ASD and OND in what concerns the processing speed capabilities, where ASD presented more difficulties, which was consistent with previous work (Mayes and Calhoun 2004). When we took into account the presence or absence of ID, the results differed. Actually, when comparing both subgroups with ID, the ASD individuals showed lower scores for VCI and PSI and similar results to OND in POI. In the subgroups with no ID, the results from the main groups were replicated. In fact, the core distinctive index from subjects with autism or without ASD was the ability to focus attention and quickly scan, discriminate between, and sequentially order visual information, which was assessed by PSI. This index requires persistence and planning ability, is sensitive to motivation, to difficulty working under a time pressure, and motor coordination, all deficits that are usually present in the ASD symptomatology. These abilities are related to reading performance and working memory: increased processing speed can decrease the load placed on working memory, while decreased processing speed can impair the effectiveness of working memory (Wechsler 2003a).
Our results corroborate previous findings that reported that individuals with ASD tend to have “Block design” as the subtest with highest results (Allen et al. 1991; Asarnow et al. 1987; Freeman et al. 1985; Happe 1994; Lincoln et al. 1988; Rumsey and Hamburger 1988; Siegel et al. 1996; Venter et al. 1992; Bailey et al. 1996; Shah and Frith 1993; Szatmari et al. 1990; Lockyer and Rutter 1970; Bowler 1992; Dennis et al. 1999; Koyama et al. 2007; Mayes and Calhoun 2003) and the lowest results in “Comprehension” (Siegel et al. 1996; Freeman et al. 1985; Asarnow et al. 1987; Narita and Koga 1987; Ohta 1987; Rumsey and Hamburger 1988; Lincoln et al. 1988; Allen et al. 1991; Venter et al. 1992; Happe 1994; Bailey et al. 1996; Dennis et al. 1999; Koyama et al. 2007; Mayes and Calhoun 2003). This is evident when comparing the main groups (ASD vs. OND), and also when analysing the ASD profile. Despite that fact, in the subgroups, the results were different. We did not find the lowest result in “Comprehension” or the highest result on “Block design” on the ASD group without ID, although they differ in these subtests when compared to age and IQ matched individuals without ASD. In this subgroup (ASD_NID), the highest score was on “Similarities” and the lowest in “Coding”. This means that the ASD subjects with no ID show good abstract, logical thinking and reasoning, and have difficulties in visual-motor dexterity, associative nonverbal learning and nonverbal short-term memory. In the subgroup with ID, ASD showed the lowest results in “Comprehension” and the highest in “Object Assembly”, which denoted better capacity to visualize component parts of a concrete object and reassemble these parts into the whole (making “puzzles”), as well as difficulties in social knowledge, practical judgment in social situations and moral conscience, a core feature of ASD.
Previous studies reported deficits in the ability to interpret action as depicted by pictures, in recognizing their sequence in a story, and in arranging these in sequential order to tell a story (“Picture Arrangement” subtest) (Allen et al. 1991; Lincoln et al. 1988; Ohta 1987; Rumsey and Hamburger 1988; Venter et al. 1992; Szatmari et al. 1990; Shah and Frith 1993), and strengths in the subtest “Digit Span”, which is a measure of short-term verbal memory and attention (Allen et al. 1991; Lincoln et al. 1988; Narita and Koga 1987; Ohta 1987; Rumsey and Hamburger 1988; Siegel et al. 1996; Bailey et al. 1996; Szatmari et al. 1990; Dennis et al. 1999), which we did not replicate in our study.
In sum, we can conclude that the WISC-III subtests that better discriminate between ASD and OND are “Comprehension” and “Coding”, which were significantly lower in all ASD individuals. Although some previous studies tried to differentiate the ASD subjects by their strengths, these difficulties were the ones which could separate ASD from the other neurodevelopmental disorders in our large sample. We can also conclude that our ASD patients exhibit a more heterogeneous intellectual profile than other neurodevelopmental disorders.
In what concerns the empirically oriented classification systems, such as Bannatyne’s (1974) categories and Kaufman’s factors (Kaufman 1975, 1994), our study showed that the SCAD (Symbol Search, Coding, Arithmetic and Digit Span subtests) can differentiate ASD subjects when referring to groups with intellectual disability. Bannatyne’s Spatial Ability showed strengths in the ASD group and in the ASD subgroup with no ID (as well as Acquired Knowledge), while in the subgroup with ID, the ASD showed difficulties in Verbal Conceptualisation Ability and Acquired Knowledge.
In our study, we replicated the results from previous work on adaptive behaviour (Mouga et al. 2015). In fact, the associations between WISC SS, Kaufman’s Factors and Bannatyne’s categories with VABS domains show that adaptive functioning is positively correlated with intellectual profile, especially in the Communication domain. The Communication domain relates not only to the ability to use the spoken language, but also to learning capabilities, especially in school-aged children. Therefore, it was expected that the cognitive ability, in some way, would modulate this domain—communication learning (Mouga et al. 2015). However, these associations seem to differ in particular aspects. In the ASD sample, the strongest associations were between VABS Communication and global and verbal intellectual ability, verbal comprehension, Kaufman’s factors (ACID, SCAD, and FDI), verbal conceptualisation and sequencing ability, acquired knowledge and verbal subtests, such as Information, Similarities, Arithmetic and Vocabulary. Whereas in OND patients, there were also associations between global adaptive behaviour (ABC) and performance IQ, verbal comprehension and perceptual organization, and spatial abilities. In fact, as shown in previous work (Mouga et al. 2015), verbal abilities seem to determine the adaptive functioning in school aged ASD individuals, highlighting the importance of the development of functional language skills for later outcome, and supporting recent findings (Howlin et al. 2014). Conversely, in OND sample, the verbal abilities did not have a determining value in the adaptive behaviour, it only occurs in the autism subjects.
We can conclude that WISC-III is recommended as a reliable IQ measure for children with autism spectrum or other neurodevelopmental disorders, albeit additional characterisation with factors and categories, such as Kaufman’s factors and Bannatyne’s categories, may add significant information.
An accurate evaluation of the intellectual profile of ASD children is important for many reasons, namely the fact that intelligence has proven to be a good predictor of outcome in terms of academic progress (Gillberg and Steffenburg 1987), which cannot be mistaken for an adequate adaptive behaviour, that is, an ability to cope in the everyday life, that is usually considerably impaired, even for the most high functioning individual (Charman et al. 2011b). The assessment of the IQ is also very important to the selection of the intervention type, school adaptations and curriculum, but also to adopt realistic perspectives for the future. On the contrary, an underestimation of intelligence may further increase the stigma that some individuals with ASD experience and may negatively affect opportunities in everyday life, for instance the opportunity of having an employment.
Wechsler scales, although they are not a diagnostic measure for ASD, are used as a criteria to match ASD individuals in research studies and affect how their potential and progress are assessed and predicted in the clinical practice (Nader et al. 2014).
Our study, with a large and well characterized sample, was able to answer some questions: the strengths and deficits are not the same in high and low-functioning ASD and that intellectual profile is associated with adaptive behaviour and not with core ASD features, as measured by ADI-R and ADOS.
In conclusion, enhanced knowledge of the cognitive phenotype, a frequent comorbidity of ASD, may contribute to our understanding of the complex links between genes, brain and neurodevelopment, as well as to inform approaches to therapeutics.
References
Allen, M. H., Lincoln, A. J., & Kaufman, A. S. (1991). Sequential and simultaneous processing abilities of high-functioning autistic and language-impaired children. Journal of Autism and Developmental Disorders, 21(4), 483–502.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington: American Psychiatric Publishing.
Asarnow, R. F., Tanguay, P. E., Bott, L., & Freeman, B. J. (1987). Patterns of intellectual functioning in non-retarded autistic and schizophrenic children. Journal of Child Psychology and Psychiatry, 28(2), 273–280.
Bailey, A., Phillips, W., & Rutter, M. (1996). Autism: Towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. Journal of Child Psychology and Psychiatry, 37(1), 89–126.
Bannatyne, A. (1968). Diagnosing learning disabilities and writing remedial prescriptions. Journal of Learning Disabilities, 1(4), 242–249.
Bannatyne, A. (1974). Diagnosis: A note on recategorization of the WISC scaled scores. Journal of Learning Disabilities, 7(2), 272–273.
Baron-Cohen, S. (2001). Theory of mind and autism: A review. International Review of Research in Mental Retardation, 23(23), 169–184.
Black, D. O., Wallace, G. L., Sokoloff, J. L., & Kenworthy, L. (2009). Brief report: IQ split predicts social symptoms and communication abilities in high-functioning children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(11), 1613–1619. doi:10.1007/s10803-009-0795-3.
Bowler, D. M. (1992). “Theory of mind” in Asperger’s syndrome. Journal of Child Psychology and Psychiatry, 33(5), 877–893.
Bramer, G. R. (1988). International statistical classification of diseases and related health problems. Tenth revision. World Health Statistics Quarterly, 41(1), 32–36.
Carothers, D. E., & Taylor, R. L. (2013). Differential effect of features of autism on IQs reported using Wechsler scales. Focus on Autism and Other Developmental Disabilities, 28(1), 54–59. doi:10.1177/1088357612457988.
Centers for Disease Control and Prevention. (2009). Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveillance Summaries, 58(10), 1–20.
Centers for Disease Control and Prevention. (2012). Prevalence of autism spectrum disorders–Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveillance Summaries, 61(3), 1–19.
Centers for Disease Control and Prevention. (2014). Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveillance Summaries, 63(2), 1–21.
Charman, T., Jones, C. R., Pickles, A., Simonoff, E., Baird, G., & Happe, F. (2011a). Defining the cognitive phenotype of autism. Brain Research, 1380, 10–21. doi:10.1016/j.brainres.2010.10.075.
Charman, T., Pickles, A., Simonoff, E., Chandler, S., Loucas, T., & Baird, G. (2011b). IQ in children with autism spectrum disorders: Data from the Special Needs and Autism Project (SNAP). Psychological Medicine, 41(3), 619–627. doi:10.1017/S0033291710000991.
Dennis, M., Lockyer, L., Lazenby, A. L., Donnelly, R. E., Wilkinson, M., & Schoonheyt, W. (1999). Intelligence patterns among children with high-functioning autism, phenylketonuria, and childhood head injury. Journal of Autism and Developmental Disorders, 29(1), 5–17.
Fombonne, E. (2003). Epidemiological surveys of autism and other pervasive developmental disorders: An update. Journal of Autism and Developmental Disorders, 33(4), 365–382.
Freeman, B., Lucas, J., Fomess, S., & Ritvo, E. (1985). Cognitive processing of high-functioning autistic children: Comparing the K-ABC and the WlSC-R. Journal of Psychoeducational Assessment, 4, 357–362.
Ghaziuddin, M., & Mountain-Kimchi, K. (2004). Defining the intellectual profile of Asperger syndrome: Comparison with high-functioning autism. Journal of Autism and Developmental Disorders, 34(3), 279–284.
Gillberg, C., & Steffenburg, S. (1987). Outcome and prognostic factors in infantile autism and similar conditions: A population-based study of 46 cases followed through puberty. Journal of Autism and Developmental Disorders, 17(2), 273–287.
Goldstein, G., Allen, D. N., Minshew, N. J., Williams, D. L., Volkmar, F., Klin, A., et al. (2008a). The structure of intelligence in children and adults with high functioning autism. Neuropsychology, 22(3), 301–312. doi:10.1037/0894-4105.22.3.301.
Goldstein, S., Naglieri, J. A., & Ozonoff, S. (2008b). Assessment of autism spectrum disorders. New York: Guilford Press.
Happe, F. G. (1994). Wechsler IQ profile and theory of mind in autism: A research note. Journal of Child Psychology and Psychiatry, 35(8), 1461–1471.
Happe, F., & Frith, U. (2006). The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders, 36(1), 5–25. doi:10.1007/s10803-005-0039-0.
Howlin, P., Savage, S., Moss, P., Tempier, A., & Rutter, M. (2014). Cognitive and language skills in adults with autism: A 40-year follow-up. Journal of Child Psychology and Psychiatry, 55(1), 49–58. doi:10.1111/jcpp.12115.
Hynd, G. W., Cohen, M. J., Riccio, C. A., & Arceneaux, J. M. (1988). Neuropsychological basis of intelligence and the WISC-III. In D. H. S. Prifitera (Ed.), WISC-III clinical use and interpretation: Scientist–practitioner perspectives (pp. 203–226). San Diego, CA: Academic Press.
Joseph, R. M. (2011). The significiance of IQ and differential cognitive abilities. In D. A. Fein (Ed.), The neuropsychology of autism. Oxford: Oxford University Press.
Kaufman, A. S. (1975). Factor-analysis of Wisc-R at 11 age levels between 6-1/2 and 16-1/2 Years. Journal of Consulting and Clinical Psychology, 43(2), 135–147. doi:10.1037/H0076502.
Kaufman, A. S. (1994). Intelligent testing with the WISC-III. New York: Wiley.
Kaufman, A. S., & Lichtenberger, E. O. (2000). Essentials of WISC-III and WPPSI-R assessment. New York: Wiley.
Koegel, L. K., Koegel, R. L., & Smith, A. (1997). Variables related to differences in standardized test outcomes for children with autism. Journal of Autism and Developmental Disorders, 27(3), 233–243.
Koyama, T., Tachimori, H., Osada, H., & Kurita, H. (2006). Cognitive and symptom profiles in high-functioning pervasive developmental disorder not otherwise specified and attention-deficit/hyperactivity disorder. Journal of Autism and Developmental Disorders, 36(3), 373–380. doi:10.1007/s10803-006-0075-4.
Koyama, T., Tachimori, H., Osada, H., Takeda, T., & Kurita, H. (2007). Cognitive and symptom profiles in Asperger’s syndrome and high-functioning autism. Psychiatry and Clinical Neurosciences, 61(1), 99–104. doi:10.1111/j.1440-1819.2007.01617.x.
Le Couteur, A., Rutter, M., Lord, C., Rios, P., Robertson, S., Holdgrafe, M., et al. (1989). Autism diagnostic interview: A semi-structured interview for parents and caregivers of autistic persons. Journal of Autism and Developmental Disorders, 19, 363–387.
Lincoln, A. J., Courchesne, E., Kilman, B. A., Elmasian, R., & Allen, M. (1988). A study of intellectual abilities in high-functioning people with autism. Journal of Autism and Developmental Disorders, 18(4), 505–524.
Lockyer, L., & Rutter, M. (1970). A five- to fifteen-year follow-up study of infantile psychosis. IV. Patterns of cognitive ability. British Journal of Social and Clinical Psychology, 9(2), 152–163.
Lord, C., Rutter, M., Goode, S., Heemsbergen, J., Jordan, H., Mawhood, L., et al. (1989). Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders, 19(2), 185–212.
Lord, C., Rutter, M., & Le Couteur, A. (1994). Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685.
Mayes, S. D., & Calhoun, S. L. (2003). Analysis of WISC-III, Stanford-Binet:IV, and academic achievement test scores in children with autism. Journal of Autism and Developmental Disorders, 33(3), 329–341.
Mayes, S. D., & Calhoun, S. L. (2004). Similarities and differences in Wechsler Intelligence Scale for Children-Third Edition (WISC-III) profiles: Support for subtest analysis in clinical referrals. The Clinical Neuropsychologist, 18(4), 559–572. doi:10.1080/13854040490888530.
Mayes, S. D., & Calhoun, S. L. (2008). WISC-IV and WIAT-II profiles in children with high-functioning autism. Journal of Autism and Developmental Disorders, 38(3), 428–439. doi:10.1007/s10803-007-0410-4.
Minshew, N. J., Goldstein, G., Muenz, L. R., & Payton, J. B. (1992). Neuropsychological functioning in nonmentally retarded autistic individuals. Journal of Clinical and Experimental Neuropsychology, 14(5), 749–761. doi:10.1080/01688639208402860.
Mottron, L. (2004). Matching strategies in cognitive research with individuals with high-functioning autism: Current practices, instrument biases, and recommendations. Journal of Autism and Developmental Disorders, 34(1), 19–27.
Mouga, S., Almeida, J., Cafe, C., Duque, F., & Oliveira, G. (2015). Adaptive profiles in autism and other neurodevelopmental disorders. Journal of Autism and Developmental Disorders, 45(4), 1001–1012. doi:10.1007/s10803-014-2256-x.
Nader, A. M., Courchesne, V., Dawson, M., & Soulieres, I. (2014). Does WISC-IV underestimate the intelligence of autistic children? Journal of Autism and Developmental Disorders,. doi:10.1007/s10803-014-2270-z.
Narita, T., & Koga, Y. (1987). Neuropsychological assessment of childhood autism. Advances in Biological Psychiatry, 16, 156–170.
Nyden, A., Billstedt, E., Hjelmquist, E., & Gillberg, C. (2001). Neurocognitive stability in Asperger syndrome, ADHD, and reading and writing disorder: A pilot study. Developmental Medicine and Child Neurology, 43(3), 165–171.
Ohta, M. (1987). Cognitive disorders of infantile autism: A study employing the WISC, spatial relationship conceptualization, and gesture imitations. Journal of Autism and Developmental Disorders, 17(1), 45–62.
Oliveira, G., Ataide, A., Marques, C., Miguel, T. S., Coutinho, A. M., Mota-Vieira, L., et al. (2007). Epidemiology of autism spectrum disorder in Portugal: Prevalence, clinical characterization, and medical conditions. Developmental Medicine and Child Neurology, 49(10), 726–733. doi:10.1111/j.1469-8749.2007.00726.x.
Oliveras-Rentas, R. E., Kenworthy, L., Roberson, R. B, 3rd, Martin, A., & Wallace, G. L. (2012). WISC-IV profile in high-functioning autism spectrum disorders: Impaired processing speed is associated with increased autism communication symptoms and decreased adaptive communication abilities. Journal of Autism and Developmental Disorders, 42(5), 655–664. doi:10.1007/s10803-011-1289-7.
Ottem, E. (1999). The structures of the WISC-R subtests: A comparison of the IQ-profiles of reading impaired and autistic subjects. Scandinavian Journal of Psychology, 40(1), 1–9.
Pennington, B. F., & Ozonoff, S. (1996). Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry, 37(1), 51–87. doi:10.1111/j.1469-7610.1996.tb01380.x.
Pisula, E. (2010). The autistic mind in the light of neuropsychological studies. Acta Neurobiologiae Experimentalis, 70(2), 119–130.
Reynolds, C. R., & Kaufman, A. S. (1990). Assessment of children’ s intelligence with the Wechsler Intelligence Scale for Children—Revised (WISC-R). In C. R. Reynolds & R. W. Kamphaus (Eds.), Handbook of psychological and educational assessment of children: Intelligence and achievement (pp. 127–165). New York: Guilford.
Rivet, T. T., & Matson, J. L. (2011). Review of gender differences in core symptomatology in autism spectrum disorders. Research in Autism Spectrum Disorders, 5(3), 957–976.
Rumsey, J. M., & Hamburger, S. D. (1988). Neuropsychological findings in high-functioning men with infantile autism, residual state. Journal of Clinical and Experimental Neuropsychology, 10(2), 201–221. doi:10.1080/01688638808408236.
Rutter, M., Bailey, A., & Lord, C. (2003). Social communication questionnaire. Los Angeles: Western Psychological Services.
Ryland, H. K., Hysing, M., Posserud, M., Gillberg, C., & Lundervold, A. J. (2014). Autistic features in school age children: IQ and gender effects in a population-based cohort. Research in Autism Spectrum Disorders, 8(3), 266–274. doi:10.1016/j.rasd.2013.12.001.
Schneider, S. G., & Asarnow, R. F. (1987). A comparison of cognitive/neuropsychological impairments of nonretarded autistic and schizophrenic children. Journal of Abnormal Child Psychology, 15(1), 29–45.
Shah, A., & Frith, U. (1993). Why do autistic individuals show superior performance on the block design task? Journal of Child Psychology and Psychiatry, 34(8), 1351–1364.
Siegel, D. J., Minshew, N. J., & Goldstein, G. (1996). Wechsler IQ profiles in diagnosis of high-functioning autism. Journal of Autism and Developmental Disorders, 26(4), 389–406.
Sparrow, S., Balla, D., & Cicchetti, D. (1984). Vineland adaptative behaviour scales: interview edition, survey form. Circle Pines, MN: American Guidance Service.
Szatmari, P., Tuff, L., Finlayson, M. A., & Bartolucci, G. (1990). Asperger’s syndrome and autism: Neurocognitive aspects. Journal of the American Academy of Child and Adolescent Psychiatry, 29(1), 130–136.
Venter, A., Lord, C., & Schopler, E. (1992). A follow-up study of high-functioning autistic children. Journal of Child Psychology and Psychiatry, 33(3), 489–507.
Wechsler, D. (1949). Wechsler intelligence scale for children. New York: The Psychological Corporation.
Wechsler, D. (1991). Wechsler Intelligence Scale for Children—Third edition (WISC-III). San Antonio, TX: The Psychological Corporation.
Wechsler, D. (1999). Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: Psychological Corporation.
Wechsler, D. (2003a). Wechsler Intelligence Scale for Children–Fourth edition. San Antonio, TX: The Psychological Corporation.
Wechsler, D. (2003b). Wechsler Intelligence Scale for Children–Third edition (WISC-III)—Portuguese version (M. R. Simões, A. M. Rocha, and C. Ferreira). Lisbon: Cegoc-Tea.
Acknowledgments
This research was supported by the Portuguese Foundation for Science and Technology (individual scholarship: SFRH/BD/102779/2014 to SM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
Conceived and designed the study: SM GO. Performed the study: SM. Analyzed the data: SM GO. Contributed with data collection and tools: SM CC JA CM FD GO. Wrote the paper: SM GO. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Rights and permissions
About this article
Cite this article
Mouga, S., Café, C., Almeida, J. et al. Intellectual Profiles in the Autism Spectrum and Other Neurodevelopmental Disorders. J Autism Dev Disord 46, 2940–2955 (2016). https://doi.org/10.1007/s10803-016-2838-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-016-2838-x