Abstract
Purpose
To investigate the effects of laser photocoagulation (LPC) and intravitreal bevacizumab (IVB) therapy used in the treatment of retinopathy of prematurity (ROP) on the first age refraction values in our center.
Methods
The preterm infants who received LPC (Group I) and IVB therapy (Group II) for ROP were evaluated, and the refraction results were retrospectively compared.
Results
The study included 86 eyes of 45 infants with a mean birth week of 26.5 ± 2.1 weeks and a mean birth weight of 904 ± 223 g. Treatments were administered up to a mean PMA of 36.0 ± 2.4 and 35.3 ± 2.6 weeks in Group I and Group II, respectively. In the follow-up examinations, 1-year spherical, cylindrical, and spherical equivalent (SE) values were 0.1 ± 2.2 D, − 1.2 ± 0.9 D, and − 0.5 ± 2.0 D in Group I and 1.3 ± 1.7 D, − 1.1 ± 0.8 D, and 0.8 ± 1.7 D in Group II, respectively (P = 0.018 for spherical; P = 0.772 for cylindrical, and P = 0.009 for SE). The mean spherical power and SE were significantly higher in Group II for zone II disease (p = 0.005 and p = 0.002). In addition, according to the ROP stage, infants with Stage 3 ROP were found to be significantly more myopic than infants in Stage 2 ROP in Group I (p = 0.03).
Conclusion
In conclusion, this study supports that even 0.625 mg IVB for ROP causes less myopia compared to LPC. Consistent with the literature, it was observed that the stage and zone of ROP had a significant effect on the development of myopia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Retinopathy of prematurity (ROP) is a disease of premature infants, characterized by retinal ischemia, fibrovascular proliferation, retinal and vitreoretinal neovascularization, and progressive vitreoretinal traction. The incidences of childhood blindness due to ROP range from 3 to 10% worldwide. Depending on the developments in neonatal care, ROP is becoming increasingly important, especially in developing countries [1,2,3,4]. Following early treatment for ROP (ETROP) study, laser photocoagulation (LPC) was accepted as the standard treatment for infants in type 1 ROP [5, 6]. However, it has been shown that laser therapy causes a number of unfavorable anatomic and functional outcomes, especially in cases with zone I and posterior zone II ROP [7, 8].
On the other hand, overexpression of vascular endothelial growth factor (VEGF), which causes abnormal vascular proliferation in premature infants, is known to play an important role in the pathogenesis of ROP. This situation has increased the intravitreal use of anti-VEGF drugs in the treatment of ROP and hence the results in rapid resolution of the plus disease with regression of ROP today [9,10,11].
Long-term studies have shown that children born prematurely have much more refractive errors than those born at full term [12, 13]. On the contrary, in severe ROPs requiring treatment, myopia and other refractive errors are more common than premature infants, and this increases the risk of amblyopia [14,15,16].
The aim of the present study was to evaluate 1-year refraction values in preterm infants to whom IVB and LPC therapy were applied.
Materials and methods
The Ethical Review Committee authorized this retrospective study (2022/01), which followed the standards of the Declaration of Helsinki for research involving human subjects. The study included preterm infants who received LPC and IVB therapy for ROP.
According to the type of treatment, the patients who underwent LPC were classified as Group 1, and the patients who received IVB were classified as Group 2. Infants who received additional and combined treatment and those who underwent surgical treatment were excluded. The infants’ gestational age (GA) at birth, birth weight (BW), ROP stages and zone of the patients, treatment options, and corrected first age refraction values were recorded. All patients' spherical and cylinder power as well as spherical equivalent (SE) were recorded as diopters (D). ROP screening was performed on infants with a birth weight of ≤ 1500 g and a gestational age of ≤ 132 weeks and selected infants considered at risk by the neonatologist.
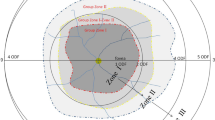
ROP status of the patients was determined according to the severity of the disease according to the criteria of the International Classification of ROP, third edition (ICROP-3) [11].
The cases that met the criteria for type I ROP (stage 2 or 3 in zone II with plus disease, stage 3 in zone I with or without plus disease, or stage 1 or 2 disease in zone I with plus disease, as defined by the ETROP study) received LPC therapy [5] and the cases diagnosed with A-ROP (rapid development of pathological neovascularization and severe plus disease without typical progression to ROP stages) received IVB therapy.
LPC was indicated for preterm infants with type 1 ROP and applied using 810-nm transpupillary diode laser (OcuLight® SL, Iridex, USA) to all avascular areas at half-spot size intervals under remifentanil sedo-analgesia. IVB treatment was indicated for cases with A-ROP, and 0.625 mg/0.025 mL IVB (Altuzan® 100 mg/dl, Roche, Sweden) was injected into the diseased eye using a sterile 32-gauge needle, at 1.5 mm from the limbus with aseptic condition using topical anesthesia. Follow-up care was conducted on postoperative day 1 and continued on a weekly basis for the first month and once every 2 weeks after 2 months until complete regression of ROP and retinal vascularization reached the temporal ora serrata.
After these examinations, all infants were scheduled to be called for the 6th month and 1-year controls and screened for refractive errors, anterior and posterior segment development, and retinal status. Refractive errors were evaluated using cycloplegic retinoscopy and automated refractometry (Welch Allyn SureSight Autorefractor, USA), following dilatation with two drops of 1% cyclopentolate hydrochloride (Sikloplejin, Abdi İbrahim, Turkey).
The SPSS 25.0 program package was used for statistical analysis. Data was presented as frequencies and percentages or as the mean ± SD. Chi-square test and Fisher’s exact test were used for categorical variables. Normal distribution fitting was checked with Kolmogorov–Smirnov test. The differences between the means were carried out by using the t-test for normally distributed data and the Mann–Whitney U test for the data that did not conform to the normal distribution. Univariable and multivariable linear regression analyses were used to investigate the effect of gestational age and birth weight on refractive results. P values of 0.05 or lower were considered to indicate statistical significance.
Results
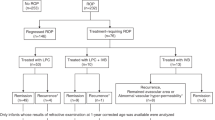
The study included 86 eyes of 45 infants with a mean ± standard deviation gestational birth age of 26.5 ± 2.1 weeks (range: 23–31 weeks) and a mean birth weight of 904 ± 223 g (range: 590–1470 g). There were 23 (51.1%) male patients and 22 (48.9%) female patients. There was no significant difference between the groups considering gestational age (GA), birth weight (BW), and gender distribution (P = 0.349 for GA, P = 0.109 for BW, and P = 0.673 for gender).
LPC therapy was performed on 59 eyes of 30 infants diagnosed with type I ROP (Group I), and IVB therapy was performed on 27 eyes of 15 infants diagnosed with A-ROP (Group II). Of the 30 infants treated with LPC, 58 eyes of 29 infants had zone II ROP and one eye of one infant had zone III ROP. Of the 15 infants treated with IVB, 14 eyes of eight infants had zone I ROP and 13 eyes of seven infants had zone II ROP.
Treatments were administered up to a mean PMA of 36.0 ± 2.4 (range: 31–42 weeks) and 35.3 ± 2.6 (range: 32–40 weeks) weeks in Group I and Group II, respectively (P = 0.32). No complications, such as iatrogenic cataract, intraocular inflammation, retinal detachment, and vitreous hemorrhage, were observed due to the treatment modality applied to the patients.
In the follow-up examinations, 1-year spherical, cylindrical, and SE values were 0.1 ± 2.2 D, − 1.2 ± 0.9 D and − 0.5 ± 2.0 D in Group I and 1.3 ± 1.7 D, − 1.1 ± 0.8 D and 0.8 ± 1.7 D in Group II, respectively (P = 0.018 for spherical, P = 0.772 for cylindrical, and P = 0.009 for SE). Myopic refraction was significantly greater in Group I than in Group II (P = 0.018). Demographic data of infants and corrected first age refraction values of infants are presented in Table 1.
The mean spherical power and spherical equivalent were significantly different between the two groups for zone II (p = 0.005 and p = 0.002). However, no significant difference was found in the mean cylindrical power (p = 0.648). Refraction values of infants according to ROP zones in Group 1 and Group 2 patients are presented in Table 2.
In eyes treated with IVB, mean spherical power and spherical equivalent were found to be significantly lower in zone I than in zone II (p = 0.04 and p = 0.034). In addition, when we compared the effects of treatment stages in the development of myopia in the eyes treated with LPC, we found that the SE values in Stage 2 and Stage 3 patients were 0.1 ± 1.2 D and − 1.1 ± 2.5 D. Infants with Stage 3 ROP were found to be significantly more myopic than infants in Stage 2 ROP (p = 0.03).
The distribution of refraction results grouped as very high myopia (≤ − 8 D) to high hyperopia (≥ + 4 D) is shown in Fig. 1. Emmetropia (− 1.0 to + 1.0 D) and low hyperopia (≥ + 1.0 to + 4.0 D) were significantly higher in eyes treated with IVB (p = 0.008 and p = 0.001). Furthermore, linear regression analysis revealed no significant correlation between mean SE of gestational age and birth weight (p = 0.25 and p = 0.54).
Discussion
The present study observed lower SE values following laser photocoagulation therapy (− 0.5 ± 2.0 D) compared to IVB therapy (0.8 ± 1.7 D). The mean spherical power and spherical equivalent were found to be significantly lower in zone I than in zone II in the group treated with IVB. In addition, when the effects of treatment stages in the development of myopia in patients treated with laser were compared, eyes with Stage 3 ROP were found to be significantly more myopic than eyes with Stage 2 ROP.
In recent years, a series of studies evaluating refractive outcomes following IVB and laser therapy have shown that IVB is associated with a lower rate of refractive errors in ROP compared to laser ablation [17,18,19,20]. Similarly, Harder et al. [18] reported that the mean SE in laser-treated infants was − 4.4 ± 1.7 D, which is much more myopic than − 1.04 ± 4.24 D in the IVB-treated preterm group.
By contrast, some studies reported that refractive errors did not differ significantly between anti-VEGF drug and laser treatment [21,22,23]. Although the cause of myopization in children treated with ROP has not been fully understood to date, Fielder and Quinn suggested that ROP treatment-related myopia may develop due to the physical restriction of anterior segment development of the damaged peripheral retina caused by laser therapy [24]. On the contrary, it has been suggested that IVB treatment blocks VEGF that is already produced and present in the vitreous [20], and after treatment, retinal development may maintain the local growth factor expression, providing the continuation of signaling pathways, and thus allowing the anterior segment to develop normally, leading to a decrease in myopia [17].
Hwang et al. [19] reported mean cylindrical values of 1.6 ± 1.5 D and 1.0 ± 0.8 D in eyes treated with laser and IVB, respectively. No significant difference was found between the groups in terms of cylindrical values in eyes with zone I and zone II ROP (for zone I p = 0.13; for zone II p = 0.19). On the other hand, Harder et al. [18] reported that refractive astigmatism was significantly lower in the single-dose IVB group compared to the conventional retinal laser photocoagulation group (−1.00 ± 1.04 D for IVB, 1.82 ± 1.41 D for laser, p = 0.03). However, in this study, no significant difference was found between LPC therapy (− 1.2 ± 0.9 D) and IVB therapy (− 1.1 ± 0.8 D) in terms of astigmatism. The difference in the distribution of patients in zone I and zone II between treatment groups and the absence of patients in zone I in patients treated with LPC may have caused the indifferences between groups in astigmatism.
In the BEAT-ROP clinical trial comparing the refractive results between IVB and laser therapy, it was reported that eyes with zone I ROP (− 1.51 D for IVB therapy and − 8.44 D for laser therapy) had a higher degree of myopia than eyes with zone II ROP (− 0.58 D and − 5.83 D for IVB therapy), but no difference was found for the IVB arm or the laser arm. Therefore, the authors thought that the severity of the disease was not the main factor influencing the severity of myopia [17]. Hwang et al. [19] evaluated refractive errors according to the zones, and it was reported that eyes with zone I ROP were more myopic than eyes with zone II ROP, especially in the IVB group. Similarly, Gunay et al. [25] reported that eyes with zone I ROP were more myopic than eyes with zone II ROP in both the IVB and laser groups. In this study, when the effects of treatment zones in the development of myopia were compared, in patients treated with IVB, eyes with zone I ROP were found to be significantly more myopic than eyes with zone II ROP (p = 0.04).
Davitt BV et al. [26] suggested that the prevalence of myopia and high myopia increased in association with the presence of plus disease and increasing ROP stage (severity).
On the other hand, Pennefather et al. [27] showed that advanced ROP (stage 3 or worse) is associated with the development of > − 0.25 D myopia, but in lesser ROP, this risk is no greater than it is in the general population.
Consistent with the literature, infants with Stage 3 ROP were found to be significantly more myopic than infants in Stage 2 ROP (p = 0.03) in our study.
This study includes limitations such as retrospective design, small sample size, lack of ocular biometry, and follow-up time. In this study, only refractive error was evaluated. Therefore, there may also be a need for other biometric measures to evaluate the myopia associated with ROP [28]. The follow-up period of approximately 1 year did not allow conclusions be drawn about the long-term effects and side effects of the treatment.
In conclusion, this study supports that even 0.625 mg intravitreal bevacizumab for ROP causes less myopia compared to laser photocoagulation and that myopia is higher in patients with advanced ROP. In future, larger and longer-term studies are needed to elucidate the effect of laser photocoagulation or IVB therapy on refractive error in eyes with ROP.
References
Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C (2013) Preterm-associated visual impairment and estimate of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res 74(1):35–49
Chen J, Smith LEH (2007) Retinopathy of prematurity. Angiogenesis 10:133–140
Hoogerwerf A, Schalij-Delfos NE, van Schooneveld MJ, Termote JU (2010) Incidence of retinopathy of prematurity over the last decade in the Central Netherlands. Neonatology 98:137–142
Isaza G, Arora S, Bal M, Chaudhary V (2013) Incidence of retinopathy of prematurity and risk factors among premature infants at a neonatal intensive care unit in Canada. J Pediatr Ophthalmol Strabismus 50:27–32
Good WV (2004) Early treatment for retinopathy of prematurity cooperative group final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc 102:233–250
Kong Q, Ming W, Mi X-S (2021) Refractive outcomes after intravitreal injection of antivascular endothelial growth factor versus laser photocoagulation for retinopathy of prematurity: a meta-analysis. BMJ Open 11:e042384
Shah PK, Ramakrishnan M, Sadat B, Bachu S, Narendran V, Kalpana N (2014) Long term refractive and structural outcome following laser treatment for zone 1 aggressive posterior retinopathy of prematurity. Oman J Ophthalmol 7(3):116–119
Suk KK, Berrocal AM, Murray TG, Rich R, Major JC, Hess D et al (2010) Retinal detachment despite aggressive management of aggressive posterior retinopathy of prematurity. J Pediatr Ophthalmol Strabismus 47:1–4
Sankar MJ, Sankar J, Chandra P (2018) Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity. Cochrane Database Syst Rev 1:Cd009734
Vander Veen DK, Melia M, Yang MB, Hutchinson AK, Wilson LB, Lambert SR (2017) Anti-vascular endothelial growth factor therapy for primary treatment of type 1 retinopathy of prematurity: a report by the American Academy of ophthalmology. Ophthalmology 124:619–633
Mintz-Hittner HA, Kennedy KA, Chuang AZ (2011) BEAT-ROP cooperative group efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 364:603
Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Paul Chan RV, Berrocal A et al (2021) International classification of retinopathy of prematurity. Third Edition Ophthalmol 128(10):e51–e68. https://doi.org/10.1016/j.ophtha.2021.05.031
Schalij-Delfos NE, de Graaf ME, Treffers WF, Engel J, Cats BP (2000) Long-term follow up of premature infants: detection of strabismus, amblyopia, and refractive errors. Br J Ophthalmol 84:963–967
Leung MP, Thompson B, Black J, Dai S, Alsweiler JM (2018) The effects of preterm birth on visual development. Clin Exp Optom 101(1):4–12
Yang CS, Wang AG, Shih YF, Hsu WM (2013) Long term biometric optic components of diode laser-treated threshold retinopathy of prematurity at 9 years of age. Acta Ophthalmol 91:276–282
Katoch D, Sanghi G, Dogra MR, Beke N, Gupta A (2011) A structural sequelae and refractive outcome 1 year after laser treatment for type 1 pre-threshold retinopathy of prematurity in Asian Indian eyes. Indian J Ophthalmol 59:423–426
Goktas A, Sener EC, Sanac AS (2012) An assessment of ocular morbidities of children born prematurely in early childhood. J Pediatr Ophthalmol Strabismus 49:236–241
Geloneck MM, Chuang AZ, Clark WL, Hunt MG, Norman AA, Packwood E et al (2014) BEAT-ROP cooperative group refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol 132(11):1327–33
Harder BC, Schlichtenbrede FC, von Baltz S, Jendritza W, Jendritza B, Jonas JB (2013) Intravitreal bevacizumab for retinopathy of prematurity: refractive error results. Am J Ophthalmol 155(6):1119–1124
Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR (2015) Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology 122(5):1008–1015
Gunay M, Celik G, Gunay BO, Aktas A, Karatekin G, Ovali F (2015) Evaluation of 2-year outcomes following intravitreal bevacizumab (IVB) for aggressive posterior retinopathy of prematurity. Arq Bras Oftalmol 78(5):300–304
Isaac M, Mireskandari K, Tehrani N (2015) Treatment of type 1 retinopathy of prematurity with bevacizumab versus laser. J AAPOS 19(2):140–144
Kuo HK, Sun IT, Chung MY, Chen YH (2015) Refractive error in patients with retinopathy of prematurity after laser photocoagulation or bevacizumab monotherapy. Ophthalmologica 234(4):211–217
Kabatas EU, Kurtul BE, Altiaylik Ozer P, Kabatas N (2017) Comparison of intravitreal bevacizumab, intravitreal ranibizumab and laser photocoagulation for treatment of type 1 retinopathy of prematurity in Turkish preterm children. Curr Eye Res 42(7):1054–1058
Fielder AR, Quinn GE (1997) Myopia of prematurity: nature, nurture, or disease? Br J Ophthalmol 81:2–3
Gunay M, Sukgen EA, Celik G, Kocluk Y (2017) Comparison of bevacizumab, ranibizumab, and laser photocoagulation in the treatment of retinopathy of prematurity in Turkey. Curr Eye Res 42(3):462–469
Davitt BV, Dobson V, Good WV, Hardy RJ, Quinn GE, Siatkowski RM et al (2005) Early treatment for retinopathy of prematurity cooperative Group Prevalence of myopia at 9 months in infants with high-risk pre-threshold retinopathy of prematurity. Ophthalmology 112(9):1564–8
Pennefather PM, Tin W, Strong NP, Clarke MP, Dutton J, Cottrell DG (1997) Refractive errors in children born before 32 weeks gestation. Eye 11:736–743
Lenis TL, Gunzenhauser RC, Fung SSM, Dhindsa YK, Sarraf D, Pineles SL et al (2020) Myopia and anterior segment optical coherence tomography findings in laser-treated retinopathy of prematurity eyes. J AAPOS 24(2):86
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by EKY, Caner Kara, and İSP. The first draft of the manuscript was written by EKY, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interest to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University of Health Science (2022/01).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kıran Yenice, E., Petriçli, İ.S. & Kara, C. One-year refractive outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity. Int Ophthalmol 43, 2197–2202 (2023). https://doi.org/10.1007/s10792-022-02615-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-022-02615-9