Abstract
Purpose
To evaluate the factors that may be associated with refractive outcome in eyes treated with intravitreal bevacizumab (IVB) injection for retinopathy of prematurity (ROP).
Methods
Retrospective case series. Refractive outcomes of 181 infants who were treated with primary IVB for Type I ROP or aggressive ROP, were retrospectively evaluated. According to the pretreatment retinal vascularization, eyes were classified into zone I, zone I–zone II, and zone II groups. The first year, third year, and final refractive error were analyzed. Univariate logistic regression test was performed to evaluate the effect of factors on the development of ≥ 1 diopter (D) myopia.
Results
At the final examination, the mean age was 22.9 ± 10.9 months. The zone II group was more hyperopic than the zone I-zone II and zone I zone groups (P = 0.001). Of the 331 eyes, 17 eyes (5.1%) had high myopia, 50 eyes (15.1%) had low myopia, and 83 eyes (25.1%) had emmetropia. During follow-up, 110 (33.2%) eyes underwent laser treatment. Gestational age, birth weight, neonatal intensive care unit type, the presence of additional laser treatment, number of injections, the type of ROP, and the dose of IVB were not associated with the development of ≥ 1 D myopia. The pretreatment and prelaser retinal zones were associated with the development of ≥ 1 D myopia.
Conclusion
The most important factors affecting the refractive outcome in infants who underwent primary IVB treatment was the extent of pretreatment and prelaser retinal vascularization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Retinopathy of prematurity (ROP) is a disease that may cause permanent vision loss due to the abnormal development of retinal vessels. Under the guidance of randomized and controlled studies conducted in the 1990s to date, anatomical outcomes have gradually improved with cryotherapy, laser, and anti-vascular endothelial growth factor (VEGF) treatments [1,2,3]. In addition to anatomical outcomes, the refractive outcomes of the treatments affect the visual rehabilitation of premature infants [4].
In a prospective randomized study, it has been shown that anti-VEGF treatment induces less myopia in Zone I and posterior Zone II cases [5]. Previously, low birth weight (BW) and ROP severity were found to be independent risk factors for myopia development, even if the disease regressed spontaneously without treatment [6, 7]. Furthermore, the refractive outcomes of anti-VEGF-treated eyes were shown to be likely affected by many factors [8, 9].
Whereas severe ROP disease is observed in infants with very low BW and gestational age (GA) in high-income countries, severe ROP disease may develop even in heavier and more mature infants in low- and middle-income countries [10,11,12,13,14]. In the clinic where the presented study was conducted, the majority of treated infants are hospitalized in neonatal intensive care units (NICUs) of private hospitals [15]. In these NICUs, severe ROP disease may develop even in high birth-weight infants [11, 15].
The aim of our study, which includes a large cohort of infants hospitalized in different types of NICUs, was to investigate the effect of GA, BW, the type of NICU, pretreatment retinal zone, presence of additional intravitreal bevacizumab (IVB), presence of additional laser treatment, prelaser retinal zone, and age at laser treatment on refractive outcomes, in eyes treated with IVB.
Methods
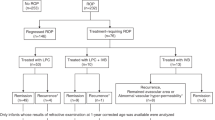
The study was conducted in accordance with the tenets of the Declaration of Helsinki. The patient charts of 283 infants who were treated (by SEB or NS) for type I ROP or Aggressive-ROP (A-ROP) between January 2016 and December 2019 were retrospectively evaluated.
The inclusion criteria were as follows: (1) Only eyes treated with IVB monotherapy as initial treatment; (2) Eyes for which at least one refractive measurement was recorded in the patient chart.
Eyes whose primary treatment was laser, vitrectomy, and laser combined with IVB were excluded.
All patients' GA, BW, IVB dose, total treatment regimen, type of NICU, pretreatment retinal zone, prelaser retinal zone, age at laser treatment, and recorded refractive parameters during follow-up were retrospectively evaluated.
In this manuscript, the term "pretreatment" has been used for describing the period, prior to the first injection of IVB in infants even if they have received additional treatments. The retinal zone was determined with a 28-diopter lens according to the guidance of the International Committee for Classification of ROP (ICROP) revisited report [16]. The location of the disease was determined by evaluating the extent of pretreatment retinal vascularization in all quadrants. As shown in Fig. 1, according to the pretreatment retinal zone, eyes were grouped into zone I, zone I–zone II, and zone II groups.
The three subgroups according to pretreatment retinal zone, each encompassing the previous one, are illustrated in the figure. A line was drawn from the nasal ora serrata to the temporal ora serrata to show the retinal zones. A circle with a central optic disk and a radius of two optic disk-to-fovea distance (ODF) is drawn to separate zone I and zone II. With the gradual evaluation, the three subgroups are described as follows. (1) Zone I: Pretreatment retinal vascularization was in zone I in all quadrants (area surrounded by red dashed line). (2) Zone I–zone II: Pretreatment retinal vascularization was in zone I in at least one quadrant and zone II in other quadrants (area surrounded by yellow dashed line). (3) Zon 2: Pretreatment retinal vascularization was in zone II in all quadrants (area surrounded by blue dashed line)
The eyes were analyzed into two groups according to the presence of additional laser treatment. The IVB group was composed of eyes treated with IVB monotherapy or repeated IVB injections. The IVB + laser group was composed of eyes treated with an additional laser after primarily bevacizumab treatment.
In the IVB + laser group, eyes were divided into three subgroups according to the age at laser treatment. Eyes that underwent laser treatment under 45 weeks postmenstrual age (PMA), between 45 and 60 weeks PMA, and after 60 weeks PMA were defined as the early, intermediate, and late laser groups.
In hospitals where ROP follow-up or treatment could not be applied, hospitalized infants were being brought to the outpatient clinic of the study center for examination or treatment with a transport incubator. NICU of the study hospital was defined as Type 1 NICU, and NICUs of the nonstudy hospitals were defined as Type 2 NICU. The NICU of the study hospital was graded as level 4, the NICUs of the nonstudy hospitals were graded as ≤ level 3 by the Ministry of Health of Turkey.
The treatment decision, zone classification, treatment, and refractive measurements of all patients included in the study were performed by two ROP experts (S.E.B. and N.S). In the study clinic, IVB monotherapy was the primary treatment method in all zone I and posterior zone II eyes, and IVB was also the partially preferable treatment method in peripheral zone II eyes. The treatment methods, follow-up procedure, and refractive measurement technique included in the study are described as follows.
After topical anesthesia, an injection of Altuzan (Roche, Basel, Switzerland) 0.625 mg or 0.3125 mg was introduced into the vitreous from 1.5 mm to the limbus with a 30-gauge 4 mm or 6 mm needle. All intravitreal injections were performed in the surgery room by providing the necessary sterility conditions [17]. Topical moxifloxacin eye drop prescribed for 1 week eight times a day. After the injection, a control examination was performed within days 1–3. Weekly visits were performed for eyes with a response to treatment. If there was no sign of recurrence at the first month of treatment, the follow-up intervals were extended by 1 week. For the infants younger than 60 weeks PMA, if recurrence was detected, IVB or laser treatment was performed according to the extent of retinal vascularization. Fluorescein angiography (FA) was performed in eyes with signs of recurrence or persistent avascular retina (PAR) after 60 weeks PMA. For the infants older than 60 weeks PMA, laser treatment was performed in all eyes with hyperfluorescent leakage and prophylactic laser was performed to some of the eyes with PAR. The main criteria for laser treatment for the eyes with PAR are as follows: (1) accompanying hyperfluorescent leakage on FA; (2) if the width of avascular retina between the vascular termination to the ora serrata was longer than four times of optic disc diameter and if the PAR is accompanied by moderate-severe pigmentary changes; (3) the patients with a risk of not continuing follow-up. Treatment decision for the eyes with PAR was constituted with the consultation and informed consent of the legal guardians of the patients and with an individualized patient-specific approach.
The cycloplegic refractive error was measured by using the Plusoptix A09 (Plusoptix GmbH, Nuremberg, Germany) device. If the measurement values were out of range or the measurements could not be taken due to excessive pupil dilation, retinoscopy was performed by using streak retinoscopy and retinoscopy bars.
The measurement recorded in the patient's chart at the nearest time to the postnatal age of 12 months and 36 months was defined as the first-year SE (between 6 and 24 months) and third-year SE (between 24 and 48 months), respectively. The last refractive measurement recorded in the patient's chart was defined as the final SE.
Refractive errors were divided into 6 categories as described in previous studies as follows: very high myopia (≤ − 8 Diopter (D), high myopia (< − 8 to − 5 D), low myopia (< − 5 to − 1 D), emmetropia (< − 1 to 1 D), low hyperopia (≥ + 1 to + 4 D), and high hyperopia (≥ + 4D) [18].
Statistical analysis
Statistical analysis was performed with SPSS (SPSS Inc, PASW Statistics for Windows, Version, 18.0, Chicago, USA) software. The distribution of parameters was checked by the Kolmogorov–Smirnov test. If data were normally distributed, parametric tests were used to analyze variables (one-way ANOVA or independent t test). If one of the groups was not normally distributed, nonparametric tests were used to analyze variables (Kruskal–Wallis or Mann–Whitney U). A P value of less than 0.05 was considered statistically significant. When a significant difference was found between groups with one-way ANOVA, the post hoc analysis test method was determined according to the homogeneity of the variances. For the analyses in which three groups were compared using the Kruskal–Wallis test, the significance of the difference between the groups was evaluated with the Mann–Whitney U test. In post hoc analysis, a P value lower than 0.017 (0.05/3 = 0.017) with Bonferroni correction was considered statistically significant. For dependent variables, nonparametric variables were evaluated using the Wilcoxon test. Univariate logistic regression analysis was performed to analyze the factors that may be effective for the development of ≥ 1 D myopia.
Results
The refraction measurements of 334 eyes of 182 infants, who had been treated with IVB, were accessible in the patient charts. Two eyes of one infant were excluded due to bilateral vitreoretinal surgery and lens extraction in one eye. Two infants had unilateral lens opacities. The eye that underwent cataract surgery was excluded because of central lens opacity that obscured the visual axis. Eye with peripheral lens opacity and whose refraction could be measured was included. Further analyses were carried out on 331 eyes of 181 infants.
The mean GA and BW of 181 infants were 28.6 ± 2.6 weeks and 1252 ± 417 g, respectively. In the first year, third year, and final examination, refraction measurements of 329, 125, and 331 eyes were evaluated, respectively. The mean ages of the first year, third year, and final examination were 12.9 ± 3.8, 31.4 ± 5.8, and 22.9 ± 10.9 months, respectively. Of the treated eyes, 104 (31%) were diagnosed with A-ROP, and 227 (69%) were diagnosed with type 1 ROP. The dose of bevacizumab was 0.625 mg in 70% of eyes and 0.3125 mg in 30% of eyes. During follow-up, 223 eyes underwent FA, and 110 eyes underwent additional laser treatment.
Fourteen infants had been hospitalized in the type 1 NICU, and 167 infants had been hospitalized in the type 2 NICU. Nonstudy hospitals consist of 45 different hospitals, and most of them were located in northwestern Turkey.
The last examination revealed high myopia in 17 eyes (5.1%), low myopia in 50 eyes (15.1%), emmetropia in 83 eyes (25.1%), low hyperopia in 167 eyes (50.5%), and high hyperopia in 14 eyes (4.2%). Logistic regression analysis was performed to determine the factors associated with ≥ 1 D myopia. Gestational age, BW, NICU type, the number of IVB injections, the dose of IVB injections, the type of ROP, the presence of additional laser treatment, and the age at laser treatment were not associated with the development of ≥ 1 myopia (Table 1). The pretreatment retinal zone and prelaser retinal zone were associated with the development of ≥ 1 myopia.
Macular dragging (straightened temporal retinal vessels and macular heterotopia) was noted in only 2 eyes during follow-up. One eye with mild straightened temporal retinal vessels had − 0.25 D myopia and the other eye with macular heterotopia had − 7.50 D myopia at final examination.
Comparison of refractive outcomes according to the pretreatment retinal zone
In comparison with pretreatment retinal vascularization, the first year and final SE were significantly different between the different zone groups (Table 2). In the post hoc analysis of the final SE outcomes, a statistically significant difference was found between the zone II and zone I groups and between the zone II and zone I-zone II groups.
Comparison of refractive outcomes according to the presence of additional laser treatment
One hundred and ten eyes underwent additional laser treatment. Two doses of IVB were administered to 15 eyes in the IVB group and 10 eyes in the IVB + laser group. The mean age at laser treatment was 62 ± 26 weeks PMA. In the IVB + laser group, GA and BW were significantly lower, but refractive results were similar in both groups (Table 3). Pretreatment zones were similar between groups.
In the IVB + laser group, prelaser retinal vascularization was in the zone II in all quadrants in 93.6% of eyes, and prelaser retinal zone was more anterior than the pretreatment retinal zone (P = 0.000 Wilcoxon).
Subgroup analysis of laser treated eyes
In the early and intermediate laser groups, laser was performed due to early treatment failure, reactivation of the disease, or hyperfluorescent leakage. In the late laser group, 20 eyes were treated due to hyperfluorescent leakage, and 26 eyes were treated due to PAR despite the absence of any significant vascular activity on FA.
The mean age at the final refraction examination was 24.6 ± 13.2 months. The average duration time between the laser and the final refraction examination was 16.8 ± 11.6 months. First-year SE, third-year SE, and final SE results were similar between the groups (Table 4). The prelaser retinal vascularization was in zone II in 86%, 93%, and 100% of the eyes in the early, intermediate and late laser groups, respectively. In all subgroups, the prelaser retinal zone was significantly more advanced than the pretreatment retinal zone (P = 0.000; Wilcoxon).
Comparison of refractive outcomes according to the type of NICU
Patients in the Type 1 NICU had significantly lower GA and BW than patients in the Type 2 NICU. There was no statistically significant difference between the two groups in terms of the first year and third year of SE; however, the final SE was found to be hyperopic in the Type 1 NICU group (Table 5). Pretreatment retinal vascularization were similar between the groups (P = 0.553).
In the Type 1 NICU group, none of the infants underwent treatment with a BW of more than 1500 g. A color fundus photograph of an infant born at 33 weeks PMA with a BW of 2200 g and underwent IVB treatment, hospitalized in the Type 2 NICU, is presented (Fig. 2).
The pretreatment photographs at 39 weeks postmenstrual age (PMA) of the large preterm infant who has been hospitalized in the neonatal intensive care unit of a nonstudy hospital are presented (2200 g, 33 weeks at birth). A, C On the left side, posterior pole focused wide-angle color photographs of both eyes are shown. B, D On the right side, extraretinal fibrovascular proliferations are shown (white dashed rectangle) on the ridge focused photographs. Infant was diagnosed as bilateral Type 1 retinopathy of prematurity in the zone II with stage 2–3 and plus disease
Comparison of refractive outcomes according to gestational age and birth weight
In 270 eyes of 146 infants, GA was lower than 32 weeks. There was no statistically significant difference between the two groups in terms of first, third year, or final SE (Table 6). Pretreatment retinal vascularization were similar between the groups (P = 0.221). In 244 eyes of 131 infants, BW was ≤ 1500 g. The mean SE at the first year, third year, and last visit were similar between the BW ≤ 1500 g and BW > 1500 g groups (Table 6). Pretreatment retinal vascularization was similar between the groups (P = 0.525).
Discussion
Our results support that the most important factor affecting refractive outcomes in eyes treated with IVB is pretreatment retinal vascularization. In our heterogeneous cohort, infants who had been hospitalized in different types of neonatal intensive care units, BW, and GA were not related to refractive outcomes. Additional laser treatment and age at laser treatment were not related to refractive outcomes. For all that, prelaser retinal vascularization was found to may affect the refractive outcomes.
It has been shown that progression of retinal vascularization, which starts from the optic nerve head, is not symmetrical, and there is nasotemporal asymmetry during the development of the retinal vasculature [19]. Nasotemporal asymmetry may cause confusion in the determination of zones [20]. In the ICROP 2005, it was not clearly specified that zone determination should be performed according to the which border (posterior–anterior) of retinal vascularization [16, 21]. At the time of clinical practice, we noted the anterior and posterior extent of the retinal vascularization in eyes whose vascularization was in both zone I and zone II. To our knowledge, it has not been investigated whether this intermediate zone differs from zone I or zone II in terms of refractive outcomes in IVB-treated eyes. In our cohort, the zone II eyes were more hyperopic than the zone I–zone II and zone I eyes. However, the final refraction did not differ between the zone I–zone II and zone I eyes.
Mueller et al. reported that myopia was higher in the posterior zone than in the peripheral zone II, regardless of the type of treatment [22]. In that study, the eyes in zone I ROP and posterior zone II ROP were considered the 'posterior zone' and were compared with the peripheral zone (the more peripheral part of zone II). In a retrospective study in which 28 eyes were treated with IVB for zone II ROP, the mean SE was reported as 1.99 D at the last visit, and this outcome was similar to zone 2 eyes of our study [23].
In the presence of recurrence or PAR in eyes treated with primary IVB, laser photocoagulation may be considered to the peripheral avascular retina [24, 25]. In a study conducted with eyes with Type 1 ROP in zone I, it has been reported that IVB with deferred laser treatment (27 eyes, mean age at laser treatment was 43 weeks PMA) resulted in less myopic refractive error than combined IVB with zone I sparing laser [26]. In infants with posterior type 1 ROP, between the comparison of 34 eyes treated with primary laser and 40 eyes treated with primary IVB with delayed laser (after 60 weeks PMA), it has been shown that primary laser eyes had significant myopia [27]. In the aforementioned study, refractive outcomes of eyes treated with IVB monotherapy (8 eyes) and with IVB plus delayed laser (after the 60 weeks PMA) were similar. The limitations of that study were the small sample size and the lack of analysis according to the age at the laser treatment. Furthermore, to the best of our knowledge, the effect of the timing of laser treatment on refractive error in eyes treated with primary IVB was not adequately investigated. In the univariate analyses of our study, the presence of additional laser treatment and the timing of laser treatment were not related with the development of ≥ 1 D myopia. In addition, final SE were similar between early, intermediate, and late laser groups. The facts that the final refraction age of the late laser group was higher than that of the early laser group, and the pretreatment retinal zone was more advanced in the late laser group compared to the other groups might also have affected group comparisons.
In our study cohort, in the presence of recurrence and if retinal vascularization was in zone I in the majority of the quadrants, the second dose of IVB was performed. In the IVB + laser group, prelaser retinal vascularization was significantly more advanced than retinal vascularization at the time of the first treatment. Univariate analysis showed that prelaser retinal vascularization was significantly associated with ≥ 1 D myopia. Based on all these data, it is conceivable that prelaser retinal vascularization affects the final refraction rather than the presence of additional laser application and the week of laser application.
In previous reports, the fact that BW and the severity of ROP were reported to be related to myopia even in untreated preterm infants complicates the interpretation of our results [6, 28]. Severe ROP disease typically occurs in infants with a very low GA and BW in developed countries, whereas it may develop in larger preterm infants in developing countries [11, 29, 30]. The development of severe ROP, even in larger preterm infants hospitalized in the Type 2 NICUs, might have increased our study cohort's average GA and BW. The fact that the same clinicians treated infants hospitalized in both types of NICUs according to the same criteria, we consider that the treatment threshold were similar between the groups. In a study previously reported in our study hospital, it was shown that mean GA and BW were significantly higher in infants from external NICUs [15]. In a multicenter, prospective study conducted in our country, it was stated that severe ROP disease that requires treatment in infants with higher GA and BW is originated in private hospital NICUs, and this situation may be related to intensive care conditions, especially excessive oxygen administration [11]. In the Type 1 NICU group, in which GA and BW were found significantly lower in our study, first-year and third-year SE were not different compared to the Type 2 NICU. Furthermore, the final SE was more hyperopic in the Type 1 NICU group. In addition, in comparisons that were performed according to the BW and GA, the refractive outcomes were similar between the groups. These findings suggest that, in our heterogeneous cohort, most of which consisted of infants hospitalized in the NICU of nonstudy hospitals, GA and BW did not affect the refractive outcome independently. In infants hospitalized in NICUs where intensive care conditions are homogeneous and adequate, GA and BW may be related to severe and posterior located ROP, and GA and BW may be related to refractive outcomes. Therefore, it would be more appropriate to limit the results of our study to infants treated with IVB in a heterogeneous cohort. In addition, it should not be ignored that there may be referral bias in infants referred for treatment. On the other hand, when each NICU is evaluated within itself, we consider that GA and BW may affect the refractive results in relation to the severity of ROP.
In randomized controlled studies, it has been demonstrated that the highest risk for the development of high myopia is narrowing of the temporal vascular arcade and macular heterotopia [6, 31]. In our study, only two of the treated eyes were noted as macular dragging, which might have contributed to the decreasing prevalence of high myopia. Performing IVB in the early stage of the disease may result in a lower rate of macular dragging. A major advantage of IVB monotherapy compared with laser treatment is the less destructive procedure and faster regression of neovascularization by blocking VEGF in the vitreous [22]. Compared to laser therapy, IVB monotherapy may lead to faster regression of acute disease, resulting in less myopia.
Consisting of a large sample size, the evaluation of the eyes whose vascularization was in both zone I and zone II in a separate subgroup, and the evaluation of the effect of additional IVB and additional laser treatments was the remarkable features of our study. Other notable aspects are evaluating the effect of age at additional laser treatment and prelaser retinal vascularization on refractive outcomes. Variation in diagnosis and classification of ROP disease was minimal because all infants were treated in a single center and by two clinicians, and most of the decisions were made together.
The retrospective and nonrandomized design of the study was the major limitation of the study. The lack of third-year refraction results in majority of the patients, and higher standard deviation of the examination age for the third year and final outcomes were the limitations of the study. In addition, in the majority of patients the refraction measurement was taken with only plusoptix device instead of in combination with retinoscopy, and visual acuity not assessed were the other limitations of our study. Zone classification was carried out according to the pretreatment examination performed by indirect ophthalmoscope. Since wide-angle fundus photography device became available at April 2018, zone determination was carried out by only examination performed by indirect ophthalmoscope before this date. Although analyzing the results of infants hospitalized in different types of NICUs provided data to understand the effect of intensive care conditions on the refractive outcome, it was also a limitation to generalize our results.
In conclusion, in cases of recurrence in eyes whose retinal vascularization has not improved adequately, a second dose of IVB may not directly affect the refractive outcome; furthermore, it may lead to the progression of retinal vascularization and reduce the myopic effect of subsequent laser treatment. In the presence of recurrence, the extent of retinal vascularization and the possible systemic risks of a second dose of IVB should be considered together. Birth weight and GA may not be independent parameters influencing refractive outcomes in infants treated with IVB. Delayed laser treatment may not affect the final refraction in eyes whose retinal vascularization was in zone II in all quadrants. In eyes with an additional laser, prelaser retinal vascularization may be a predictive parameter rather than the age at the laser treatment. Our study suggests that, in infants who underwent primary IVB injection, pretreatment retinal vascularization is the most critical determinant of refractive outcomes.
References
Cryotherapy for Retinopathy of Prematurity Cooperative Group (1990) Multicenter trial of cryotherapy for retinopathy of prematurity: one-year outcome: structure and function. Arch Ophthalmol 108(10):1408–1416. https://doi.org/10.1001/archopht.1990.01070120056029
Early Treatment for Retinopathy of Prematurity Cooperative Group (2003) Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 121:1684–1695. https://doi.org/10.1001/archopht.121.12.1684
Mintz-Hittner HA, Kennedy KA, Chuang AZ (2011) Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 364(7):603–615. https://doi.org/10.1056/NEJMoa1007374
Lok JY, Yip WW, Luk AS, Chin JK, Lau HH, Young AL (2018) Visual outcome and refractive status in first 3 years of age in preterm infants suffered from laser-treated type 1 retinopathy of prematurity (ROP): a 6-year retrospective review in a tertiary centre in Hong Kong. Int Ophthalmol 38(1):163–169. https://doi.org/10.1007/s10792-016-0439-5
Geloneck MM, Chuang AZ, Clark WL et al (2014) Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA ophthalmology 132(11):1327–1333. https://doi.org/10.1001/jamaophthalmol.2014.2772
Quinn GE, Dobson V, Repka MX et al (1992) Development of myopia in infants with birth weights less than 1251 grams. The cryotherapy for retinopathy of prematurity cooperative group. Ophthalmology 99(3):329–40. https://doi.org/10.1016/S0161-6420(92)31968-2
O’Connor AR, Stephenson TJ, Johnson A, Tobin MJ, Ratib S, Fielder AR (2006) Change of refractive state and eye size in children of birth weight less than 1701 g. Br J Ophthalmol 90(4):456–460. https://doi.org/10.1136/bjo.2005.083535
Harder BC, Schlichtenbrede FC, von Baltz S, Jendritza W, Jendritza B, Jonas JB (2013) Intravitreal bevacizumab for retinopathy of prematurity: refractive error results. Am J Ophthalmol 155(6):1119–24.e1. https://doi.org/10.1016/j.ajo.2013.01.014
Mintz-Hittner HA, Geloneck MM (2016) Review of effects of anti-VEGF treatment on refractive error. Eye and brain 8:135
Gilbert C (2008) Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Human Dev 84(2):77–82. https://doi.org/10.1016/j.earlhumdev.2007.11.009
Bas AY, Demirel N, Koc E, Ulubas Isik D, Hirfanoglu İM, Tunc T (2018) Incidence, risk factors and severity of retinopathy of prematurity in Turkey (TR-ROP study): a prospective, multicentre study in 69 neonatal intensive care units. Br J Ophthalmol 102(12):1711–1716. https://doi.org/10.1136/bjophthalmol-2017-311789
Isaza G, Arora S, Bal M, Chaudhary V (2013) Incidence of retinopathy of prematurity and risk factors among premature infants at a neonatal intensive care unit in Canada. J Pediatr Ophthalmol Strabismus 50(1):27–32. https://doi.org/10.3928/01913913-20121127-02
Nicoara S-D, Cristian C, Irimescu I, Stefanut A-C, Zaharie G (2014) Diode laser photocoagulation for retinopathy of prematurity: outcomes after 7 years of treatment. J Pediatr Ophthalmol Strabismus 51(1):39–45. https://doi.org/10.3928/01913913-20131112-02
Al-Khaled T, Valikodath NG, Patel SN et al (2021) Addressing the third epidemic of retinopathy of prematurity through telemedicine and technology: a systematic review. J Pediatr Ophthalmol Strabismus 58(4):261–269. https://doi.org/10.3928/01913913-20210223-01
Erşan HBA, Pinhan D, Kavuncuoğlu S, Sayın N, Bayramoğlu SE, Çetinkaya M (2018) The demographic and clinical characteristics of the patients treated for type 1 retinopathy of prematurity. İKSST Derg 10(2):81–86. https://doi.org/10.5222/iksst.2018.14622
International Committee for the Classification of Retinopathy of Prematurity (2005) The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 123(7):991. https://doi.org/10.1001/archopht.123.7.991
Grzybowski A, Told R, Sacu S et al (2018) 2018 update on intravitreal injections: Euretina expert consensus recommendations. Ophthalmologica 239(4):181–193. https://doi.org/10.1159/000486145
Geloneck MM, Chuang AZ, Clark WL et al (2014) Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol 132(11):1327–1333. https://doi.org/10.1001/jamaophthalmol.2014.2772
Gallagher K, Moseley MJ, Tandon A, Watson MP, Cocker KD, Fielder AR (2003) Nasotemporal asymmetry of retinopathy of prematurity. Arch Ophthalmol 121(11):1563–1568. https://doi.org/10.1001/archopht.121.11.1563
Fielder AR, Wallace DK, Stahl A, Reynolds JD, Chiang MF, Quinn GE (2019) Describing retinopathy of prematurity: current limitations and new challenges. Ophthalmology 126(5):652–654. https://doi.org/10.1016/j.ophtha.2018.12.034
Chiang MF, Quinn GE, Fielder AR et al (2021) International classification of retinopathy of prematurity. Ophthalmology. https://doi.org/10.1016/j.ophtha.2021.05.031
Mueller B, Salchow DJ, Waffenschmidt E et al (2017) Treatment of type I ROP with intravitreal bevacizumab or laser photocoagulation according to retinal zone. Br J Ophthalmol 101(3):365–370. https://doi.org/10.1136/bjophthalmol-2016-308375
Larrañaga-Fragoso P, Peralta J, Bravo-Ljubetic L, Pastora N, Abelairas-Gómez J (2016) Intravitreal bevacizumab for zone II retinopathy of prematurity. J Pediatr Ophthalmol Strabismus 53(6):375–382. https://doi.org/10.3928/01913913-20160727-01
Gonzalez JMG, Snyder L, Blair M, Rohr A, Shapiro M, Greenwald M (2018) Prophylactic peripheral laser and fluorescein angiography after bevacizumab for retinopathy of prematurity. Retina 38(4):764–772. https://doi.org/10.1097/IAE.0000000000001581
Snyder LL, Garcia-Gonzalez JM, Shapiro MJ, Blair MP (2016) Very late reactivation of retinopathy of prematurity after monotherapy with intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging Retina 47(3):280–283. https://doi.org/10.3928/23258160-20160229-12
Yoon JM, Shin DH, Kim SJ et al (2017) Outcomes after laser versus combined laser and bevacizumab treatment for type 1 retinopathy of prematurity in zone I. Retina 37(1):88–96. https://doi.org/10.1097/iae.0000000000001125
Anand N, Blair MP, Greenwald MJ, Rodriguez SH (2019) Refractive outcomes comparing primary laser to primary bevacizumab with delayed laser for type 1 ROP. J AAPOS 23(2):88. e1-88. e6. https://doi.org/10.1016/j.jaapos.2018.10.013
Quinn GE, Dobson V, Siatkowski R et al (2001) Does cryotherapy affect refractive error? Results from treated versus control eyes in the cryotherapy for retinopathy of prematurity trial. Ophthalmology 108(2):343–347. https://doi.org/10.1016/S0161-6420(00)00527-3
Quinn GE (2016) Retinopathy of prematurity blindness worldwide: phenotypes in the third epidemic. Eye Brain 8:31. https://doi.org/10.2147/EB.S94436
Akçakaya AA, Yaylali SA, Erbil HH et al (2012) Screening for retinopathy of prematurity in a tertiary hospital in Istanbul: incidence and risk factors. J Pediatr Ophthalmol Strabismus 49(1):21–25. https://doi.org/10.3928/01913913-20110208-01
Davitt BV, Dobson V, Good WV et al (2005) Prevalence of myopia at 9 months in infants with high-risk prethreshold retinopathy of prematurity. Ophthalmology 112(9):1564–1568. https://doi.org/10.1016/j.ophtha.2005.03.025
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
Ethics committee approval was obtained from the University of Health Sciences, Istanbul Kanuni Sultan Suleyman Training and Research Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bayramoglu, S.E., Sayin, N. Factors associated with refractive outcome in children treated with bevacizumab for retinopathy of prematurity: the importance of retinal vascularization. Int Ophthalmol 42, 3199–3210 (2022). https://doi.org/10.1007/s10792-022-02321-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-022-02321-6