Abstract
Research question
In a randomized, triple-blind, placebo-controlled clinical trial (RCT), we investigated the effect of astaxanthin (AST) on pro-inflammatory cytokines, oxidative stress (OS) markers, and assisted reproductive technology (ART) outcomes in 44 infertile Polycystic Ovary Syndrome (PCOS) patients.
Design
Patients with PCOS were randomly divided into two groups. The intervention group received 6 mg AST, and the control group received placebo daily for 8 weeks. Blood samples were obtained from all patients before and after intervention and follicular fluid (FF) was collected during the ART procedure. Interleukin (IL) ‐6, IL‐1β were evaluated from serum samples and FF and OS markers (malondialdehyde [MDA], catalase [CAT], superoxide dismutase [SOD], and reactive oxygen species [ROS]) were measured from FF. The groups were compared for ART outcomes as well.
Results
A significant decrease in IL-6 and IL-1β concentrations (both, P = < 0.01) serum levels was found following AST treatment. FF cytokine levels and OS markers did not differ significantly between the groups. Reproductive outcomes, including the number of oocytes retrieved (P = 0.01), the MII oocyte count (P = 0.007), oocyte maturity rate (MII %) (P = 0.02) and number of frozen embryos (P = 0.03) significantly improved after intervention. No significant differences were found in chemical, clinical and multiple pregnancies between the groups.
Conclusions
AST pretreatment may modify inflammation and improve ART outcomes in PCOS infertile patients. Further investigations are recommended to verify these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS), is an endocrine and metabolic disorder (Singh et al. 2023). The World Health Organization reports that PCOS has a prevalence rate of 2–26% worldwide (Jaripur et al. 2022). Because of its reproductive, metabolic, and psychological features, PCOS is a significant public health concern (Azziz et al. 2016).
Three major features distinguish PCOS: irregular menstruation, hyperandrogenism, and polycystic ovarian morphology (Islam, Masud, Islam, and Haque 2022). Despite decades of intensive research, pathology and etiology of this disease remain unknown. Various internal and external factors have been implicated, including insulin resistance (IR), hyperandrogenism, epigenetics, genetics, and environmental factors (Sadeghi et al. 2022). There is substantial evidence that chronic low-grade inflammation plays a crucial role in the pathogenesis of PCOS (Barrea et al. 2018; Li et al. 2019). There have been studies that indicate PCOS patients have higher levels of interleukin (IL)-6 and IL‐1β than healthy women (Bednarska and Siejka 2017; Rostamtabar et al. 2021). In several studies, reactive oxygen species (ROS) production has been demonstrated to affect ovarian physiology and negatively affect reproductive function. The imbalance of ROS production stimulates inflammatory signaling pathways, resulting in cystic ovarian dysfunction and abnormal ovulation (Boots and Jungheim 2015).
Treatment options are determined by the target patient and her priorities. There are a variety of complications, such as infertility treatment, hormonal regulation, weight loss, or management of hyperandrogenic symptoms, such as acne, androgenic alopecia and hirsutism. Individuals should receive a customized approach to achieve the most effective results. Currently, there is no one ideal treatment for all women with PCOS, so physicians must treat them symptomatically (Helvaci and Yildiz 2023; Sadeghi et al. 2022). Metformin and thiazolidinediones were previously used, but both had side effects. (Kiani, Donato, Dhuli, Stuppia, and Bertelli 2022). Natural molecules from nutritional supplements and herbal medicines have recently achieved satisfactory results with no side effects (Günalan, Yaba, and Yılmaz 2018; Kiani et al. 2022).
However, supplementation with natural molecules has a significant impact on the oxidative stress (OS) associated with PCOS as well as its associated complications (Dubey et al. 2021). Herbal medicines may be effective treatment for PCOS patients with infertility and IR (Arentz, Abbott, Smith, and Bensoussan 2014; Brenjian et al. 2020).
Astaxanthin (3,3′-dihydroxy-β, β′-carotene-4,4′-dione) (AST) is a red fat-soluble carotenoid that is known as one of the strongest antioxidants in nature. It can be found in a variety of microorganisms and marine animals (Ambati, Phang, Ravi, and Aswathanarayana 2014; Tian et al. 2021). In addition to having strong antioxidant properties, AST has anti-inflammatory, anti-apoptotic, anti-proliferative, antidiabetic, anticancer, and skin-protective, DNA repair, cell regeneration, neuroprotective and immune modulatory effects, making it a valuable multi-target pharmacological agent (Chang and Xiong 2020; Si and Zhu 2022). Anti-inflammatory mechanisms of AST involved in targeting multiple signaling pathways in addition to inflammatory biomarkers (Chang and Xiong 2020). It can downregulate inflammatory cytokine, such as IL-1β, IL-6, and tumor necrosis factor-alpha (TNF-α) (Tian et al. 2021). Supplementing with AST has been found to reduce inflammation in young soccer players due to rigorous physical training (Baralic et al. 2015). AST reduced iNOS and COX-2 expression in human keratinocytes by reducing mRNA and protein expression (Yoshihisa, Rehman, and Shimizu 2014). This natural molecule significantly inhibited COX-2, iNOS, and ICAM-1 protein expressions in streptozotocin-induced diabetic rats, indicating that it inhibits inflammation resulting from diabetic liver dysfunction (Park et al. 2015). Moreover, this ketocarotenoid reduces circulating levels of IL-6 and MDA in type 2 diabetes patients (Shokri-Mashhadi, Tahmasebi, Mohammadi-Asl, Zakerkish, and Mohammadshahi 2021).
AST targets a variety of molecules and pathways, including nuclear factor erythroid 2-related factor 2 (Nrf2), nuclear factor-kappa B (NF-κB), phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt), Mitogen activated protein kinase (MAPK), Wnt/β-catenin, Janus kinase 1(JAK1)/transduction and the activators of the transcription-3 (STAT3) (Brasil et al. 2021; Cai et al. 2019; Song et al. 2012; Xu, Zhang, Jiang, and Zhang 2015; Zarneshan, Fakhri, Farzaei, Khan, and Saso 2020).
To counteract OS, AST inhibits extracellular signal-regulated kinase (p-ERK)/ERK and stimulates nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE). This signaling pathway activates antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (Zarneshan et al. 2020). ROS stimulate NF-κB signaling pathway, which controls the production of pro-inflammatory chemokines and cytokines. AST reduces inflammation by preventing ROS-mediated induction of these transcription factors (Zarneshan et al. 2020).
Supplementation with AST for infertility management has been examined in many studies. An animal study showed that AST enhanced bovine oocyte quality and growth, while suppressing luteinization in granulosa cells (Chelenga et al. 2020). Furthermore, another study showed AST can increase the number of corpora lutea, implantation sites and fetuses (Patil, Kasabe, and Dandge 2022).Recently some research was also conducted clinical trials to examine the effect of AST on patients with infertility. The AST administration can reduce OS, inflammation and improve antioxidant response elements in female with infertility (Gharaei et al. 2022; Jabarpour, Aleyasin, Nashtaei, Lotfi, and Amidi 2023; Rostami et al. 2023). Thus, the present study examined the effects of AST supplementation on inflammation, OS, and reproductive outcomes in PCOS patients with infertility.
Materials and methods
The study design, randomization and blinding
There was a prospective randomized parallel triple-blind placebo-controlled study conducted from April 2022 to May 2023 at the Arash Hospital infertility clinic, Tehran, Iran. 44 Infertile women with PCOS aged 18–40 were enrolled according to Rotterdam criteria and recommended to undergo intracytoplasmic sperm injection (ICSI). This project registered at Iranian Registry of Clinical Trials and Tehran University of Medical Sciences’ Ethics Committee approved this study (Reference number of the ethics committee: IR.TUMS.MEDICINE.REC.1400.1110).
The subjects did not have hyperprolactinemia, congenital adrenal hyperplasia, thyroid disease, Cushing’s syndrome, ovarian tumors, autoimmune disease, systemic disorders, hyperlipidemia cardiovascular disease and severe male factor infertility. The participants did not use any type of oral contraceptive pill during the 3 month period prior to the study or any other medications that might affect their reproductive physiology.
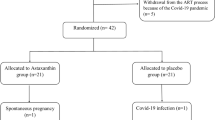
The balanced block randomization method within a block size of 4 was used by an independent statistician to assign eligible patients to the AST or placebo groups (1:1). All researchers, patients, embryologists, statisticians and laboratory personnel were blinded to each patient’s treatment plans (Fig. 1).
Intervention and controlled ovarian stimulation
Based on previous RCT studies, the therapeutic dose of AST is between 2 and 24 mg daily for at least 3 week(Brendler and Williamson 2019; Donoso, González-Durán, Muñoz, González, and Agurto-Muñoz 2021) In this study, treatment and placebo groups received 6 mg oral AST or placebo capsules (AstaReal® astaxanthin; AstaReal Co., Ltd., Tokyo, Japan) for 8 weeks before and during the routine gonadotropin-releasing hormone (GnRH) antagonist ovarian stimulation protocol. A placebo capsule had exactly the same color, size, shape, packaging, and taste as an AST capsule and made by the same company.
A flexible GnRH antagonist ovarian stimulation protocol was used in all patients during their IVF/ICSI cycles. Recombinant follicle stimulating hormone (rFSH) (150–300 IU/day, Gonal-F®, Merck Serono SA, Switzerland) was administered on day 2 or 3 of the menstrual cycle and continued until human chorionic gonadotropin (HCG) injection. In order to evaluate the ovarian response, serial transvaginal sonography (TVS) was performed. The GnRH-antagonist (Cetrotide®: 0.25 mg daily, cetrorelix acetate, Serono, Inc) was started when 2 or more follicles in size of ≥ 14 in diameter were observed. Cetrotide was stopped after at least two follicles reached a diameter of 18 mm. Final oocyte maturation was achieved by injecting 10,000 IU of HCG (Ovitrelle, Merck Serono SA, Switzerland). Under an ultrasound guide, the ovum pickup was planned 36 h after the trigger. All patients underwent ICSI.
Blood and FF collection and biochemical assessment
10 ml venous blood specimens were collected from all the patients before and after the intervention to assess pro-inflammatory cytokines (IL-1β, IL-6). After centrifuging the blood samples (1500 rpm for 10 min, 4 °C), the serum was separated and stored at – 80 °C until further analysis. During oocyte retrieval, FF was only aspirated from the first follicle to avoid blood contamination. The samples were centrifuged for 10 min at 3000 rpm(Kazemi et al. 2014; Pella et al. 2020), and the clarified supernatants were collected. The FF aliquots were also immediately stored at − 80 °C for OS marker measurement. FF was assessed for ROS, SOD, CAT activities and malondialdehyde (MDA) using human enzyme-linked immunosorbent assay (ELISA) kits (Zellbio GmbH, Germany).
The concentrations of pro-inflammatory cytokines in serum and FF were detected using human ELISA kits (Karmania Pars Gene Company; KPG, Iran). Pro-inflammatory serum cytokines were analyzed in duplicate, blinded, in pairs (pre/post-intervention) simultaneously, and in the same analytical run randomly.
ART outcomes
About 2 h after oocyte pick-up (OPU), cumulus-oocyte complexes (COCs) were denuded with hyaluronidase enzyme (Sigma®, USA). Under a stereo microscope (Olympus SZX7, Tokyo, Japan), oocytes were evaluated for quality and maturity. Based on maturity level oocytes were classified into germinal vesicles (GV), metaphase I (MI), and metaphase II (MII). The ICSI procedure was performed on MII oocytes, and the fertilization was assessed 17–20 h after insemination by observing two pronuclei (PN) and two polar bodies (PBs).
Oocyte number and quality were evaluated at the time of ICSI injection with regard to the oocyte maturity index (the ratio of MII oocytes to total oocytes collected (Uk et al. 2022)). Fertilization rate(FR) (Number of 2PN/2PB oocytes, 16-18 h post-insemination, divided by the number of MII oocytes injected (Uk et al. 2022)), number of frozen embryos, high-quality embryos (based on the ASEBIR criteria, grade A and grade B cleavage embryos (“The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting,” 2011)), chemical and clinical pregnancy rate (the number of positive serum β-hCG per number of embryo transfers (ET) after 14 days from the day of ET and the number of observed gestational sacs per number of ET by ultrasonography six weeks after ET, respectively (Zafardoust, Ansaripor, Karimi, Hosseinirad, & Ataei 2022)) and multiple pregnancy rate (the number of multiple pregnancies × 100 per the number of clinical pregnancies) were also assessed.
Statistical analysis
Statistical analysis of the data was performed using SPSS (SAS Institute Inc, version 22, Cary, NC, USA). Results are presented as mean ± standard deviation (SD). Kolmogorov–Smirnov and Shapiro-Wilk tests were used to determine data normality. The independent and paired t-tests were used to compare continuous variables with normal distributions within and between groups. Additionally, the Mann-Whitney U test was used to analyze the data without normal distribution. For the evaluation of the correlation between the parameters, Pearson's correlation was used. Fisher's exact and Pearson’s chi-squared tests were used to compare chemical, clinical, and multiple pregnancy rates. In order to test the correlation between serum and FF cytokines levels with ART outcomes, Pearson's correlation coefficient was used.
Results
A total number of 44 participants were randomly selected (Each group consists of 22 patients). The patients were enrolled between April 2022 to May 2023. Data on demographics and clinical characteristics are presented in Table 1. No statistically significant differences were found in age (29.86 ± 4.279 vs. 31.14 ± 6.213 P = 0.433), BMI (25.68 ± 2.603 vs. 25.52 ± 2.690 P = 0.841) and AMH (7.23 ± 1.494 vs. 8.04 ± 1.681 P = 0.1), duration of infertility (5.31 ± 2.798 vs. 6.23 ± 2.181 P = 0.234), FSH (4.75 ± 1.541 vs. 4.66 ± 0.849 P = 0.813), LH (8.47 ± 1.652 vs. 7.63 ± 2.481 P = 0.190) and TSH (2.56 ± 0.807 vs. 2.75 ± 0.647 P = 0.394) between the two groups at the start of the study. All 44 patients completed the research. Based on pill count-backs on returned bottles, the AST and placebo groups had adherence rates of 93% and 95%, respectively. During the intervention, no adverse effects or toxicity were reported by the patients. 80% of the AST group and 72% of the placebo group were diagnosed with primary Table 2 infertility.
Serum and FF cytokine parameters
The baseline and post-treatment values for the two groups (placebo and AST) are shown in Table2. Before AST supplementation, no statistically significant differences were found between the two groups in serum cytokine levels. A significant decrease in IL-6 (4.70 ± .794 vs 3.45 ± 1.112 P = <0.01) and IL-1β concentrations (5.64 ± .758 vs. 4.61 ± 0.561 P =<0.01) serum levels was found following AST treatment. After the intervention, the AST and placebo groups did not have significant differences in FF IL-1β and IL-6 levels (all P > 0.05).
FF oxidative stress profile
All the OS indicators are shown in Table 3. Assessment of OS parameters in FF showed that there was no statistically significant difference between the two groups (P > 0.05).
ART outcomes
After AST therapy, reproductive outcomes, including the number of oocytes retrieved (15.14 ± 4.794 vs. 11.36 ± 4.991 P = 0.01), the MII oocyte count (9.59 ± 3.459 vs. 6.50 ± 3.700 P = 0.007), oocyte maturity rate (MII %) (62.58 ± 12.246 vs. 53.63 ± 13.854 P = 0.02) and number of frozen embryos (7.41 ± 2.667 vs. 5.59 ± 2.806 P = 0.03) significantly improved. The high-quality embryos (P = 0.58) and fertilization rate (P =0.69) showed similar results in both groups. In addition, there were no significant differences in chemical (9/22 (40.9%) vs. 7/22 (31.8%) two-tailed P = 0.531), clinical (7/22 (31.8%) vs. 5/22(22.7%) two-tailed P = 0.498) and multiple (3/22 (13.6%) vs. 2/22 (9.1%) two-tailed P = 0.635) pregnancy rates between the groups.
Correlation analysis
According to correlation analysis, Serum IL6 correlated Table 4 positively with the number of oocytes retrieved (rs = 0.481; P=0.02), MII count (rs = 0.456; P = 0.03) and negatively with multiple pregnancy (rs = − 0.356; P = 0.01). SerumIL-1β correlated positively with the number of oocytes retrieved (rs = − 0.453; P = 0.03) and fertilization rate (rs = − 0.418; P = 0.005). There was positive correlation between FF IL-1β and High-quality embryos (rs = 0.320; P = 0.03). FF MDA and clinical pregnancy correlated negatively (rs = − 0.312; P = 0.03). FF SOD positively correlated with chemical pregnancy rates (rs = 0.395; P = 0.008) and clinical pregnancy rates (rs = 0.379; P = 0.01).
Discussion
In this randomized, triple-blind, placebo-controlled clinical Table 5 trial investigating the antioxidant, anti-inflammatory, and fertility-promoting effects of AST supplementation on infertile patients with PCOS candidate for ART. The results showed that supplementation with 6 mg/day of AST for 8 weeks alleviated inflammation and improved ART outcomes.
The study indicated that AST reduced serum levels of IL‐1β, IL‐6; meanwhile, the concentration of these inflammatory markers in FF remains unchanged after AST administration.
Although there is growing prevalence of PCOS worldwide, the exact etiology and pathophysiology of PCOS remain unclear. There were two major factors responsible for this disorder. Insulin resistance, hyperandrogenism, inflammation, oxidative stress, and obesity are internal factors, whereas epigenetics, stress, environmental toxicants and diet are external factors (Sadeghi et al. 2022).
Inflammatory markers and low-grade inflammation have been associated with PCOS in recent studies (Rostamtabar et al. 2021).Women with PCOS have higher serum levels of IL6 and IL‐1β than healthy women. Research has shown that IR and androgen levels are related to IL-6 levels. IL‐1β regulates steroidogenesis, which is essential for ovulation and fertilization (Lin et al. 2011; Zafari Zangeneh, Naghizadeh, and Masoumi 2017).
A clinical trial demonstrated that AST supplementation could decrease plasma IL-6 levels in patients with type 2 diabetes(Shokri-Mashhadi et al. 2021). In a clinical trial study on women with endometriosis shown that serum IL-1β levels decreased after 6mg/day AST supplementation (Rostami et al. 2023). The mRNA expression levels of IL-1β, IL-6, CCL2, and CXCL2 decreased Table 6 significantly with AST in the colorectal carcinogenesis model of mice (Kochi et al. 2014). In addition, it has been reported that AST can block the NF-κB-dependent signaling pathway and inhibit the expression of downstream inflammatory mediators such as IL-1β, IL-6, and tumor necrosis factor-α (TNF-α) (Speranza et al. 2012).
OS is an internal factor that participates in PCOs etiology(Sadeghi et al. 2022; J. Zhang, Bao, Zhou, & Zheng 2019). Several studies reported that AST can ameliorate OS in several diseases (Kishimoto, Yoshida, and Kondo 2016; Wu, Xu, Chen, and Zhang 2020; X. S. Zhang et al. 2021);The present study did not find any significant changes in FF OS markers after AST supplementation. The results are in line with an RCT on PCOs which found no significant improvement in FF levels of the SOD and MDA after AST supplementation (Gharaei et al. 2022). Furthermore, another RCT showed that AST consumption had no effect on OS markers in the FF of endometriosis patients (Rostami et al. 2023). Also a study reported Growth hormone treatment did not significantly alter FF levels of TAC, MDA, and SOD in PCOS patients. However, treatment with growth hormone could reduce total oxidant status (TOS) and the OS Index (OSI) of the FF (Gong, Luo, et al., 2020). Therefore, Further studies will be required to determine TOS, OSI, total antioxidant status (TAS), pro-oxidant-antioxidant balance (PAB), and other biomarkers of OS following antioxidant therapy. Furthermore, the presence of AST and its oxidation fragments in FF also needs to be assessed in future studies. In addition, these findings can be explained by the following that FF serves as a complex microenvironment, derived from the diffusion of serum, plasma, and metabolites synthesized in the follicle wall. Granulosa cells and theca cells will modify this microenvironment later (Brinca et al. 2022). It is thus evident that the levels of OS markers in this fluid reflect the OS level of the ovaries and the systemic OS level (Bianchi et al. 2016). Furthermore, systemic OS occurs in association with hyperandrogenism, obesity, and IR. Recent studies have shown that PCOS patients with different phenotypes respond differently to various treatments and have different ART results (Clark et al. 2014; De Vos et al. 2018). Thus, the accuracy of the results may be increased by classifying the participants based on their PCOS phenotypic characteristics.
Despite the limited sample size, AST therapy resulted in a significant increase in number of oocytes, mature oocytes, and frozen embryos. The results of this study are consistent with those of some previous studies reporting that antioxidant supplementation improves ART outcomes in women with or without PCOS (Choi, Youn, and Shin 2011; Gharaei et al. 2022; Rostami et al. 2023). Thus, AST may provide promising protection against oxidative and inflammatory damage to oocytes and embryos.
High-quality embryo and FR were not significantly different between the groups. Despite excluding patients with male factors, fertilization may be affected by the quality of sperm. In addition, FR and High-quality embryo provide insight into the quality and maturity of gametes, as well as the competence of operators.
Moreover, in the current study, chemical, clinical, and multiple pregnancy rates were not significantly different between groups. A variety of factors can influence pregnancy outcome, such as male gamete quality, endometrial receptivity, the way embryos are handled, frozen and stored and Embryologist abilities in ET (Vlaisavljevic et al. 2021). There may be a need for further research comparing outcomes between fresh and frozen cycles with a larger sample size.
Based on a correlation analysis, serum IL-6 concentration was positively related to the number of retrieved oocytes as well as MII count, but negatively related to multiple pregnancy. Studies regarding serum IL-6 levels and the number of oocytes obtained from ART are rare. However, some authors suggested no relationship between serum level of pro-inflammatory factors and pregnancy achievement following ART(Fıçıcıoğlu et al. 2009; Ozgu-Erdinc et al. 2021). Serum IL-1β positively associated with number of retrieved oocytes and fertilization rate. According to the previous studies, an increase in serum IL-1β is associated with higher implantation and pregnancy (Karagouni, Chryssikopoulos, Mantzavinos, Kanakas, and Dotsika 1998; Kreines et al. 2018). Another study showed that IL-1β concentrations increased significantly in women who had a pregnancy through ART than in those who did not (Sequeira, Espejel-Núñez, Vega-Hernández, Molina-Hernández, and Grether-González 2015). We also found FF IL-1β was positively correlated with high-quality embryos. Previous studies suggested that serum and FF IL-1β concentrations associated with pregnancy success (Fıçıcıoğlu et al. 2009; Karagouni et al. 1998). However, the findings are controversial. In another study, it was found that FF IL-1β concentrations did not correlate with fertilization and embryo cleavage rates (Barrionuevo et al. 2000).
It was found that FF MDA was negatively correlated with clinical pregnancy. Study was reported that the quality of embryos in IVF-ET was strongly correlated with oxidative markers in FF, especially MDA and TOC. The quality of embryos is usually a good indicator of pregnancy success (Liu et al. 2021). In another study, females with negative IVF outcomes had significantly higher FF MDA (Kumar et al. 2018).
In our study, FF SOD was positively correlated with chemical and clinical pregnancy rates. SOD is an essential antioxidant in the body that protects against OS. Enzymes are effective therapeutic agents against ROS-related diseases (Younus 2018). A study found that pretreatment with growth hormone reduced OS and increased SOD levels, improved oocyte quality and IVF outcomes (Gong, Zhang, et al. 2020a, b).
It is the first randomized, triple blind, placebo-controlled clinical trial to evaluate the effects of AST supplementation on OS markers, inflammatory cytokines, and ART outcomes in infertile PCO patients. Our study has some limitations that need to be considered. PCOS women have different phenotypic characteristics. As a result, a larger sample size is required to analyze different phenotypes separately. In addition, for more effective results different doses of AST and durations should be tried. We were also unable to assess late pregnancy outcomes, including live birth rate, the most relevant measure of ART success.
Conclusion
As a result, AST administration could be a promising supplement for improving PCO complications. Additionally, it may enhance ART outcomes in infertile PCO patients.
Data availability
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
References
Ambati RR, Phang SM, Ravi S, Aswathanarayana RG (2014) Astaxanthin: sources, extraction, stability, biological activities and its commercial applications–a review. Mar Drugs 12(1):128–152. https://doi.org/10.3390/md12010128
Arentz S, Abbott JA, Smith CA, Bensoussan A (2014) Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. BMC Complement Altern Med 14:511. https://doi.org/10.1186/1472-6882-14-511
Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Yildiz BO (2016) Polycystic ovary syndrome. Nat Rev Dis Prim. https://doi.org/10.1038/nrdp.2016.57
Baralic I, Andjelkovic M, Djordjevic B, Dikic N, Radivojevic N, Suzin-Zivkovic V, Pejic S (2015) Effect of astaxanthin supplementation on salivary IgA, oxidative stress, and inflammation in young soccer players. Evid Based Complement Alternat Med. https://doi.org/10.1155/2015/783761
Barrea L, Marzullo P, Muscogiuri G, Di Somma C, Scacchi M, Orio F, Savastano S (2018) Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome. Nutr Res Rev 31(2):291–301. https://doi.org/10.1017/s0954422418000136
Barrionuevo MJ, Schwandt RA, Rao PS, Graham LB, Maisel LP, Yeko TR (2000) Nitric oxide (NO) and interleukin-1beta (IL-1beta) in follicular fluid and their correlation with fertilization and embryo cleavage. Am J Reprod Immunol 44(6):359–364. https://doi.org/10.1111/j.8755-8920.2000.440607.x
Bednarska S, Siejka A (2017) The pathogenesis and treatment of polycystic ovary syndrome: what’s new? Adv Clin Exp Med 26(2):359–367. https://doi.org/10.17219/acem/59380
Bianchi L, Gagliardi A, Landi C, Focarelli R, De Leo V, Luddi A, Piomboni P (2016) Protein pathways working in human follicular fluid: the future for tailored IVF? Expert Rev Mol Med. https://doi.org/10.1017/erm.2016.4
Boots CE, Jungheim ES (2015) Inflammation and human ovarian follicular dynamics. Semin Reprod Med 33(4):270–275. https://doi.org/10.1055/s-0035-1554928
Brasil FB, Bertolini Gobbo RC, Souza de Almeida FJ, Luckachaki MD, Dall’Oglio EL, de Oliveira MR (2021) The signaling pathway PI3K/Akt/Nrf2/HO-1 plays a role in the mitochondrial protection promoted by astaxanthin in the SH-SY5Y cells exposed to hydrogen peroxide. Neurochem Int 146:105024. https://doi.org/10.1016/j.neuint.2021.105024
Brendler T, Williamson EM (2019) Astaxanthin: How much is too much? Safety Rev Phytother Res 33(12):3090–3111. https://doi.org/10.1002/ptr.6514
Brenjian S, Moini A, Yamini N, Kashani L, Faridmojtahedi M, Bahramrezaie M, Amidi F (2020) Resveratrol treatment in patients with polycystic ovary syndrome decreased pro-inflammatory and endoplasmic reticulum stress markers. Am J Reprod Immunol 83(1):e13186. https://doi.org/10.1111/aji.13186
Brinca AT, Ramalhinho AC, Sousa Â, Oliani AH, Breitenfeld L, Passarinha LA, Gallardo E (2022) Follicular fluid: a powerful tool for the understanding and diagnosis of polycystic ovary syndrome. Biomedicines. https://doi.org/10.3390/biomedicines10061254
Cai X, Chen Y, Xie X, Yao D, Ding C, Chen M (2019) Astaxanthin prevents against lipopolysaccharide-induced acute lung injury and sepsis via inhibiting activation of MAPK/NF-κB. Am J Transl Res 11(3):1884–1894
Chang MX, Xiong F (2020) Astaxanthin and its effects in inflammatory responses and inflammation-associated diseases: recent advances and future directions. Molecules. https://doi.org/10.3390/molecules25225342
Chelenga M, Sakaguchi K, Abdel-Ghani MA, Yanagawa Y, Katagiri S, Nagano M (2020) Effect of increased oxygen availability and astaxanthin supplementation on the growth, maturation and developmental competence of bovine oocytes derived from early antral follicles. Theriogenology 157:341–349. https://doi.org/10.1016/j.theriogenology.2020.07.023
Choi HD, Youn YK, Shin WG (2011) Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Foods Hum Nutr 66(4):363–369. https://doi.org/10.1007/s11130-011-0258-9
Clark NM, Podolski AJ, Brooks ED, Chizen DR, Pierson RA, Lehotay DC, Lujan ME (2014) Prevalence of polycystic ovary syndrome phenotypes using updated criteria for polycystic ovarian morphology: an assessment of over 100 consecutive women self-reporting features of polycystic ovary syndrome. Reprod Sci 21(8):1034–1043. https://doi.org/10.1177/1933719114522525
Donoso A, González-Durán J, Muñoz AA, González PA, Agurto-Muñoz C (2021) Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials. Pharmacol Res 166:105479. https://doi.org/10.1016/j.phrs.2021.105479
Dubey P, Reddy S, Boyd S, Bracamontes C, Sanchez S, Chattopadhyay M, Dwivedi A (2021) Effect of nutritional supplementation on oxidative stress and hormonal and lipid profiles in PCOS-affected females. Nutrients. https://doi.org/10.3390/nu13092938
Fıçıcıoğlu C, Kumbak B, Akcin O, Attar R, Yıldırım G, Tecellioğlu N, Yeşildağlar N (2009) Cytokine and nitric oxide concentrations in follicular fluid and blood serum of patients undergoing assisted reproductive treatment: relationship to outcome. J Turk Ger Gynecol Assoc 10(3):132–136
Gharaei R, Alyasin A, Mahdavinezhad F, Samadian E, Ashrafnezhad Z, Amidi F (2022) Randomized controlled trial of astaxanthin impacts on antioxidant status and assisted reproductive technology outcomes in women with polycystic ovarian syndrome. J Assist Reprod Genet 39(4):995–1008. https://doi.org/10.1007/s10815-022-02432-0
Gong Y, Zhang K, Xiong D, Wei J, Tan H, Qin S (2020) Growth hormone alleviates oxidative stress and improves the IVF outcomes of poor ovarian responders: a randomized controlled trial. Reprod Biol Endocrinol 18(1):91. https://doi.org/10.1186/s12958-020-00648-2
Gong Y, Luo S, Fan P, Jin S, Zhu H, Deng T, Huang W (2020) Growth hormone alleviates oxidative stress and improves oocyte quality in Chinese women with polycystic ovary syndrome: a randomized controlled trial. Sci Rep 10(1):18769. https://doi.org/10.1038/s41598-020-75107-4
Günalan E, Yaba A, Yılmaz B (2018) The effect of nutrient supplementation in the management of polycystic ovary syndrome-associated metabolic dysfunctions: a critical review. J Turk Ger Gynecol Assoc 19(4):220–232. https://doi.org/10.4274/jtgga.2018.0077
Helvaci N, Yildiz BO (2023) Current and emerging drug treatment strategies for polycystic ovary syndrome. Expert Opin Pharmacother 24(1):105–120. https://doi.org/10.1080/14656566.2022.2108702
Islam H, Masud J, Islam YN, Haque FKM (2022) An update on polycystic ovary syndrome: a review of the current state of knowledge in diagnosis, genetic etiology, and emerging treatment options. Womens Health 18:17455057221117966. https://doi.org/10.1177/17455057221117966
Jabarpour M, Aleyasin A, Nashtaei MS, Lotfi S, Amidi F (2023) Astaxanthin treatment ameliorates ER stress in polycystic ovary syndrome patients: a randomized clinical trial. Sci Rep 13(1):3376. https://doi.org/10.1038/s41598-023-28956-8
Jaripur M, Ghasemi-Tehrani H, Askari G, Gholizadeh-Moghaddam M, Clark CCT, Rouhani MH (2022) The effects of magnesium supplementation on abnormal uterine bleeding, alopecia, quality of life, and acne in women with polycystic ovary syndrome: a randomized clinical trial. Reprod Biol Endocrinol 20(1):110. https://doi.org/10.1186/s12958-022-00982-7
Karagouni EE, Chryssikopoulos A, Mantzavinos T, Kanakas N, Dotsika EN (1998) Interleukin-1beta and interleukin-1alpha may affect the implantation rate of patients undergoing in vitro fertilization-embryo transfer. Fertil Steril 70(3):553–559. https://doi.org/10.1016/s0015-0282(98)00243-x
Kazemi A, Ramezanzadeh F, Nasr Esfahani MH, Saboor-Yaraghi AA, Nejat SN, Rahimi-Foroshani A (2014) Relationship between energy expenditure related factors and oxidative stress in follicular fluid. Int J Fertil Steril 8(2):175–182
Kiani AK, Donato K, Dhuli K, Stuppia L, Bertelli M (2022) Dietary supplements for polycystic ovary syndrome. J Prev Med Hyg 63(2 Suppl 3):E206-e213. https://doi.org/10.15167/2421-4248/jpmh2022.63.2S3.2762
Kishimoto Y, Yoshida H, Kondo K (2016) potential anti-atherosclerotic properties of astaxanthin. Mar Drugs. https://doi.org/10.3390/md14020035
Kochi T, Shimizu M, Sumi T, Kubota M, Shirakami Y, Tanaka T, Moriwaki H (2014) Inhibitory effects of astaxanthin on azoxymethane-induced colonic preneoplastic lesions in C57/BL/KsJ-db/db mice. BMC Gastroenterol 14:212. https://doi.org/10.1186/s12876-014-0212-z
Kreines FM, Nasioudis D, Minis E, Irani M, Witkin SS, Spandorfer S (2018) IL-1β predicts IVF outcome: a prospective study. J Assist Reprod Genet 35(11):2031–2035. https://doi.org/10.1007/s10815-018-1296-0
Kumar S, Mishra V, Thaker R, Gor M, Perumal S, Joshi P, Verma Y (2018) Role of environmental factors & oxidative stress with respect to in vitro fertilization outcome. Indian J Med Res 148(Suppl):S125-s133. https://doi.org/10.4103/ijmr.IJMR_1864_17
Li Y, Zheng Q, Sun D, Cui X, Chen S, Bulbul A, Yan Q (2019) Dehydroepiandrosterone stimulates inflammation and impairs ovarian functions of polycystic ovary syndrome. J Cell Physiol 234(5):7435–7447. https://doi.org/10.1002/jcp.27501
Lin YS, Tsai SJ, Lin MW, Yang CT, Huang MF, Wu MH (2011) Interleukin-6 as an early chronic inflammatory marker in polycystic ovary syndrome with insulin receptor substrate-2 polymorphism. Am J Reprod Immunol 66(6):527–533. https://doi.org/10.1111/j.1600-0897.2011.01059.x
Liu Y, Yu Z, Zhao S, Cheng L, Man Y, Gao X, Zhao H (2021) Oxidative stress markers in the follicular fluid of patients with polycystic ovary syndrome correlate with a decrease in embryo quality. J Assist Reprod Genet 38(2):471–477. https://doi.org/10.1007/s10815-020-02014-y
Ozgu-Erdinc AS, Gozukara I, Kahyaoglu S, Yilmaz S, Yumusak OH, Yilmaz N, Engin-Ustun Y (2021) Is there any role of interleukin-6 and high sensitive C-reactive protein in predicting IVF/ICSI success? A prospective cohort study. Horm Mol Biol Clin Investig 43(1):35–40. https://doi.org/10.1515/hmbci-2021-0039
Park CH, Xu FH, Roh SS, Song YO, Uebaba K, Noh JS, Yokozawa T (2015) Astaxanthin and Corni Fructus protect against diabetes-induced oxidative stress, inflammation, and advanced glycation end product in livers of streptozotocin-induced diabetic rats. J Med Food 18(3):337–344. https://doi.org/10.1089/jmf.2014.3174
Patil AD, Kasabe PJ, Dandge PB (2022) Pharmaceutical and nutraceutical potential of natural bioactive pigment: astaxanthin. Nat Prod Bioprospect 12(1):25. https://doi.org/10.1007/s13659-022-00347-y
Pella R, Suárez-Cunza S, Orihuela P, Escudero F, Pérez Y, García M, Romero S (2020) Oxidative balance in follicular fluid of ART patients of advanced maternal age and blastocyst formation. JBRA Assist Reprod 24(3):296–301. https://doi.org/10.5935/1518-0557.20200012
Rostami S, Alyasin A, Saedi M, Nekoonam S, Khodarahmian M, Moeini A, Amidi F (2023) Astaxanthin ameliorates inflammation, oxidative stress, and reproductive outcomes in endometriosis patients undergoing assisted reproduction: a randomized, triple-blind placebo-controlled clinical trial. Front Endocrinol 14:1144323. https://doi.org/10.3389/fendo.2023.1144323
Rostamtabar M, Esmaeilzadeh S, Tourani M, Rahmani A, Baee M, Shirafkan F, Nouri HR (2021) Pathophysiological roles of chronic low-grade inflammation mediators in polycystic ovary syndrome. J Cell Physiol 236(2):824–838. https://doi.org/10.1002/jcp.29912
Sadeghi HM, Adeli I, Calina D, Docea AO, Mousavi T, Daniali M, Abdollahi M (2022) Polycystic ovary syndrome: a comprehensive review of pathogenesis, management, and drug repurposing. Int J Mol Sci. https://doi.org/10.3390/ijms23020583
Sequeira K, Espejel-Núñez A, Vega-Hernández E, Molina-Hernández A, Grether-González P (2015) An increase in IL-1β concentrations in embryo culture-conditioned media obtained by in vitro fertilization on day 3 is related to successful implantation. J Assist Reprod Genet 32(11):1623–1627. https://doi.org/10.1007/s10815-015-0573-4
Shokri-Mashhadi N, Tahmasebi M, Mohammadi-Asl J, Zakerkish M, Mohammadshahi M (2021) The antioxidant and anti-inflammatory effects of astaxanthin supplementation on the expression of miR-146a and miR-126 in patients with type 2 diabetes mellitus: a randomised, double-blind, placebo-controlled clinical trial. Int J Clin Pract 75(5):e14022. https://doi.org/10.1111/ijcp.14022
Si P, Zhu C (2022) Biological and neurological activities of astaxanthin (review). Mol Med Rep. https://doi.org/10.3892/mmr.2022.12816
Singh S, Pal N, Shubham S, Sarma DK, Verma V, Marotta F, Kumar M (2023) Polycystic ovary syndrome: etiology, current management, and future therapeutics. J Clin Med. https://doi.org/10.3390/jcm12041454
Song X, Wang M, Zhang L, Zhang J, Wang X, Liu W, Lv C (2012) Changes in cell ultrastructure and inhibition of JAK1/STAT3 signaling pathway in CBRH-7919 cells with astaxanthin. Toxicol Mech Methods 22(9):679–686. https://doi.org/10.3109/15376516.2012.717119
Speranza L, Pesce M, Patruno A, Franceschelli S, Lutiis MA, Grilli A, Felaco M (2012) Astaxanthin treatment reduced oxidative induced pro-inflammatory cytokines secretion in U937: SHP-1 as a novel biological target. Mar Drugs 10(4):890–899. https://doi.org/10.3390/md10040890
The Istanbul consensus workshop on embryo assessment (2011) proceedings of an expert meeting. Hum Reprod 26(6):1270–1283. https://doi.org/10.1093/humrep/der037
Tian L, Wen Y, Li S, Zhang P, Wang Y, Wang J, Jie Y (2021) Benefits and safety of astaxanthin in the treatment of mild-to-moderate dry eye disease. Front Nutr 8:796951. https://doi.org/10.3389/fnut.2021.796951
Uk A, Decanter C, Grysole C, Keller L, Béhal H, Silva M, Barbotin AL (2022) Polycystic ovary syndrome phenotype does not have impact on oocyte morphology. Reprod Biol Endocrinol 20(1):7. https://doi.org/10.1186/s12958-021-00874-2
Vlaisavljevic V, Apter S, Capalbo A, D’Angelo A, Gianaroli L, Griesinger G, Tilleman K (2021) The Maribor consensus: report of an expert meeting on the development of performance indicators for clinical practice in ART. Hum Reprod Open. https://doi.org/10.1093/hropen/hoab022
De Vos M, Pareyn S, Drakopoulos P, Raimundo JM, Anckaert E, Santos-Ribeiro S, Blockeel C (2018) Cumulative live birth rates after IVF in patients with polycystic ovaries: phenotype matters. Reprod Biomed Online 37(2):163–171. https://doi.org/10.1016/j.rbmo.2018.05.003
Wu D, Xu H, Chen J, Zhang L (2020) Effects of astaxanthin supplementation on oxidative stress. Int J Vitam Nutr Res 90(1–2):179–194. https://doi.org/10.1024/0300-9831/a000497
Xu Y, Zhang J, Jiang W, Zhang S (2015) Astaxanthin induces angiogenesis through Wnt/β-catenin signaling pathway. Phytomedicine 22(7–8):744–751. https://doi.org/10.1016/j.phymed.2015.05.054
Yoshihisa Y, Rehman MU, Shimizu T (2014) Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced apoptosis in keratinocytes. Exp Dermatol 23(3):178–183. https://doi.org/10.1111/exd.12347
Younus H (2018) Therapeutic potentials of superoxide dismutase. Int J Health Sci 12(3):88–93
Zafardoust S, Ansaripor S, Karimi A, Hosseinirad H, Ataei M (2022) Effects of adjuvant growth hormone therapy on poor ovarian responders in assisted reproductive technology. Maedica 17(2):336–343. https://doi.org/10.26574/maedica.2022.17.2.336
Zafari Zangeneh F, Naghizadeh MM, Masoumi M (2017) Polycystic ovary syndrome and circulating inflammatory markers. Int J Reprod Biomed 15(6):375–382
Zarneshan SN, Fakhri S, Farzaei MH, Khan H, Saso L (2020) Astaxanthin targets PI3K/Akt signaling pathway toward potential therapeutic applications. Food Chem Toxicol 145:111714. https://doi.org/10.1016/j.fct.2020.111714
Zhang J, Bao Y, Zhou X, Zheng L (2019) Polycystic ovary syndrome and mitochondrial dysfunction. Reprod Biol Endocrinol 17(1):67. https://doi.org/10.1186/s12958-019-0509-4
Zhang XS, Lu Y, Li W, Tao T, Peng L, Wang WH, Li W (2021) Astaxanthin ameliorates oxidative stress and neuronal apoptosis via SIRT1/NRF2/Prx2/ASK1/p38 after traumatic brain injury in mice. Br J Pharmacol 178(5):1114–1132. https://doi.org/10.1111/bph.15346
Acknowledgements
We would like to thank the peer reviewers for their time and effort in reviewing the manuscript.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest
Informed Consent
Informed consent was obtained from all subjects involved in the study
Institutional review board statement
The experimental procedures used in this study were approved by Tehran University of Medical Sciences (Reference number of the ethics committee: IR.TUMS.MEDICINE.REC.1400.1110).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fereidouni, F., Kashani, L., Amidi, F. et al. Astaxanthin treatment decreases pro‐inflammatory cytokines and improves reproductive outcomes in patients with polycystic ovary syndrome undergoing assisted reproductive technology: A randomized clinical trial. Inflammopharmacol 32, 2337–2347 (2024). https://doi.org/10.1007/s10787-024-01504-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-024-01504-0