Abstract
Purpose
Retrospective cohort studies have shown a relationship between maternal serum interleukin-1β (IL-1β) and interleukin-1 receptor antagonist (IL-1Ra) levels and in vitro fertilization (IVF) cycle outcome. The objective of this investigation was to explore the correlation between serum IL-1β and/or IL-1Ra levels obtained prospectively and IVF outcomes.
Methods
Sera from 205 women were collected just prior to initiation of their IVF cycle, at the time of human chorionic gonadotropin administration, day 24 of IVF cycle, day 28, and day 35. Sera were analyzed for IL-1β and IL-1Ra using commercially available ELISA kits. Cycle outcomes were followed prospectively. Data were analyzed using Friedman analysis of variance by ranks and chi-square analysis.
Results
Among women with a viable pregnancy, IL-1β serum levels increased over time for those that proceeded to deliver or had an ongoing pregnancy. There was no increase in serum levels for those with subsequent pregnancy loss. Of the women that had an embryo transfer, detectable IL-1β levels at the start of the cycle were associated with successful IVF outcome (p = 0.027). Of women with a positive pregnancy test, undetectable IL-1β at the start of the cycle were associated with subsequent pregnancy loss (p = 0.046). For all IL1-Ra serum analysis, there were no significant results.
Conclusions
The increasing levels of IL-1β over time are consistent with the known role of the IL-1 cytokine family in implantation and pregnancy. Additionally, we confirm in a prospective investigation the positive relationship between detectable serum IL-1β at the start of IVF cycle and outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite advancements in the field of in vitro fertilization (IVF) over the past few decades, the national rate of live births per cycle (using fresh non-donor eggs or embryos) has remained relatively constant at around 27% [1]. After confirmation of a clinical pregnancy, the rate of pregnancy loss in IVF cycles is around 16% [1]. Failures of embryo implantation as well as subsequent early pregnancy losses likely have multiple etiologies involving fetal and/or maternal factors.
One potential underlying mechanism for IVF failure is an inadequate immunological response at the maternal-fetal interface [2, 3]. Because the human fetus is a semi-allograft, regulation and adjustment of the maternal immune system is necessary for fetal implantation and development [4]. Although the initial hypothesis surrounding the role of the immune system in pregnancy was that of immune suppression, recent evidence has clearly shown that a more complex balance between pro- and anti-inflammatory signaling is required for successful embryo implantation and subsequent pregnancy progression [4,5,6,7].
The interleukin-1 (IL-1) family is a central component of the innate immune response and is composed of 11 different ligands [8]. Two members of this family, IL-1b and IL-1 receptor antagonist (IL-1Ra), have been shown to be associated with successful embryo implantation, ectopic pregnancies, and preterm birth [9,10,11,12,13,14,15,16,17,18,19]. IL-1β is a potent pro-inflammatory cytokine, while IL-1Ra binds to the same receptor as IL-1β but has no agonist activity and thereby inhibits IL-1 signaling [8].
This relationship between IL-1 components and implantation/pregnancy progression has been demonstrated in multiple murine studies. Both IL-1β and the IL-1 receptor (IL-1R) are expressed in the endometrium and increase in expression during the secretory phase of the menstrual cycle [14]. Similarly, IL-1R, IL-1β, and IL-1Ra are all present in embryos [10]. Interestingly, the release of IL-1 family components from the embryos is dependent on co-culture with maternal endometrial components [10]. Furthermore, this expression of IL-1R, IL-1β, and IL-1Ra is both temporally [9, 12] and spatially [9, 13] related to embryonic implantation onto the endometrium.
The influence of IL-1 components at the embryo-endometrial interface on implantation and fertility seems to involve a balance between its pro- and anti-inflammatory components. The blockade of IL-1β pro-inflammatory activity by IL-1Ra has been shown to prevent embryonic implantation [9]. However, fertility is also reduced in IL-1Ra knockout mice. Interestingly, their fertility was improved by the simultaneous knockout of IL-1R, thereby preventing IL-1β-mediated inflammation [11]. Studies of supernatants from in vitro-cultured human embryos have demonstrated associations between higher IL-1Ra/IL-1b ratios and better embryo quality [15, 16].
Retrospective studies in humans have suggested a relationship between serum IL-1β and IL-1Ra levels and IVF outcome [17]. However, this has not been investigated prospectively. In this study, we prospectively investigated the association between maternal IL-1β and IL-1Ra levels and IVF cycle outcome. We hypothesized that serum IL-1β levels would be prospectively related to successful IVF outcome.
Methods
Subjects
Two hundred and sixty-five women who were undergoing an IVF cycle at the Weill Cornell Center for Reproductive Medicine between 2015 and 2017 were prospectively enrolled in this study before the start of their cycles. Sixty-five women who initially enrolled were subsequently excluded from analysis due to cryopreservation of their embryos and/or their decision to proceed with preimplantation genetic diagnosis. The study was approved by the Institutional Review Board of Weill Cornell Medical College and all subjects gave informed written consent.
IVF
Standard ovulation induction protocols were employed based on the patient’s history and IVF-ET was performed as previously cited [20]. Most started gonadotropin on day 2 with a baseline sonogram and blood samples, followed by the start of an antagonist when the lead follicle was 13 mm or when E2 was > 300 pg/mL. Employing a step-down protocol ovarian stimulation was performed with a combination of gonadotropins (HMG and/or recombinant FSH [rFSH]). When at least two follicles reached or exceeded a mean diameter of 17 mm, as measured by transvaginal ultrasound, HCG was administered (3300–10,000 IU). Transvaginal ultrasound-guided follicular puncture was performed to harvest oocytes 35-36 h after HCG administration. Oocyte insemination or intracytoplasmic sperm injection (ICSI) was executed as indicated. Following retrieval, the best morphologically appearing embryos were transferred into the uterine cavity on day 3 or day 5, as clinically appropriate. Maternal age, according to our standard protocol, dictated the number of embryos transferred. On the third day after HCG administration, progesterone supplementation (50 mg intramuscular/day) was initiated and was continued until the ultrasound assessment of the pregnancy at 47–51 days of gestation, designated by the day of oocyte insemination (day 14). Transvaginal techniques were utilized for ultrasound.
Serum assays
Peripheral blood was collected on day 2 (start of cycle), day of the HCG trigger administration (around day 14), day 24 (10 days after oocyte retrieval, 5–7 days after embryo transfer), day 28 (14 days after oocyte retrieval, 9–11 days after embryo transfer, and the time of the initial pregnancy test), and day 35 (21 days after oocyte retrieval, 16–18 days after embryo transfer, and the time of the second pregnancy test) of the IVF cycle. These time points were chosen because of the availability of blood samples simultaneously obtained for clinical indications and to allow for the tracking of cytokine levels over the course of early pregnancy. Following clot formation, bloods were centrifuged (1000×g for 15 min), and the supernatants were stored at − 80 °C in aliquots until assayed. Serum levels of IL-1β and IL-1Ra were determined by commercial ELISAs (Quantikine Immunoassay, R&D systems, Minneapolis, MN, USA). The lower limit of sensitivity of the assays was < 3.91 pg/mL for IL-1β and < 39.1 pg/mL for IL-1ra.
Statistical analysis
The statistical analysis was performed using IBM SPSS Statistics 22.0 software. Data were analyzed using Friedman analysis of variance by ranks and chi-square analysis. A p value < 0.05 was considered significant. Data are presented as mean (± standard error of mean).
Results
As shown in Table 1, the mean age of patients was 37.8 ± 0.3 years and mean BMI was 24.3 ± 0.3 kg/m2. Forty-two percent of patients were nulliparous prior to initiation of their cycle. Mean parity was 0.29 ± 0.03, and mean previous number of IVF cycles 1.4 ± 0.1. Mean anti-Müllerian hormone level was 2.1 ± 0.2 ng/mL. Mean antral follicle count before cycle start was 9.3 ± 0.5 follicles. The mean number of harvested oocytes was 9.9 ± 0.5. Of those with successful fertilization, the mean number of two pronuclear (2PN) embryos was 5.9 ± 0.4. Of those who underwent transfer, mean number of embryos transferred was 2.1 ± 0.1. One hundred and twenty-seven (75.1%) of the transferred embryos were transferred on day 3, and 42 (24.9%) were transferred on day 5.
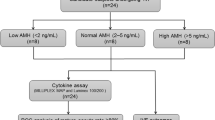
As shown in Fig. 1, 27 women (13.2%) had their cycles canceled, 9 (4.4%) did not have an embryo transfer, while 169 (82.4%) proceeded to embryo transfer. Among those with transferred embryos, 83 (49.1%) had an initial positive pregnancy test, 71 (85.5%) of whom proceeded to a clinical pregnancy and 52 (62.7%) delivered or currently have an ongoing pregnancy. Only one woman had an ectopic pregnancy.
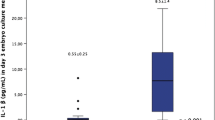
As shown in Fig. 2, when all women who had a confirmed clinical pregnancy were considered, the mean serum IL-1β values among patients who continued to have ongoing pregnancies increased over the five time points, as opposed to those who experienced pregnancy loss (R2 = 0.88, and 0.03, respectively).
For the patients who underwent embryo transfer, there was also a significant association between detectable serum IL-1β at the initiation of their IVF cycles and pregnancies that were ongoing or proceeded to delivery. Of those with undetectable serum IL-1β levels on day 2, 30/114 (26.3%) went on to deliver or have an ongoing pregnancy, compared to 11/22 (50.0%) of those with detectable serum IL-1β levels on day 2 (Pearson’s chi-square p = 0.027).
When evaluating patients with an initial positive pregnancy test, there was a significant association between undetectable serum IL-1β at the start of their cycles and subsequent pregnancy loss. Of those with undetectable serum IL-1β levels on day 2, 25/55 (45.5%) went on to lose their pregnancies after the initial positive test, compared to 2/13 (15.4%) of those with detectable serum IL-1β levels on day 2 (Pearson’s chi-square p = 0.046).
For all of the analysis including IL-1Ra serum levels over all times points, there were no associations with any outcome variables. There was additionally no association between IL-1Ra to IL-1β ratio and any outcome variables.
Discussion
Our analysis demonstrates a prospective relationship between detectable serum IL-1β at the start of an IVF cycle and increasing levels of IL-1β over time and successful IVF outcome. Among patients with a confirmed clinical pregnancy, increasing serum IL-1β levels over the first 35 days of their IVF cycle were associated with ongoing pregnancy or successful delivery. This finding was consistent with the correlation found between detectable levels of IL-1β on day 2 of the cycles and positive pregnancy outcome (both when all patients who underwent embryo transfer and only those with an initial positive pregnancy test were considered).
These results are particularly interesting when viewed in relation to previous retrospective serum studies that demonstrated a correlation between elevated levels of the pro-inflammatory components of the IL-1 family and negative IVF cycle outcome. In the retrospective serum study from Lekovich et al. in 2015, lower IL-1β levels were associated with positive pregnancy outcome (i.e., live birth) [17]. Conversely, this same study demonstrated a relationship between lower IL-1Ra and a negative pregnancy test. Both of these findings are contrary to what was found in our current study, as our results point to a relationship between elevated pro-inflammatory signaling and positive IVF cycle outcome. An important difference between the two studies is the day of serum analysis. Whereas our study analyzed IL-1β levels at the start of IVF cycle on day 2, their study analyzed cytokine levels only on day 24 and 28 of the cycles. Our current prospective study did not find any correlation between cytokine levels on days 24 and 28 and clinical outcome. However, since cytokine levels are known to vary throughout the cycle, these findings may not necessarily be contradictory.
The study from Lekovich et al. was consistent with findings from Spandorfer et al. in 2000 and 2003 [15, 16]. In the 2003 study, elevated levels of the IL-1β antagonist, IL-1Ra, was found to correlate with better embryo quality and pregnancy outcome [16]. The 2000 study similarly demonstrated a negative relationship between IL-1β and embryonic development [15]. It is important to note, however, that the study from 2000 was in vitro; it analyzed cytokine levels in the supernatant of culture medium taken from embryos grown in endometrial co-culture. Similarly, the serum analyzed in the 2003 study measured cytokine levels in serum used to supplement human tubal fluid media for in vitro embryo growth. Because these two studies were done in vitro and measured the immediate environment surrounding the embryo rather than in the peripheral circulation, it is difficult to compare these findings directly to the current results.
Other research concerning the effect of IL-1 signaling on pregnancy has pointed to a more nuanced role of this cytokine on outcome. Molecular murine studies have demonstrated a relationship between IL-1 family signaling and embryo implantation [9, 10, 12, 13]. A 1994 study from Simon et al. demonstrated that IL-1 signaling blockade prevented embryo implantation in mice, which highlights the importance of the pro-inflammatory IL-1 components in successful pregnancy outcome, particularly at early stages [9]. All of these findings taken together point to a complex relationship between IL-1 family signaling and pregnancy outcome. Because there is likely a delicate and incompletely understood balance between the pro-inflammatory and anti-inflammatory components of the IL-1 family system in pregnancy, this may account for the seemingly contradictory results between the retrospective and prospective serum studies.
A strength of the present study is that it was prospective and patients were enrolled and followed clinically. Although past studies have evaluated serum IL-1 family cytokines and IVF cycle outcome retrospectively [16, 17], this is the first prospective study that we could identify on this topic. However, there are several limitations that need to be considered. Only 83 women achieved a positive pregnancy test after embryo transfer. These small numbers limited the statistical analysis and may have disguised other potentially significant findings or confounding factors that may have appeared with a larger patient population. It will be important for future research on this topic to include more patients. Nevertheless, despite the small sample size of this study, starting to elucidate the prospective relationship between pro-inflammatory cytokines and IVF outcome is an important step towards understanding how the embryo’s environment affects development. Similarly, only one patient was diagnosed with an ectopic pregnancy. Despite past research pointing to a relationship between the pro-inflammatory components of the IL-1 family and rate of ectopic pregnancy, this prevented our study from reaching any conclusions about ectopic pregnancy.
Another limitation of this study is the unknown relationship between serum cytokine levels and the activity of cytokines at the level of the endometrium. Although there is good reason to believe that cytokine activity at the embryo-endometrium interface would manifest proportionally in serum, there is no data available specifically describing this relationship. Finally, this study is limited by the lack of knowledge of embryo quality. Because all patients whose embryos underwent preimplantation genetic diagnosis were excluded from the study, we do not know what effect the quality of the embryos had on cycle outcome, independent of external cytokine environment. It will be important for future studies to investigate this relationship further by controlling for embryo quality.
Conclusion
Overall, despite a small sample size, this study provides additional information regarding the relationship between the IL-1 family and IVF cycle outcome. Because of its prospective nature, the results are more robust than prior retrospective serum studies. With additional data, the hope is to allow clinicians to use cytokine serum markers as predictors of IVF outcomes, thereby guiding management.
References
Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2014 Assisted reproductive technology national summary report Atlanta, GA; 2016 Available from: http://www.cdc.gov/art/reports/.
Ghaebi M, Nouri M, Ghasemzadeh A, Farzadi L. Immune regulatory network in successful pregnancy and reproductive failures. Biomed Pharmacother. 2017;88:61–73.
Makrigiannakis A, Petsas G, Toth B, Relakis K. Recent advances in understanding immunology of reproductive failure. J Reprod Immunol. 2011;90:96–104.
Hyde KJ, Schust DJ. Immunologic challenges of human reproduction: an evolving story. Fertil Steril. 2016;106:499–510.
Ramhorst R, Grasso E, Paparini D, Hauk V, Calo G, Vota D, et al. Decoding the chemokine network that links leukocytes with decidual cells and the trophoblast during early implantation. Cell Adhes Migr. 2016;10:197–207.
Dekel N, Gnainsky Y, Granot I, Racicot K, Mor G. The role of inflammation for a successful implantation. Am J Reprod Immunol. 2014;72:141–7.
Granot I, Gnainsky Y, Dekel N. Endometrial inflammation and effect on implantation improvement and pregnancy outcome. Reproduction. 2012;144:661–8.
van de Veerdonk FL, Netea MG. New insights in the immunobiology of IL-1 family members. Front Immunol. 2013;4:1–11.
Simon C, Frances A, Piquette GN, el Danasouri I, Zurawski G, Dang W, et al. Embryonic implantation in mice is blocked by interleukin-1 receptor antagonist. Endocrinology. 1994;134:521–8.
Simon C, Mercader A, Gimeno MJ, Pellicer A. The interleukin-1 system and human implantation. Am J Reprod Immunol. 1997;37:64–72.
Irikura VM, Lagraoui M, Hirsh D. The epistatic interrelationships of IL-1, IL-1 receptor antagonist, and the type I IL-1 receptor. J Immunol. 2002;169:393–8.
Kruessel JS, Huang HY, Wen Y, Kloodt AR, Bielfeld P, Polan ML. Different pattern of interleukin-1beta-(IL-1beta), interleukin-1 receptor antagonist- (IL-1ra) and interleukin-1 receptor type I-(IL-1R tI) mRNA-expression in single preimplantation mouse embryos at various developmental stages. J Reprod Immunol. 1997;34:103–20.
Simon C, Frances A, Piquette G, Hendrickson M, Milki A, Polan ML. Interleukin-1 system in the materno-trophoblast unit in human implantation: immunohistochemical evidence for autocrine/paracrine function. J Clin Endocrinol Metab. 1994;78:847–54.
Simon C, Piquette GN, Frances A, Polan ML. Localization interleukin-1 type I receptor and interleukin-1b in human endometrium throughout menstrual cycle. J Clin Endocrinol Metab. 1993;77:549–55.
Spandorfer SDD, Neuer A, Liu H-CC, Bivis L, Clarke R, Veeck L, et al. Interleukin-1 levels in the supernatant of conditioned media of embryos grown in autologous endometrial coculture: correlation with outcome after in vitro fertilization. Am J Reprod Immunol. 2000;43:6–11.
Spandorfer SD, Neuer A, Liu HC, Rosenwaks Z, Witkin SS. Involvement of interleukin-1 and the interleukin-1 receptor antagonist in in vitro embryo development among women undergoing in vitro fertilization-embryo transfer. J Assist Reprod Genet. 2003;20:502–5.
Lekovich J, Witkin SS, Doulaveris G, Orfanelli T, Shulman B, Pereira N, et al. Elevated serum interleukin-1β levels and interleukin-1β-to-interleukin-1 receptor antagonist ratio 1 week after embryo transfer are associated with ectopic pregnancy. Fertil Steril. 2015;104:1190–4.
Fasouliotis SJ, Spandorfer SD, Schattman G, Liu HC, Roberts JE, Rosenwaks Z. Maternal serum levels of interferon-gamma and interleukin-2 soluble receptor-alpha predict the outcome of early IVF pregnancies. Hum Reprod. 2004;19:1357–63.
Huang H, Chan S, Sc B, Wu C. Interleukin-1 system messenger ribonucleic acid and protein expression in human fallopian tube may be associated with ectopic pregnancy. Fertil Steril. 2005;84:1484–92.
Spandorfer S, Avrech O, Colombero L, Palermo G, Rosenwaks Z. Effect of parental age on fertilization and pregnancy characteristics in couples treated by intracytoplasmic sperm injection. Hum Reprod. 1998;13:334–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Kreines, F.M., Nasioudis, D., Minis, E. et al. IL-1β predicts IVF outcome: a prospective study. J Assist Reprod Genet 35, 2031–2035 (2018). https://doi.org/10.1007/s10815-018-1296-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1296-0