Abstract
Inflammation is a multifaceted biological reaction to a wide range of stimuli, and it has been linked to the onset and progression of chronic diseases such as heart disease, cancer, and diabetes. Inflammatory markers found in the blood, including C-reactive protein, serum amyloid A, fibrinogen, plasma viscosity, erythrocyte sedimentation rate, interleukin-6, and soluble adhesion molecules (like intercellular adhesion molecule-1 and vascular cell adhesion molecule-1), are risk factors for cardiovascular diseases such as coronary heart disease, stroke, and peripheral arterial disease. These markers play a crucial role in understanding and assessing cardiovascular health. Due to this complicated relationship between inflammation and cardiovascular disease, anti-inflammatory agents of natural origin have been the subject of many preclinical and clinical studies in recent years. Eugenol is a natural phenolic compound found in clove oil, nutmeg oil, cinnamon oil, and bay leaf oil, as well as other essential oils. Eugenol has been shown to have anti-inflammatory properties in many forms of experimental inflammation. It may scavenge free radicals, which contribute to inflammation and tissue damage. Various studies also suggest that eugenol can limit the production of inflammatory mediators such as prostaglandins, cytokines, and chemokines. Animal models of arthritis, colitis, and lung damage, as well as human clinical studies, have shown that eugenol has phenomenal anti-inflammatory properties. These properties suggest that eugenol may be able to reduce the risk of cardiovascular diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural medicinal products have made significant contributions to the development of new drugs for the treatment of a wide variety of conditions, including cardiovascular disease, cancer, multiple sclerosis, and infectious disorders, among others. These products are valued for their high efficacy and safety profile. In fact, more than 250,000 natural substances have been documented in the course of drug discovery (Cragg and Newman 2013).
The therapeutic effects of natural products can be attributed to a rich array of compounds such as alkaloids, terpenoids, sesquiterpenoids, lactones, glycosides, steroids, peptides, amino acids, flavonoids, essential oils, carotenoids, and more. Traditional medicines, primarily plant-based, have played a vital role in global healthcare, with the World Health Organization (WHO) reporting that 80% of people rely on these remedies for primary healthcare needs. Remarkably, 80% of the 122 plant-derived drugs are still used for their original ethno-pharmacological purposes. Medicinal plants offer a unique combination of safety, affordability, potency, and accessibility, making them a cornerstone of traditional medicine practice (Hosseinzadeh et al. 2015).
The concept of mild chronic vascular inflammation as part of the pathophysiology of cardiovascular disease, indeed there are links between vascular inflammation, endothelial dysfunction and oxidative stress.
Recent years have witnessed a surge in research dedicated to exploring the therapeutic potential of non-nutritional compounds found in the diet, particularly in the prevention and treatment of degenerative diseases such as cardiovascular diseases and infectious diseases (Tedeschi et al. 2021; Kumar et al. 2021; Singh and Singh 2022). Phytochemicals, a diverse group of bioactive elements, encompass vitamins, carotenoids, food polyphenols (including flavonoids), phytoalexins, phenolic chemicals, indoles, sulphur-rich substances, and more. Among these, flavonoids are commonly found in fruits, vegetables, and beverages like tea, wine, and beer. They are also prevalent in various dietary supplements and herbal remedies due to their potential cardioprotective effects (Das and Das 2007; Patel et al. 2018).
Medicinal plants are a rich source of essential oils and secondary metabolites. These essential oils are volatile liquids that readily dissolve in lipids and organic solvents (Jirovetz et al. 2006; Hosseinzadeh et al. 2015). Over the past few decades, Indian scientists and researchers have conducted numerous studies highlighting the involvement of eugenol and essential oils in the medicinal properties of various plants (Hosseinzadeh et al. 2015). Aromatic plants such as Eugenia caryophyllus, Syzygium aromaticum, Dicipelium cariophyllatum, Pimenta dioica, Croton zehntneri, and Ocimum gratissimum contain substantial amounts of eugenol as one of their chemical constituents (Jirovetz et al. 2006). Additionally, fragrant plants like basil, bay, marjoram, mace, and nutmeg also contain significant levels of eugenol. These plants have gained recognition for their antioxidant, anti-inflammatory, and antimicrobial properties, making them popular choices for treating chronic inflammation and various serious health conditions (Jirovetz et al. 2006; Hosseinzadeh et al. 2015).
Eugenol was first identified as an aromatic component extracted from Eugenia caryophyllus, is derived from clove oil, and serves as a phenolic aromatic ingredient (Marchese et al. 2017). It is a transparent or pale-yellow oil that exhibits mild solubility in both water and organic solvents (Nisar et al. 2021). Eugenol has found applications as a topical analgesic, and it is commonly used in dental plasters, fillings, and cements, as well as in local anaesthetics and antiseptics. The fragrance and flavouring industries also utilize clove and eugenol (Pramod et al. 2010; Isaksson et al. 2020). Pharmacological research has explored the effects of eugenol on various systems, including the immunological, reproductive, cardiovascular, gastric, central nervous, and urinary systems, as well as its impact on blood biochemistry. Clove essential oil has demonstrated anti-inflammatory, antinociceptive, cytotoxic, anti-carcinogenic, hepatoprotective, analgesic, antibacterial, antioxidant, antifungal, and antiviral properties (Kouidhi et al. 2010; Hussain et al. 2017; Han and Parker 2017; Ugbogu et al. 2021). Eugenol, a common phenylpropanoid, plays essential roles in the food and cosmetic industries (Mohammadi Nejad et al. 2017).
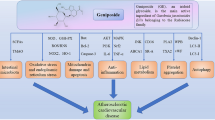
Eugenol exhibits antioxidative, anti-inflammatory, and cardiovascular characteristics, along with analgesic and localised anaesthetic effects. Research on the pharmacokinetics and pharmacodynamics of eugenol in human subjects has provided valuable insights. Eugenol has even been investigated as a substance that enhances penetration. Its multifaceted applications make it a promising candidate for the development of novel pharmaceuticals targeting diverse cardiovascular disease risk factors through distinct pathways. In this review, we will delve into the effectiveness of eugenol in mitigating various risk factors associated with cardiovascular disease (Fig. 1).
Role of Eugenol in different pharmacological pathways
Angiotensin converting enzyme inhibitory action
Persistent hypertension is a significant risk factor for various diseases such as cardiovascular diseases, chronic renal failure, and diabetes (Sarnak and Levey 2000; Kumar et al. 2021; Corb Aron et al. 2021). In hypertension, the renin-angiotensin system regulates blood pressure as well as salt and water balance. Inhibiting the angiotensin converting enzyme (ACE) has become a new therapeutic technique for treating high blood pressure in both diabetic and non-diabetic patients (Sankhyan and Pawar 2013; Mnafgui et al. 2013; Gheorghe et al. 2020). In a study using alloxan-induced diabetic rats, eugenol was found to inhibit ACE. Additionally, an in-vitro study showed that eugenol can inhibit pancreatic α-amylase (IC50 = 62.53 µg/mL), lipase (IC50 = 130.67 µg/mL), and ACE activity (Rathod and Yadav 2021). Furthermore, an in-vivo study revealed that eugenol decreased the activity of amylase in diabetic rats (Hamdin et al. 2019).
Hypotensive effect of eugenol
Eugenol has been found to have a hypotensive effect in various studies. In an animal study, eugenol was found to decrease the blood pressure in hypertensive rats. Another study reported that eugenol could lower blood pressure in normotensive and hypertensive animals by inhibiting the activity of certain enzymes involved in blood pressure regulation. In human studies, eugenol has been found to have a relaxant effect on blood vessels, leading to a reduction in blood pressure. However, more research is needed to establish the hypotensive effects of eugenol in humans and determine the optimal dose and duration of treatment. The administration of Eugenol intravenously leads to hypotension and bradycardia, which are dose dependent. This indicates the presence of a functional parasympathetic nerve drive to the heart. The hypotension is a result of active vascular relaxation rather than the withdrawal of sympathetic tone (Elsebai and Albalawi 2022). It has been observed that the release of nitric oxide (NO) from vascular endothelial cells does not play a role in mediating Eugenol-induced hypotension. When Eugenol is administered intravenously in rats, it reduces the Mean Arterial pressure even in the absence of central sympathetic nerve drive, which normally contributes to maintaining blood pressure. This effect persists even when ganglionic blockade is induced by hexamethonium (de Fátima Leal Interaminense de Andrade 2007). It was also noted that the essential oil derived from the leaves of Ocimum gratissimum, along with its primary component, eugenol, exhibited amplified hypotensive effects in conscious rats with DOCA-salt-induced hypertension. Hypotensive effects were observed in hypertensive rats when administered eugenol at concentrations ranging from 0.006 to 6 mM. These effects can be attributed to the relaxation of blood vessels (de Fátima Leal Interaminense de Andrade 2007; Savsani 2020).
Vasorelaxant action of eugenol
Vasorelaxation refers to the relaxation of smooth muscles in blood vessels, resulting in the dilation of blood vessels and a decrease in blood pressure. The ability of eugenol to induce vasorelaxation has been demonstrated in various studies (Touyz et al. 2018). In an animal study, eugenol was found to reduce blood pressure in hypertensive rats by inducing vasorelaxation (Damiani et al. 2003). Another study reported that eugenol could lower blood pressure in normotensive and hypertensive animals by inhibiting the activity of certain enzymes involved in blood pressure regulation (Rathod and Yadav 2021).
The vasorelaxant action of eugenol is believed to be mediated by various mechanisms. One of the mechanisms is the activation of endothelial nitric oxide synthase (eNOS), leading to the production of NO. NO is a potent vasodilator that relaxes smooth muscles in blood vessels, resulting in vasorelaxation. Eugenol has been found to increase the production of NO in endothelial cells, leading to vasorelaxation (Damiani et al. 2003; Rameshrad et al. 2016).
Eugenol has demonstrated the ability to stimulate potassium channels within smooth muscle cells, thereby promoting the outward movement of potassium ions. This phenomenon, in turn, induces hyperpolarization of the smooth muscle cells, ultimately leading to vasorelaxation. The activation of potassium channels by eugenol has been observed in multiple studies, emphasizing its potential as a therapeutic agent for addressing vascular dysfunctions in humans, including conditions such as preeclampsia (Dantas et al. 2022). These findings open up new possibilities for the utilization of eugenol in the treatment of such disorders.
Furthermore, eugenol has been found to induce significant vasorelaxation specifically in uterine arteries of pregnant goats. Notably, its vasorelaxant effect is amplified approximately three-fold during pregnancy, indicating its potential as both a nutraceutical agent and a therapeutic candidate for managing hypertensive disorders associated with pregnancy (Parija et al. 2020).
In addition to its vasorelaxant action, eugenol has also been found to have antioxidant and anti-inflammatory properties, which may contribute to its potential therapeutic applications in the treatment of hypertension and cardiovascular diseases. Oxidative stress and inflammation are known to play a crucial role in the development of hypertension and cardiovascular diseases. Eugenol has been found to reduce oxidative stress and inflammation, leading to a decrease in blood pressure and improvement in cardiovascular function.
Calcium channel blocker action
There is evidence that certain types of High Voltage ion channels are involved in inflammation. High Voltage calcium channels are abundantly expressed in smooth muscle cells. Yamamoto et al. demonstrated that Ca++ influx is controlled by the transient receptor potential melastin 2 (TRPM 2) that further controls ROS induced chemokine production in monocytes. These ROS are responsible for inflammatory reactions (Yamamoto et al. 2008). Increased Ca++ leak from the sarcoplasmic reticulum (SR) via RYR2. This, in turn, causes diastolic SR Ca2+ release events, which activate the Na+/Ca2+ exchanger (NCX), producing a transient-inward current that causes membrane depolarization. Numerous studies have implicated hyperphosphorylation of RYR2 via calmodulin-dependent protein kinase II (CaMKII) in arrhythmogenic SR Ca2+ leak in ventricular and atrial myocytes of diseased hearts. CAMKII, which typically requires the binding of Ca2+ to calmodulin for its activation, is also triggered by oxidation. However, eugenol induces vasorelaxation is by inhibiting the influx of calcium ions into smooth muscle cells. By inhibiting the influx of calcium ions, eugenol prevents smooth muscle contraction, leading to vasorelaxation that can lead to lower the blood pressure, and in the case of angina, this vasorelaxation can also reduce the frequency and severity of spasms in coronary arteries, improving blood flow to the heart (Dantas et al. 2022). Eugenol has been found to inhibit the influx of calcium ions in smooth muscle cells, leading to cardioprotective (Chen et al. 1997). Eugenol exhibits dose-dependent and reversible vasodilator effects, with its potency varying within the range of 0.2–20 µmol. These vasodilator responses are partially reliant on the presence of the endothelium (Damiani et al. 2003). Notably, a study conducted by Sensch and colleagues revealed that eugenol, at concentrations ranging from 60 to 600 µmol/L, demonstrated negative inotropic effects on Guinea pig heart muscle. The magnitude of this effect was comparable to that of nifedipine, a calcium channel blocker (Sensch et al. 2000). The smooth muscle relaxant properties of eugenol are attributed to its ability to block voltage-sensitive and receptor-operated channels. Additionally, Endothelia cells Ca2+ influx then causes the inflammatory process. Eugenol blocks these actions mediated through the NO generated in the endothelial cells (Earley and Brayden 2015; Hu et al. 2016).
Eugenol has been found to inhibit high-voltage-activated calcium channel currents in both capsaicin-sensitive and capsaicin-insensitive dental primary afferent neurons (Earley and Brayden 2015). Interestingly, this inhibitory action of eugenol remains unaffected by capsazepine, suggesting that it does not involve the activation of transient receptor potential vanilloid 1. Furthermore, eugenol has demonstrated the ability to inhibit N-type calcium currents in the C2D7 cell line (Hwang et al. 2020). This inhibition of high-voltage-activated calcium channel currents in dental primary afferent neurons is responsible for the dental analgesic action of eugenol. In terms of anaesthetic action, eugenol has been shown to inhibit sodium channel currents in rat dental primary afferent neurons through the whole-cell patch-clamp method (Comunanza et al. 2011). Both capsaicin-sensitive and capsaicin-insensitive neurons are inhibited by eugenol, and its excitatory effect is potentially due to the inhibition of voltage-gated potassium channels. Additionally, Yang and colleagues have indicated that eugenol exerts its effects on sensory nerve endings in teeth, at least partially, through vanilloid receptor 1 (Yang et al. 2003; Pramod et al. 2010). The combination of eugenol and QX-314 irreversibly and selectively blocked voltage-gated sodium channels in trigeminal ganglion neurons expressing TRPV1 (Hwang et al. 2020).
The analgesic effect of eugenol is likely attributed to its inhibition of voltage-gated calcium channels. Importantly, the mechanism of eugenol’s inhibition of voltage-gated calcium channels is distinct from that of capsaicin and does not involve transient receptor potential vanilloid 1 (Chung et al. 2008). It appears that both capsaicin receptor-mediated and capsaicin receptor-independent pathways are involved in the activation of calcium channels by eugenol (Ohkubo and Kitamura 1997).
Anti-inflammatory action
Inflammation is the complicated process that causes the three primary symptoms of inflammation-pain, oedema, and heat. Local blood flow, vasodilation, fluid extravasation, and mediators that promote inflammation were all induced by inflammation (Foudah et al. 2022). Both oxidative stress and inflammation are related processes that are present in a variety of clinical disorders, including cancer, renal, liver, and cardiovascular disease. Increased ROS generation in the inflamed inflammatory tissue during inflammation significantly contributes to the synthesis of pro-inflammatory mediators such cytokines, chemokines, etc. that are involved in the migration of inflammatory cells (Tan et al. 2022).
Various studies have investigated the anti-inflammatory effects of eugenol, focusing on leukocyte migration and employing different stimuli such as N-formyl-methionyl-leucyl-phenylalanine, leukotriene B4, and carrageenan. The recruitment of polymorphonuclear leukocytes to the site of inflammation involves a complex response that relies on interactions between the endothelium and leukocytes, leading to their extravasation at the inflamed site (Kummer et al. 2015). Research indicates that eugenol significantly reduces leukocyte migration both in vitro and in vivo by modulating the rolling and adherence of leukocytes to perivascular tissue. This effect has been observed in studies examining leukocyte migration in response to various stimuli (Estevão-Silva et al. 2014). Furthermore, Pan and Dong demonstrated that eugenol administration inhibits eosinophilia induced by ovalbumin (OVA) in lung tissue, preventing the increase of Interleukin-4 &-5 (IL-4 & IL-5) levels. It also reduces the activation of Nuclear factor kappa B (NF-κB) signaling pathways in an experimental model of allergic asthma induced by OVA (Pan and Dong 2015). The anti-inflammatory response of eugenol plays a significant role in its anti-asthmatic effect, leading to a decrease in airway resistance.
Moreover, eugenol has shown beneficial effects in lipopolysaccharide (LPS)-induced acute lung injury. Its administration inhibits inflammation and the recruitment of leukocytes into lung tissue by downregulating the expression of proinflammatory cytokines [IL-6 and Tumour necrosis factor-α (TNF-α)], as well as suppressing NF-κB signaling and neutrophil infiltration. These findings suggest that eugenol has anti-inflammatory properties that contribute to its therapeutic potential in various inflammatory conditions (Magalhães et al. 2010; Barboza et al. 2018).
Eugenol exhibits protective effects against hepatic ischemia/reperfusion (I/R) injury by reducing liver damage and suppressing inflammatory mediators. This is achieved by reducing myeloperoxidase activity, TNF-α levels, and NF-κB expression. Additionally, eugenol modulates the redox status by increasing glutathione (GSH) levels and altering oxidative markers while reducing malondialdehyde levels. These actions collectively contribute to the amelioration of hepatic I/R injury (Wong et al. 2017; Barboza et al. 2018). In the context of cardiac remodelling following myocardial infarction, eugenol has been evaluated as a preventive agent. It effectively reduces cardiac injury biomarkers, including troponin-T, creatine kinase-muscle/brain, and lactate dehydrogenase (LDH). This leads to improvements in electrocardiographic and hemodynamic parameters, highlighting its potential as an antithrombotic, anti-inflammatory, and anti-ischemic agent (Wong et al. 2017).
Furthermore, eugenol inhibits the expression of inducible nitric oxide synthase (iNOS) in macrophages upon exposure to LPS, resulting in a reduction in NO levels (Yeh et al. 2011). Additionally, eugenol promotes the downregulation of TNF-α in LPS-activated macrophages, which is associated with antigenotoxic activity when DNA damage is induced with doxorubicin. These findings highlight the molecular mechanisms through which eugenol exerts its anti-inflammatory effects, particularly in regulating the production of inflammatory mediators by macrophages (Feng et al. 2018).
According to another study, eugenol exerts cardioprotective effects on the transplanted heart in rat heterotopic heart transplantation through alleviating myocardial oedema, downregulating the myocardial inflammatory response and inhibiting myocardial apoptosis (Feng et al. 2018). In a study conducted by Rodrigues and colleagues, the impact of a hydroalcoholic extract of clove on the production of pro-inflammatory cytokines by macrophages in mice was investigated. It was observed that clove oil, at a dosage of 200 mg/kg, exhibited cytokine inhibition, which could be attributed to the presence of eugenol (Rodrigues et al. 2009). Eugenol, as a constituent of clove oil, possesses anti-inflammatory properties, contributing to this activity. Furthermore, the anti-inflammatory activity of eugenol in the context of LPS-induced lung injury has been demonstrated in an in vivo study. Administration of eugenol at a dose of 160 mg/kg, intraperitoneally (ip), exhibited significant anti-inflammatory effects (Magalhães et al. 2010; Bittencourt-Mernak et al. 2021).
Antioxidative action
Via antioxidant elements and scavenging free radicals
Eugenol plays a significant role as a potent antioxidant and exhibits radical-scavenging activity. The compound aspirin eugenol ester mitigates H2O2-induced oxidative stress in human umbilical vein endothelial cells by acting through the Mitochondria-Lysosome Axis. Additionally, aspirin eugenol ester ameliorates paraquat-induced oxidative damage by modulating the ROS/p38-MAPK-mediated mitochondrial apoptosis pathway (Huang et al. 2019).
At a concentration of 15 μg/mL, eugenol inhibits lipid peroxidation in a linoleic acid emulsion. Its inhibitory activity is approximately five times higher than that of α-tocopherol and about ten times lower than that of BHT (Xiao-Rong et al. 2021). Eugenol completely inhibits both iron and Fenton reagent-mediated lipid peroxidation induced in mitochondria by (Fe(II)-ascorbate) or (Fe(II) + H2O2). The measurement of thiobarbituric acid reactive substances (TBARS) formation is used to assess lipid peroxidation (Nagababu et al. 2010). In an in vivo study, eugenol provides significant hepatic protection against lipid peroxidation-mediated liver damage induced by CCl4 administration in rats. Furthermore, eugenol inhibits the increase in SGOT activity and cell necrosis but does not prevent endoplasmic reticulum (ER) damage, as indicated by the decrease in cytochrome P450 and G-6-phosphatase activity (Nagababu et al. 1995). The protective action of eugenol is attributed to its interception of secondary radicals derived from ER lipids rather than its interference with primary radicals of CCl4 (Nagababu et al. 1995; Halliwell 2001). Eugenol undergoes enzymatic or non-enzymatic oxidation via the one-electron pathway, resulting in the formation of a phenoxyl radical and potentially leading to eugenol quinonemethide. The cytotoxicity of eugenol-related compounds is significantly associated with the production activity of phenoxyl radicals, the stability of subsequent quinonemethide (QM), and their hydrophobicity (Fujisawa et al. 2002).
Via nuclear factor erythroid 2–related factor 2 (Nrf2) pathway
Nrf2 is primarily known for its role in maintaining cellular redox balance and protecting cells from oxidative stress, which is a significant contributor to heart diseases and cardiac damage. It regulates the production of antioxidant genes, which ultimately provide anti-inflammatory actions. Nrf2 and its primary negative regulator, the E3 ligase adaptor Kelch-like ECH-associated protein 1 (Keap1), play critical roles in intracellular redox homeostasis and inflammatory regulation. It has been reported that animal and cell models of cardiotoxicity show a downregulation of the expression of Nrf2 in these models (Hu et al. 2023; Adeyemi et al. 2023; Jiao et al. 2023). Thus, regulating the Nrf2 signalling pathway through pharmacological interventions can mitigate the negative consequences of cardiotoxicity and be employed as a therapeutic candidate in the prevention and treatment of cardiovascular illnesses. Furthermore, Eugenol has been shown to modulate the Nrf2 signalling pathway in several in vitro and in vivo studies of various diseases. For instance, in IR-induced acute kidney injury mice model, methyl eugenol modulates the AMPK/GSK3β axis to regulate the cytoplasmic–nuclear translocation of Nrf2, resulting in Nrf2 nuclear retention and thereby enhancing antioxidant target gene transcription that protects the kidney from oxidative damage (Kuang et al. 2023). Similarly, eugenols potential in cardioprotection also been explored in animal as well as cell models. In a hamster model of atherosclerosis induced by a high-fat diet (HFD), Aspirin eugenol ester (AEE) significantly reduced HFD-induced malondialdehyde levels, restored SOD activity and GSH/GSSG ratio, and decreased the overexpression of inducible NOS (iNOS) in the aorta and in an in vitro model of oxidative stress (H2O2-induced apoptosis of HUVECs). Moreover, in vitro studies showed that AEE protected vascular endothelial cells from oxidative damage by reducing NO production via NOS and increasing Nrf2 expression (Huang et al. 2019).
Analgesic action
Analgesic action denotes the capacity of a substance or medicine to alleviate pain without inducing unconsciousness. These pain-relieving agents, often referred to as painkillers, function by specifically affecting the pain pathways within the nervous system (Thakur et al. 2016). Their primary goal is to decrease or obstruct the transmission of pain signals from the location of injury or inflammation to the brain, thereby easing the feeling of pain and providing relief to the person experiencing it. Eugenol exerts its analgesic effect by inhibiting cyclooxygenase and prostaglandin H synthase, suggesting its involvement in the modulation of these pain-related pathways. This inhibition may occur through eugenol’s competition with arachidonic acid (Markowitz et al. 1992). Moreover, eugenol demonstrates the ability to suppress the release of proinflammatory mediators such as interleukin-1β, tumour necrosis factor-α, and prostaglandin E2 from macrophages (das Chagas Pereira de Andrade and Mendes 2020). Additionally, eugenol has been revealed to possess analgesic action via reduction of inflammatory response in few studies (Daniel et al. 2009). As well as, eugenol holds promise as an anti-inflammatory agent for managing acute inflamed dental pulps, apical periodontitis and cardiomyopathy in diabetic rats (Qar et al. 2022). For instance, in an arthritis-induced male SD rat model, eugenol ameliorated both paw and joint swelling by inhibiting inflammatory response (Sharma et al. 1994). Even, Eugenol not only shows analgesic effect in rabbits but also showed greater fever reducing potential than paracetamol (Nisar et al. 2021). Therefore, eugenol can be considered a potential therapeutic agent for managing pain in various cardiovascular disorders caused by inflammatory mediators.
Anti-atherosclerosis activity
Hyperlipidemia and hypercholesterolemia are major contributors to the development of systemic atherosclerosis, which leads to a variety of cardiovascular disorders (Gheorghe et al. 2020; Behl et al. 2020). Eugenol has been found to reduce expressions of CRP, iNOS, TNF-α, IL-1β, and NF-κB in atherogenic diet fed rat’s hepatic tissues (Karuppasamy et al. 2022). This shows the cardioprotective activity of eugenol through the inhibition of inflammatory mediators. Furthermore, eugenol reduces steatosis and hepatic inflammation in liver sections, as well as lowering hepatomegaly, and decreases the activity of hepatic marker enzymes alanine aminotransferase (ALT) and alkaline phosphatase (ALP) in hypercholesterolemic rats (Venkadeswaran et al. 2016). While eugenol does not inhibit hepatic 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase, it downregulates transient receptor potential vanilloid (TRPV1) channels in the liver (Harb et al. 2019). Eugenol pretreatment prevented liver injury by decreasing CYP2E1 activity, lipid peroxidation indices, protein oxidation and inflammatory markers and by improving the antioxidant status. Eugenol decreased the expression of COX-2 gene induced by thioacetamide (Yogalakshmi et al. 2010). Similarly in cardiovascular issues, eugenol prevents the lipid peroxidation indices, protein oxidation and inflammatory markers.
Aspirin eugenol ester, a novel drug compound combining aspirin and eugenol, exhibits anti-thrombotic, anti-atherosclerotic, and anti-oxidative effects. It potentially protects vascular endothelial cells from oxidative injury by modulating NOS and Nrf2 signaling pathways (Huang et al. 2019). The anti-atherosclerotic effects of aspirin eugenol ester on vascular endothelial dysfunction involve the inhibition of oxidative stress and reduction in the expression of adhesion molecules. Furthermore, eugenol increases the levels of superoxide dismutase, catalase, glutathione peroxidase, and glutathione-S-transferase, which are crucial antioxidative enzymes (Srivastava and Mustafa 1989).
In a study conducted by Choudhary et al., eugenol was observed to counteract isoproterenol-induced cardiac hypertrophy in male Wister rats, using a dose of 1 mg/kg twice daily (Choudhary et al. 2006). The researchers demonstrated that eugenol suppressed serum calcineurin activity in vitro and reduced oxidative stress and apoptosis induced by isoproterenol. Moreover, eugenol restored cardiac calcineurin and protein kinase C activity in ventricular tissue to normal levels (Choudhary et al. 2006; Pramod et al. 2010; Nisar et al. 2021).

Pharmacokinetics and pharmacodynamics of eugenol
Eugenol possesses the capacity to inhibit proinflammatory mediators, including NOS, lipoxygenase, and cyclooxygenase. Its analgesic effects are exerted through the inhibition of Na+ channels and cyclooxygenase. Damiani et al. investigated the inotropic effects of eugenol in rat left ventricular papillary muscles, revealing a decrease in contraction force without impacting the contractile machinery (Ohkubo and Kitamura 1997). A concentration of 0.5 mM of eugenol completely blocked inward Ca2+ channels. Eugenol has also been found to express transient receptor potential vanilloid 3, a warm-sensitive Ca2+-permeable cation channel present in the skin, tongue, and nose, as explained by (Xu et al. 2007). Moreover, eugenol has been reported to enhance the response of GABAA receptors, which can modulate neural transmission in the brain similar to benzodiazepines and barbiturates (Johnston 2005; Pramod et al. 2010). Salah et al. demonstrated that eugenol induces relaxation of the rat ileal strip, reduces intestinal transit in rats, and enhances the diarrhoea-inducing effect of castor oil (Cho et al. 2008). They proposed that these effects are mediated through the modulation of Ca2+ channels. In isolated canine and human ventricular cardiomyocytes, eugenol accelerates the inactivation of the Ca2+ current (Xu et al. 2007).
Fischer et al. conducted an investigation on the metabolism of eugenol in male and female healthy volunteers, revealing its rapid absorption and metabolism following oral administration. Within 24 h, almost all of the administered dose is excreted in the urine, with less than 0.1% being excreted unchanged. Eugenol was detected in the urine as conjugates and metabolites, with eugenol glucuronide and sulphate accounting for 55% of the conjugate metabolites (Fischer et al. 1990). The metabolism of eugenol in humans was also found to involve pathways such as the epoxide-diol pathway, allylic oxidation, synthesis of a thiophenol and a substituted propionic acid, and migration of the double bond (Mohammadi Nejad et al. 2017). Guénette et al. conducted a study using non-compartmental analysis to explore the pharmacokinetic parameters of eugenol after oral administration in male Sprague–Dawley rats at a dose of 40 mg/kg (Guenette et al. 2006; Guénette et al. 2007).
Marketed preparations of eugenol
Eugenol is already incorporated into various marketed preparations for different purposes. These are some formulations which are available in the market (Table 1).
Conclusion
Inflammation has been identified as a critical factor in nearly every chronic disease aetiology, including cardiovascular disease. Multiple pro-inflammatory mediators and regulatory systems are involved in the physiology of inflammation. The transition from fatty streak to complex plaque is facilitated by inflammation. T cells excite macrophages through cyto-signaling or CD40 ligation, resulting in the generation of cytokines and MMPs that contribute to the formation of the fibrous cap, which protects the plaque. However, as time passes, the fibrous cap thins and becomes more prone to fragmentation, allowing a thrombus to form and cause a myocardial infarction or other cardiovascular issues. This series of events deviates dramatically from the traditional understanding of cardiovascular disease as a lipid storage problem. As our understanding of the pathophysiological causes grows, it is critical that risk assessment and treatment for cardiovascular disease progress in tandem. C-reactive protein, serum amyloid A, fibrinogen, plasma viscosity, erythrocyte sedimentation rate, and cytokines such as interleukin-6, as well as soluble adhesion molecules (e.g., intercellular adhesion molecule-1, vascular cell adhesion molecule-1) must all be carefully considered before being included in risk assessment algorithms. Lifestyle changes and complementary and alternative therapy may be critical in avoiding coronary bypass surgery and other cardiovascular problems.
Eugenol has proven anti-inflammatory effects in preclinical and clinical studies. It has already been proven to have a vasorelaxant effect via activating eNOS, inhibiting calcium influx, and activating potassium channels. Eugenol’s vasorelaxant effect, as well as its antioxidant and anti-inflammatory properties, could have therapeutic significance in the treatment of hypertension and cardiovascular disease. However, more detailed preclinical and clinical evidence is required to define the appropriate therapy dose, formulation, and duration. It should be highlighted that eugenol should not be used as a replacement for approved hypertension medications without first consulting a healthcare professional, since additional research is needed to fully understand the molecular mechanisms underlying its efficacy.
Availability of data and materials
Not applicable.
References
Adeyemi DH, Obembe OO, Hamed MA, Akhigbe RE (2023) Sodium acetate ameliorates doxorubicin-induced cardiac injury via upregulation of Nrf2/HO-1 signaling and downregulation of NFkB-mediated apoptotic signaling in Wistar rats. Naunyn Schmiedebergs Arch Pharmacol. https://doi.org/10.1007/s00210-023-02620-4
Barboza JN, da Silva-Maia-Bezerra-Filho C, Silva RO et al (2018) An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid Med Cell Longev 2018:3957262. https://doi.org/10.1155/2018/3957262
Behl T, Bungau S, Kumar K et al (2020) Pleotropic effects of polyphenols in cardiovascular system. Biomed Pharmacother 130:110714. https://doi.org/10.1016/j.biopha.2020.110714
Bittencourt-Mernak MI, Pinheiro NM, da Silva RC et al (2021) Effects of eugenol and dehydrodieugenol b from nectandra leucantha against lipopolysaccharide (LPS)-induced experimental acute lung inflammation. J Nat Prod 84:2282–2294. https://doi.org/10.1021/acs.jnatprod.1c00386
Chen S-J, Huang Y-C, Wu B-N, Chen I-J (1997) Eugenolol: an eugenol-derived β-adrenoceptor blocker with partial β2-agonist and calcium mobilization inhibition associated vasorelaxant activities. Drug Dev Res 40:239–250. https://doi.org/10.1002/(SICI)1098-2299(199703)40:3%3c239::AID-DDR4%3e3.0.CO;2-L
Cho JS, Kim TH, Lim J-M, Song J-H (2008) Effects of eugenol on Na+ currents in rat dorsal root ganglion neurons. Brain Res 1243:53–62. https://doi.org/10.1016/j.brainres.2008.09.030
Choudhary R, Mishra KP, Subramanyam C (2006) Interrelations between oxidative stress and calcineurin in the attenuation of cardiac apoptosis by eugenol. Mol Cell Biochem 283:115–122. https://doi.org/10.1007/s11010-006-2386-3
Chung G, Rhee JN, Jung SJ et al (2008) Modulation of CaV2.3 calcium channel currents by eugenol. J Dent Res 87:137–141. https://doi.org/10.1177/154405910808700201
Comunanza V, Carbone E, Marcantoni A et al (2011) Calcium-dependent inhibition of T-type calcium channels by TRPV1 activation in rat sensory neurons. Pflugers Arch 462:709–722. https://doi.org/10.1007/s00424-011-1023-5
Corb Aron RA, Abid A, Vesa CM et al (2021) Recognizing the benefits of pre-/probiotics in metabolic syndrome and type 2 diabetes mellitus considering the influence of akkermansia muciniphila as a key gut bacterium. Microorganisms 9:618. https://doi.org/10.3390/microorganisms9030618
Cragg GM, Newman DJ (2013) Natural products: a continuing source of novel drug leads. Biochim Biophys Acta 1830:3670–3695. https://doi.org/10.1016/j.bbagen.2013.02.008
Damiani CEN, Rossoni LV, Vassallo DV (2003) Vasorelaxant effects of eugenol on rat thoracic aorta. Vascul Pharmacol 40:59–66. https://doi.org/10.1016/s1537-1891(02)00311-7
Daniel AN, Sartoretto SM, Becerra-Schmidt G et al (2009) Anti-inflammatory and antinociceptive activities A of eugenol essential oil in experimental animal models. Rev Bras. https://doi.org/10.1590/S0102-695X2009000200006
das Chagas Pereira de Andrade F, Mendes AN (2020) Computational analysis of eugenol inhibitory activity in lipoxygenase and cyclooxygenase pathways. Sci Rep 10:16204. https://doi.org/10.1038/s41598-020-73203-z
Das S, Das DK (2007) Resveratrol: a therapeutic promise for cardiovascular diseases. Recent Pat Cardiovasc Drug Discov 2:133–138. https://doi.org/10.2174/157489007780832560
de Dantas DM, de Silva AA, Pereira-de-Morais L et al (2022) Characterization of the vasodilator effect of eugenol in isolated human umbilical cord arteries. Chemico-Biol Interact 359:109890. https://doi.org/10.1016/j.cbi.2022.109890
de Fátima Leal Interaminense de Andrade L (2007) RI UFPE: Estudo dos efeitos cardiovasculares do óleo essencial do Ocimum gratissimum e de seu principal constituinte, Eugenol, em ratos hipertensos DOCA-sal, acordados
Earley S, Brayden JE (2015) Transient receptor potential channels in the vasculature. Physiol Rev 95:645–690. https://doi.org/10.1152/physrev.00026.2014
Elsebai MF, Albalawi MA (2022) Essential oils and COVID-19. Molecules. https://doi.org/10.3390/molecules27227893
Estevão-Silva CF, Kummer R, Fachini-Queiroz FC et al (2014) Anethole and eugenol reduce in vitro and in vivo leukocyte migration induced by fMLP, LTB4, and carrageenan. J Nat Med 68:567–575. https://doi.org/10.1007/s11418-014-0839-7
Feng W, Jin L, Xie Q et al (2018) Eugenol protects the transplanted heart against ischemia/reperfusion injury in rats by inhibiting the inflammatory response and apoptosis. Exp Ther Med 16:3464–3470. https://doi.org/10.3892/etm.2018.6598
Fischer IU, von Unruh GE, Dengler HJ (1990) The metabolism of eugenol in man. Xenobiotica 20:209–222. https://doi.org/10.3109/00498259009047156
Foudah AI, Devi S, Alqarni MH et al (2022) Quercetin attenuates nitroglycerin-induced migraine headaches by inhibiting oxidative stress and inflammatory mediators. Nutrients 14:4871. https://doi.org/10.3390/nu14224871
Fujisawa S, Atsumi T, Kadoma Y, Sakagami H (2002) Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology 177:39–54. https://doi.org/10.1016/s0300-483x(02)00194-4
Gheorghe G, Toth PP, Bungau S et al (2020) Cardiovascular risk and statin therapy considerations in women. Diagnostics (basel) 10:483. https://doi.org/10.3390/diagnostics10070483
Guenette SA, Beaudry F, Marier JF, Vachon P (2006) Pharmacokinetics and anesthetic activity of eugenol in male Sprague-Dawley rats. J Vet Pharmacol Ther 29:265–270. https://doi.org/10.1111/j.1365-2885.2006.00740.x
Guénette SA, Ross A, Marier J-F et al (2007) Pharmacokinetics of eugenol and its effects on thermal hypersensitivity in rats. Eur J Pharmacol 562:60–67. https://doi.org/10.1016/j.ejphar.2007.01.044
Halliwell B (2001) Food-derived antioxidants: how to evaluate their importance in food and in vivo. Handbook of antioxidants, 2nd edn. CRC Press, p 46
Hamdin CD, Utami SW, Muliasari H et al (2019) Histological pattern on pancreas and liver of diabetic rats after treatment of eugenol isolated from leaves of Syzygium aromaticum. AIP Conf Proc 2199:060004. https://doi.org/10.1063/1.5141313
Han X, Parker TL (2017) Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharm Biol 55:1619–1622. https://doi.org/10.1080/13880209.2017.1314513
Harb AA, Bustanji YK, Almasri IM, Abdalla SS (2019) Eugenol reduces LDL cholesterol and hepatic steatosis in hypercholesterolemic rats by modulating TRPV1 receptor. Sci Rep 9:14003. https://doi.org/10.1038/s41598-019-50352-4
Hosseinzadeh S, Jafarikukhdan A, Hosseini A, Armand R (2015) The application of medicinal plants in traditional and modern medicine: a review of Thymus vulgaris. Int J Clin Med 06:635. https://doi.org/10.4236/ijcm.2015.69084
Hu G, Li X, Zhang S, Wang X (2016) Association of rat thoracic aorta dilatation by astragaloside IV with the generation of endothelium-derived hyperpolarizing factors and nitric oxide, and the blockade of Ca2+ channels. Biomed Rep 5:27–34. https://doi.org/10.3892/br.2016.680
Hu S, Liu B, Yang M et al (2023) Carnosic acid protects against doxorubicin-induced cardiotoxicity through enhancing the Nrf2/HO-1 pathway. Food Funct 14:3849–3862. https://doi.org/10.1039/D2FO03904D
Huang M-Z, Yang Y-J, Liu X-W et al (2019) Aspirin eugenol ester attenuates oxidative injury of vascular endothelial cells by regulating NOS and Nrf2 signalling pathways. Br J Pharmacol 176:906–918. https://doi.org/10.1111/bph.14592
Hussain S, Rahman R, Mushtaq A, Zerey-Belaskri AE (2017) Clove: a review of a precious species with multiple uses
Hwang S-M, Lee K, Im S-T et al (2020) Co-application of eugenol and QX-314 elicits the prolonged blockade of voltage-gated sodium channels in nociceptive trigeminal ganglion neurons. Biomolecules 10:1513. https://doi.org/10.3390/biom10111513
Isaksson M, Rustemeyer T, Antelmi A (2020) Dental materials and implants. In: Contact dermatitis. pp 1–40
Jiao K, Su P, Li Y (2023) FGFR2 modulates the Akt/Nrf2/ARE signaling pathway to improve angiotensin II-induced hypertension-related endothelial dysfunction. Clin Exp Hypertens 45:2208777. https://doi.org/10.1080/10641963.2023.2208777
Jirovetz L, Buchbauer G, Stoilova I et al (2006) Chemical composition and antioxidant properties of clove leaf essential oil. J Agric Food Chem 54:6303–6307. https://doi.org/10.1021/jf060608c
Johnston GAR (2005) GABA(A) receptor channel pharmacology. Curr Pharm Des 11:1867–1885. https://doi.org/10.2174/1381612054021024
Karuppasamy V, Sekar SK, Kandhasamy S, et al (2022) The anti-integrity activity of eugenol on inflammatory markers of chronic atherosclerosis (In Review)
Kouidhi B, Zmantar T, Bakhrouf A (2010) Anticariogenic and cytotoxic activity of clove essential oil (Eugenia caryophyllata) against a large number of oral pathogens. Ann Microbiol 60:599–604. https://doi.org/10.1007/s13213-010-0092-6
Kuang B, Wang Z, Hou S et al (2023) Methyl eugenol protects the kidney from oxidative damage in mice by blocking the Nrf2 nuclear export signal through activation of the AMPK/GSK3β axis. Acta Pharmacol Sin 44:367–380. https://doi.org/10.1038/s41401-022-00942-2
Kumar S, Behl T, Sachdeva M et al (2021) Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus. Life Sci 264:118661. https://doi.org/10.1016/j.lfs.2020.118661
Kummer R, Estevão-Silva CF, Bastos RL et al (2015) Alpha-pinene reduces in vitro and in vivo leukocyte migration during acute inflammation. Int J Appl Res Nat Prod 8:12–17
Magalhães CB, Riva DR, DePaula LJ et al (2010) In vivo anti-inflammatory action of eugenol on lipopolysaccharide-induced lung injury. J Appl Physiol 108:845–851. https://doi.org/10.1152/japplphysiol.00560.2009
Marchese A, Barbieri R, Coppo E et al (2017) Antimicrobial activity of eugenol and essential oils containing eugenol: a mechanistic viewpoint. Crit Rev Microbiol 43:668–689. https://doi.org/10.1080/1040841X.2017.1295225
Markowitz K, Moynihan M, Liu M, Kim S (1992) Biologic properties of eugenol and zinc oxide-eugenol. A clinically oriented review. Oral Surg Oral Med Oral Pathol 73:729–737. https://doi.org/10.1016/0030-4220(92)90020-q
Mnafgui K, Kaanich F, Derbali A et al (2013) Inhibition of key enzymes related to diabetes and hypertension by eugenol in vitro and in alloxan-induced diabetic rats. Arch Physiol Biochem 119:225–233. https://doi.org/10.3109/13813455.2013.822521
Mohammadi Nejad S, Özgüneş H, Başaran N (2017) Pharmacological and toxicological properties of eugenol. Turk J Pharm Sci 14:201–206. https://doi.org/10.4274/tjps.62207
Nagababu E, Sesikeran B, Lakshmaiah N (1995) The protective effects of eugenol on carbon tetrachloride induced hepatotoxicity in rats. Free Radic Res 23:617–627. https://doi.org/10.3109/10715769509065281
Nagababu E, Rifkind JM, Boindala S, Nakka L (2010) Assessment of antioxidant activity of eugenol in vitro and in vivo. Methods Mol Biol 610:165–180. https://doi.org/10.1007/978-1-60327-029-8_10
Nisar MF, Khadim M, Rafiq M et al (2021) Pharmacological properties and health benefits of eugenol: a comprehensive review. Oxid Med Cell Longev 2021:2497354. https://doi.org/10.1155/2021/2497354
Ohkubo T, Kitamura K (1997) Eugenol activates Ca(2+)-permeable currents in rat dorsal root ganglion cells. J Dent Res 76:1737–1744. https://doi.org/10.1177/00220345970760110401
Pan C, Dong Z (2015) Antiasthmatic effects of eugenol in a mouse model of allergic asthma by regulation of vitamin D3 upregulated protein 1/NF-κB pathway. Inflammation 38:1385–1393. https://doi.org/10.1007/s10753-015-0110-8
Parija SC, Jandhyam H, Mohanty BP, et al (2020) Eugenol induces potent vasorelaxation in uterine arteries from pregnant goats—a promising natural therapeutic agent for hypertensive disorders of pregnancy. 2020.09.22.307629
Patel RV, Mistry BM, Shinde SK et al (2018) Therapeutic potential of quercetin as a cardiovascular agent. Eur J Med Chem 155:889–904. https://doi.org/10.1016/j.ejmech.2018.06.053
Pramod K, Ansari SH, Ali J (2010) Eugenol: a natural compound with versatile pharmacological actions. Nat Prod Commun 5:1999–2006
Qar J, Al-Trad B, Khmaiseh A et al (2022) The effect of eugenol treatment on diabetic cardiomyopathy in streptozotocin-induced diabetic rats. Biomed Pharmacol J 15:623–633
Rameshrad M, Babaei H, Azarmi Y, Fouladi DF (2016) Rat aorta as a pharmacological tool for in vitro and in vivo studies. Life Sci 145:190–204. https://doi.org/10.1016/j.lfs.2015.12.043
Rathod P, Yadav RP (2021) Anti-diabesity potential of various multifunctional natural molecules. J Herb Med 27:100430. https://doi.org/10.1016/j.hermed.2021.100430
Rodrigues TG, Fernandes A, Sousa JPB et al (2009) In vitro and in vivo effects of clove on pro-inflammatory cytokines production by macrophages. Nat Prod Res 23:319–326. https://doi.org/10.1080/14786410802242679
Sankhyan A, Pawar PK (2013) Metformin loaded non-ionic surfactant vesicles: optimization of formulation, effect of process variables and characterization. Daru 21:7. https://doi.org/10.1186/2008-2231-21-7
Sarnak MJ, Levey AS (2000) Cardiovascular disease and chronic renal disease: a new paradigm. Am J Kidney Dis 35:S117-131. https://doi.org/10.1016/s0272-6386(00)70239-3
Savsani HH (2020) Pharmacological modulation of calcium in selective cardiovascular disorders through store operated calcium entry inhibitors. Vadodara
Sensch O, Vierling W, Brandt W, Reiter M (2000) Effects of inhibition of calcium and potassium currents in guinea-pig cardiac contraction: comparison of beta-caryophyllene oxide, eugenol, and nifedipine. Br J Pharmacol 131:1089–1096. https://doi.org/10.1038/sj.bjp.0703673
Sharma JN, Srivastava KC, Gan EK (1994) Suppressive effects of eugenol and ginger oil on arthritic rats. Pharmacology 49:314–318. https://doi.org/10.1159/000139248
Singh A, Singh KS (2022) Bioactive compounds from polar regions: an account of chemical ecology and biotechnological applications. Curr Org Chem 26:1055–1087
Srivastava KC, Mustafa T (1989) Spices: antiplatelet activity and prostanoid metabolism. Prostaglandins Leukot Essent Fatty Acids 38:255–266. https://doi.org/10.1016/0952-3278(89)90129-4
Tan J, Yadav MK, Devi S, Kumar M (2022) Neuroprotective effects of arbutin against oxygen and glucose deprivation-induced oxidative stress and neuroinflammation in rat cortical neurons. Acta Pharm 72:123–134. https://doi.org/10.2478/acph-2022-0002
Tedeschi LO, Muir JP, Naumann HD et al (2021) Nutritional aspects of ecologically relevant phytochemicals in ruminant production. Front Vet Sci 8:628445. https://doi.org/10.3389/fvets.2021.628445
Thakur G, Singh A, Singh I (2016) Chitosan-montmorillonite polymer composites: formulation and evaluation of sustained release tablets of aceclofenac. Sci Pharm 84:603–618. https://doi.org/10.3390/scipharm84040603
Touyz RM, Alves-Lopes R, Rios FJ et al (2018) Vascular smooth muscle contraction in hypertension. Cardiovasc Res 114:529–539. https://doi.org/10.1093/cvr/cvy023
Ugbogu OC, Emmanuel O, Agi GO et al (2021) A review on the traditional uses, phytochemistry, and pharmacological activities of clove basil (Ocimum gratissimum L.). Heliyon 7:e08404. https://doi.org/10.1016/j.heliyon.2021.e08404
Venkadeswaran K, Thomas PA, Geraldine P (2016) An experimental evaluation of the anti-atherogenic potential of the plant, Piper betle, and its active constitutent, eugenol, in rats fed an atherogenic diet. Biomed Pharmacother 80:276–288. https://doi.org/10.1016/j.biopha.2016.03.028
Wong ZW, Thanikachalam PV, Ramamurthy S (2017) Molecular understanding of the protective role of natural products on isoproterenol-induced myocardial infarction: a review. Biomed Pharmacother 94:1145–1166. https://doi.org/10.1016/j.biopha.2017.08.009
Xiao-Rong L, Ning M, Xi-Wang L et al (2021) Untargeted and targeted metabolomics reveal the underlying mechanism of aspirin eugenol ester ameliorating rat hyperlipidemia via inhibiting FXR to induce CYP7A1. Front Pharmacol 12:733789. https://doi.org/10.3389/fphar.2021.733789
Xu G-Y, Winston JH, Shenoy M et al (2007) Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology 133:1282–1292. https://doi.org/10.1053/j.gastro.2007.06.015
Yamamoto S, Shimizu S, Kiyonaka S et al (2008) TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 14:738–747. https://doi.org/10.1038/nm1758
Yang BH, Piao ZG, Kim Y-B et al (2003) Activation of vanilloid receptor 1 (VR1) by eugenol. J Dent Res 82:781–785. https://doi.org/10.1177/154405910308201004
Yeh JL, Hsu JH, Hong YS et al (2011) Eugenolol and glyceryl-isoeugenol suppress LPS-induced iNOS expression by down-regulating NF-kappaB AND AP-1 through inhibition of MAPKS and AKT/IkappaBalpha signaling pathways in macrophages. Int J Immunopathol Pharmacol 24:345–356. https://doi.org/10.1177/039463201102400208
Yogalakshmi B, Viswanathan P, Anuradha CV (2010) Investigation of antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology 268:204–212. https://doi.org/10.1016/j.tox.2009.12.018
Acknowledgements
The authors are grateful to the Chitkara College of Pharmacy, Chitkara University, Rajpura, Patiala, Punjab, India for providing the necessary facilities to carry out the research work.
Funding
Nil.
Author information
Authors and Affiliations
Contributions
TGS, SD conceptualising, editing and execution of manuscript. AM, SC: literature survey, wrote manuscript, medical writing and designing the review. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Ethical approval
Not applicable.
Consent to Publish
The paper is submitted with the consent of all listed authors.
Compliance with ethical standards
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Devi, S., Chauhan, S., Mannan, A. et al. Targeting cardiovascular risk factors with eugenol: an anti-inflammatory perspective. Inflammopharmacol 32, 307–317 (2024). https://doi.org/10.1007/s10787-023-01392-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01392-w