Abstract
The aim of the study was to investigate the antiasthmatic effects of eugenol (EUG) and the possible mechanisms. Asthma model was established by ovalbumin induction. A total of 50 mice were randomly assigned to five experimental groups: control, OVA, OVA + dexamethasone (2 mg/kg), OVA + EUG (10 mg/kg), and OVA + EUG (20 mg/kg). Airway resistance (Raw) were measured, histological studies were evaluated by the hematoxylin and eosin (HE) staining, interleukin-4 (IL-4) and interleukin-5 (IL-5) were evaluated by enzyme-linked immunosorbent assay (ELISA), Vitamin D3 upregulated protein 1 (VDUP1), IκBα, P-IκBα, NF-κBP65, and p-NF-κBP65 were measured by Western blotting. Our study demonstrated that EUG inhibited OVA-induced increases in Raw and eosinophil count; IL-4 and IL-5 were recovered. Histological studies demonstrated that EUG substantially inhibited OVA-induced eosinophilia in the lung tissue. Western blotting studies demonstrated that EUG substantially inhibited P-IκBα, NF-κBP65, and p-NF-κBP65 protein levels and increased VDUP1 and IκBα protein levels. These findings suggest that EUG may effectively ameliorate the progression of asthma and could be used as a therapy for patients with allergic asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Allergic diseases of the airway such as asthma or allergic rhinitis are on the rise throughout the world, especially in Western countries [1]. One of the most important diseases is bronchial asthma that is defined as a chronic inflammation of the bronchial airways and characterized by reversible airway obstruction, increased mucus production, and infiltration of the airway with eosinophils, neutrophils, mast cells, and T-lymphocytes [2].
Interleukin (IL)-4 and IL-5, expressed by T-helper type 2 (Th2) cells, are key cytokines in the pathogenesis of atopy and atopic asthma [3, 4]. Both IL-4 and IL-13 promote acute inflammatory processes and underlying structural changes to the airways, and their receptors are expressed on a number of cell types [5].
Vitamin D3 upregulated protein 1 (VDUP1) is a multifunctional 46-kDa protein that was originally identified as a differentially expressed gene in 1α,25-dihydroxyvitamin D3 (1,25[OH]2D3)-treated HL-60 leukemia cells (15) and B16 melanoma cells [6]. VDUP1 interacts with the antioxidant thioredoxin (Trx) to inhibit the reducing activity of Trx and blocks the interactions of Trx with other factors, such as ASK-1 and PAG, thereby increasing the vulnerability of cells to oxidative stress [7]. The NF-κB transcription factor family is the crucial mediator of the inflammatory process and plays an important role in other processes, including development, cell growth and survival, and proliferation. Cytokines and pathogen-associated molecular patterns (PAMPs) stimulate cell surface receptors including toll-like receptors (TLRs) to initiate a signaling cascade resulting in the activation of NF-κB, which drives expression of target genes that mediate the release of antimicrobial molecules and cytokines to activate the immune response. There are many upstream signaling molecules involved in NF-kB inactivation. Previous studies suggest that inactivation of NF-kB by VDUP1 indicates a potential role for this pathway in inflammation responses [8]. So, we chose VDUP1/NF-kB pathway as a potential therapeutic target to inhibit inflammation response in OVA-induced asthma.
The Piper betle plant is widely grown in the tropical humid climate of Southeast Asia, and its leaves, with a strong pungent and aromatic flavor, are widely consumed as a mouth freshener. The leaves of P. betle, which are reported to possess medicinal properties, have been widely used as a traditional medicine for treating several diseases including catarrhal and pulmonary infections [9]. Eugenol (4-allyl-1-hydroxy-2-methoxybenzene), a natural food-flavoring agent found in plant extracts of P. betle, cinnamon, clove, basil, and nutmeg, has been found to ameliorate oxidative stress by preventing oxidative tissue damage in different experimental models [10].
Hence, we hypothesized that EUG, which have protective effects in ovalbumin-sensitized asthma in vivo, might also be associated with the inhibition of inflammation. The present study was undertaken to evaluate the protective effects of EUG and to elucidate whether its protective effects related to VDUP1/NF-κB pathway.
MATERIALS AND METHODS
Materials
EUG (pure 95 %) was purchased from National Institutes for Food and Drug Control (Beijing, China). Dexamethasone was purchased from Xiansheng drug Store (Nanjing, China), ovalbumin (OVA) (Sigma Chemical Co., St Louis, MO), aluminum hydroxide (Pierce Biotechnology, Rockford, USA), Wright–Giemsa staining (Nanjing Jiancheng Bioengineering institute, Nanjin, China). ELISA kits (R&D, Minneapolis, MN, USA).
Animals
Specific pathogen-free female BALB/c mice, aged 6–8 weeks, which were routinely screened serologically for relevant respiratory pathogens, were purchased from the Experimental Animal Center of China Pharmaceutical University (Nanjing, China). Animal experiment was carried out in accordance with the Guidelines for Animal Experimentation of China Pharmaceutical University. A total of 60 female BALB/c mice were obtained from Experimental Animal Center of China Pharmaceutical University (Nanjing, China). Mice were maintained in an animal facility under standard laboratory conditions for 1 week prior to experiments and provided with water and standard chow ad libitum. All experimental procedures were carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals, and animal handling followed the dictates of the National Animal Welfare Law of China.
Sensitization, Airway Challenge, and Treatment
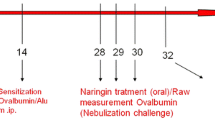
Mice were divided into five groups (each of ten animals): (1) normal control, (2) asthma–model control, (3) asthma treated with dexamethasone (2 mg/kg, administered by gavage), and (4 and 5) asthma treated with EUG (10, 20, administered by gavage). Allergic asthma was induced by ovalbumin (grade V) in five of the groups, using the method described by Oh et al. [11]. Mice were immunized via intraperitoneal (i.p.) injection with 10-μg chicken OVA and 2 mg aluminum hydroxide in 200 μl phosphate-buffered saline (PBS) (pH 7.4), on days 0 and 14. Mice were exposed to a 1 % (w/v) OVA solution in PBS, for 20 min, using an ultrasonic nebulizer (NE-U12; Omron Corp., Tokyo, Japan) on days 28, 29, and 30 after initial sensitization. Animals were sacrificed 48 h after the last challenge (thus, on day 32) to characterize the suppressive effects of EUG. A schematic diagram of the treatment schedule is shown in Fig. 1.
Measurement of Airway Resistance
Airway resistance was assessed as an increase in pulmonary resistance in response to increasing concentrations of aerosolized acetylcholine (Sigma) in anesthetized mice. Briefly, mice were anesthetized with 70–90-mg/kg pentobarbital sodium (Sigma). Trachea was exposed and cannulated with an 18-gauge tracheal tube. A suture around the trachea was then tied to prevent air leak. Mice were mechanically ventilated using a computer-controlled small animal ventilator (flexiVent; Scireq, Montreal, Canada) with the following parameters: respiratory rate of 150 breaths/min, tidal volume of 10 ml/kg, inhalation: exhaustion ratio of 2:3, and positive end-expiratory pressure of 2–3 cm H2O. Aerosolized PBS and increasing concentrations of acetylcholine (1, 2, 3, 4, and 5 mg/ml) were delivered to the animal, and readings were recorded every 4 min for 12 s at each concentration. Pulmonary resistance was calculated using a software program (flexiVent; Scireq).
Collection of Bronchoalveolar Lavage Fluid
Mice were sacrificed using an overdose of 50 mg/kg of pentobarbital 48 h after the last challenge, and tracheotomy was performed. After instilling ice-cold PBS (0.5 ml) into a lung, bronchoalveolar lavage (BAL) fluid was obtained by three successive aspirations (total volume 1.5 ml) via tracheal cannulation [12]. BAL fluid (BALF) samples were centrifuged at 1500 rpm for 10 min at 4 °C, the supernatants were stored in −80 °C for analysis of cytokine concentrations, and the pellet was resuspended in 100 μl of saline, centrifuged onto slides and stained for 8 min with Wright–Giemsa staining. The slides were quantified for differential cell count by counting a total of 200 cells/slide at × 40 magnification.
Histological Assessment
Lung tissues and airway tissues were detached from the mice and fixed with Carnoy’s solution overnight at 4 °C. The fixed tissues were embedded in paraffin and cut into 4-μm sections with amicrotome (Leica, Nussloch, Germany). The sections were placed on slide glasses, deparaffinized, and stained with hematoxylin and eosin (Sigma, Korea) in order to examine the cells that had infiltrated into the peribronchial connective tissues and airway tissues. Peribronchial and airway cell count based on a five-point scoring system was performed as previously described to estimate the severity of leukocyte infiltration [13]. The scoring system was as follows: 0, no cells; 1, a few cells; 2, a ring of cells 1 cell layer deep; 3, a ring of cells two to four cell layers deep; and 4, a ring of cells more than four cell layers deep.
Enzyme-Linked Immunosorbent Assay Detection of BALF Cytokines IL-4 and IL-5
BALF levels of IL-4 and IL-5 were measured by ELISA according to the manufacturer’s instructions (R&D, Minneapolis, MN, USA). All measurements were performed in duplicate. Briefly, the BALF samples were added in duplicate to 96-well plates with 100 ml per well. The appropriate biotin-conjugated antibodies were added to each well. The samples were incubated at room temperature for 2 h. The wells were then aspirated, and each well was washed five times. The substrate solutions were added to each well and were incubated for 30 min at room temperature in the dark. The optical density (O.D.) of each well was determined using a microplate reader (Bio-Rad Model 680, USA) that was set to 450 nm. A standard curve was created of the average of the O.D. duplicate readings. The results were calculated using Excel 2007 (Microsoft Office, USA).
Measurement of OVA-Specific Serum and BALF Levels of IgE
OVA-specific serum and BALF IgE levels were determined by enzyme-linked immunosorbent assay (ELISA) using samples collected 48 h after the last OVA challenge, as described previously (Jain et al., 2002). In brief, a 96-well microtiter plate was coated with OVA (10 mg/ml) and then treated with mouse sera followed by biotin-conjugated rat antimouse IgE (Pharmingen, San Diego, CA). Then, avidine horseradish peroxidase (HRP) solution was added to each well. Units are optical density readings at 405 nm.
Western Blotting of VDUP1/NF-κB Pathway
The lung tissues were homogenized, washed with PBS, and incubated in lysis buffer in addition to a protease inhibitor cocktail (Sigma, St. Louis, MO) to obtain extracts of lung proteins. The samples were loaded to 10 % SDS-PAGE gels and were electrotransferred to nitrocellulose. The blots were incubated with the appropriate concentration of specific antibody. After washing, the blots were incubated with horseradish peroxidase-conjugated second antibody. The membranes were stripped and reblotted with anti-GAPDH antibody (Sigma) to verify the equal loading of protein in each lane. Quantification of protein expression was normalized to β-actin using a densitometer (Imaging System).
Statistical Analysis
All values were expressed as the mean ± S.D. and analyzed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test using SPSS version 13.0 software; a p value of less than 0.05 was considered significant and p < 0.01 was considered to be statistically very significant.
RESULTS
Effect of EUG on Airway Resistance
Measurements of airway resistance (Raw) during methacholine-induced constriction are shown in Fig. 2. The Raw value of the OVA-challenged group was significantly higher than that of the control group (p < 0.05). The treatment groups showed a significant decrease with acetylcholine 0.5 mg/ml and 1 mg/ml (p < 0.05 and 0.01, compared with model group, respectively).
Effect of EUG on OVA-Induced Eosinophilia and BAL Fluid Composition
We examined changes in total cell levels in the BAL fluid to determine the effects of EUG on asthma. Suppression of eosinophilia by EUG in OVA-challenged mice was estimated by counting of cells recruited to the BAL fluid 24 h after the last challenge. As shown in Fig. 3, OVA induced a marked influx of leukocytes into the BAL fluid in the control group. Eosinophil levels markedly increased in the BAL fluid of OVA-challenged mice. OVA-sensitized and OVA-challenged mice treated with EUG displayed significantly reduced leukocytosis, a fall in absolute eosinophilia, and decreased lymphocytosis, upon asthma induction.
Effect of EUG on OVA-Induced Eosinophilia in Lung Tissue
We next examined the antiasthmatic effects of the EUG. Lung tissue was collected 24 h after the final OVA challenge. In OVA-induced asthmatic lung tissue, we observed marked infiltration of inflammatory cells into perivascular, peribronchial connective tissues, compared with the normal tissue. Moreover, most leukocytes were eosinophils. The control group displayed no change in the extent of inflammatory cell infiltration, similar to what was seen in dexamethasone-treated-positive control mice. Eosinophilia in OVA-sensitized and OVA-challenged mice treated with EUG was significantly attenuated compared with the level seen in OVA-challenged mice as shown in Fig. 4.
Pathological changes of the lung tissues observed by HE staining (light microscopy, ×200): a control, b OVA, c dexamethasone, 2 mg/kg, d EUG (10 mg/kg), and e EUG (20 mg/kg). Scoring of the extent of inflammation via quantitative analysis of inflammatory cell infiltration in lung sections. Values are expressed as means ± SDs. Compared with control: # p < 0.05; ## p < 0.01. Compared with model: *p < 0.05; **p < 0.01.
Effect of EUG on Cytokine Levels in BAL Fluid
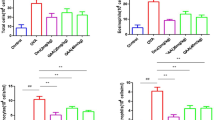
To determine whether EUG influenced cytokine secretion into BAL fluid, IL-4 and IL-5 in the BAL fluid were measured by ELISA 24 h after the final challenge. The results are shown in Fig. 5; from Fig. 5, as for BALF levels of IL-4 and IL-5, compared with control group, levels of IL-4 and IL-5 in model group were markedly increased (p < 0.05), while, compared with model group, the treatment groups can markedly decrease levels of IL-4 and IL-5.
Effect of EUG on OVA-Specific Serum and BALF Levels of IgE
The cross-linking of allergen-specific IgE on the surfaces of mast cells upon allergen challenge is relevant to the initiation of the early asthmatic reaction. The serum and BALF levels of OVA-specific immunoglobulins were measured 24 h after the final airway challenge. We determined that sensitization and challenge with OVA resulted in increased serum and BALF levels of OVA-specific IgE, when compared with the control mice. The treatment of sensitized mice with EUG resulted in reductions in OVA-specific IgE (Fig. 6). These results support the conclusion that EUG suppressed the generation of a Th2-type immune response in this animal asthma model.
Effect of EUG on VDUP1/NF-κB Pathway
The expression of VDUP1/NF-κB pathway proteins changed by OVA in the lung. As shown in Fig. 7, compared with the control group, the protein P-IκBα, NF-κBP65, and p-NF-κBP65 protein levels in the model group were significantly increased (p < 0.05). In EUG, the protein level of p-IκBα, NF-κBP65, and p-NF-κBP65 protein levels were significantly decreased compared to the model group, respectively (p < 0.05); compared with the control group, VDUP1 and IκBα protein levels in the model group were significantly decreased (p < 0.05). In EUG, the protein level of VDUP1 and IκBα protein levels were significantly increased compared to the model group, respectively (p < 0.05).
DISCUSSION
Asthma, a chronic inflammatory disease of the airways, affects many individuals worldwide. The disease may cause severe morbidity, and even mortality if exacerbated. To date, a variety of drugs have been developed to treat asthma; these include steroids, leukotriene inhibitors, mast cell stabilizers, and β2 adrenergic agonists. Many herbal medicines, including EUG, were commonly used to treat asthma, but the therapeutic efficacies and modes of action of such medicines are currently unclear. Here, using an OVA-induced mouse model of asthma, we show for the first time that EUG can be effective as a therapeutic drug for the treatment of allergic asthma.
Asthma is defined as a variable level of airway obstruction usually accompanied by airway hyper-responsiveness (AHR). Bronchoconstriction attributable to contraction or hypertrophy of airway smooth muscle (ASM), and inflammation within the airway, leads to decreased lung function [14]. AHR is a measure of the bronchial constriction commonly observed in individuals with asthma. We monitored the effects of EUG on AHR by measuring Raw. These experiments disclosed that EUG inhibited the OVA-induced AHR in response to inhaled methacholine.
The mechanisms of recruitment of inflammatory cells associated with and presumably causing AHR have been well-studied. Migration of inflammatory cells, specifically eosinophils and lymphocytes, into the lung is a major contributor to the development of allergic airway inflammation [15]. A rise in the number of eosinophils in the BAL fluid is a characteristic of asthma. The present results clearly demonstrate that EUG significantly reduces eosinophil numbers in the lung tissue.
Allergic asthma is recognized as a TH2-mediated immune system disease. Asthma and inflammation are associated with enhanced production of TH2 cytokines (IL-4 and IL-5). Our data show that EUG significantly suppresses IL-4 and IL-5 cytokine levels. The present results clearly demonstrate that EUG significantly regulated VDUP1/NF-κB pathway. Our findings support the possible use of EUG as a therapeutic drug for patients with allergic asthma. Also, further and comprehensive studies are needed before clinical application.
References
Beasley, R., J. Crane, C.K. Lai, and N. Pearce. 2000. Prevalence and etiology of asthma. J Allergy Clin Immunol 105: 466–472.
Busse, W.W., and R.F. Lemanske Jr. 2001. Asthma. N Engl J Med 344: 350–362.
Brightling, C.E., F.A. Symon, S.S. Birring, et al. 2002. TH2 cytokine expression in bronchoalveolar lavage fluid T lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitis. J Allergy Clin Immunol 110: 899–905.
Robinson, D.S., Q. Hamid, S. Ying, et al. 1992. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 326: 298–304.
Hershey, G.K. 2003. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol 111: 677–690.
Song, H., D. Cho, J.H. Jeon, S.H. Han, D.Y. Hur, Y.S. Kim, and I. Choi. 2003. Vitamin D(3) up-regulating protein 1 (VDUP1) antisense DNA regulates tumorigenicity and melanogenesis of murine melanoma cells via regulating the expression of fas ligand and reactive oxygen species. Immunol Lett 86: 235–247.
Junn, E., S.H. Han, J.Y. Im, Y. Yang, E.W. Cho, H.D. Um, D.K. Kim, K.W. Lee, P.L. Han, S.G. Rhee, and I. Choi. 2000. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol 164: 6287–6295.
Kwon, H.-J., Y.-S. Won, and H.-W. Suh. 2014. Vitamin D3 upregulated protein 1 suppresses TNF-α-induced NF-kB activation in hepatocarcinogenesis. J Immunol 185: 3980–3989.
Ahmad, I., Z. Mehmood, and F. Mohammad. 1998. Screening of some Indian medicinal plants for their antimicrobial properties. J Ethnopharmacol 62(2): 183–193.
Nagababu, E., and N. Lakshmaiah. 1992. Inhibitory effect of eugenol on non-enzymatic lipid peroxidation in rat liver mitochondria. Biochem Pharmacol 43(11): 2393–2400.
Oh, S.R., M.Y. Lee, K. Ahn, B.Y. Park, O.K. Kwon, H. Joung, J. Lee, D.Y. Kim, S. Lee, J.H. Kim, and H.K. Lee. 2006. Suppressive effect of verproside isolated from Pseudolysimachion longifolium on airway inflammation in a mouse model of allergic asthma. Int Immunopharmacol 6: 978–986.
Djukanović, R., W.R. Roche, J.W. Wilson, C.R. Beasley, O.P. Twentyman, R.H. Howarth, and S.T. Holgate. 1990. Mucosal inflammation in asthma. Am J Respir Cirtical Care Med 142: 434–457.
Duan, W., J.H. Chan, C.H. Wong, B.P. Leung, and W.S. Wong. 2004. Anti inflammatory effects of mitogen-activated protein kinase inhibitor U0126 in an asthma mouse model. J Immunol 172: 7053–7059.
Wenzel, S.E. 2006. Asthma: defining of the persistent adult phenotypes. Lancet 368: 804–813.
Elsner, J., and A. Kapp. 1999. Regulation and modulation of eosinophil effector functions. Allergy 54: 15–22.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pan, C., Dong, Z. Antiasthmatic Effects of Eugenol in a Mouse Model of Allergic Asthma by Regulation of Vitamin D3 Upregulated Protein 1/NF-κB Pathway. Inflammation 38, 1385–1393 (2015). https://doi.org/10.1007/s10753-015-0110-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0110-8