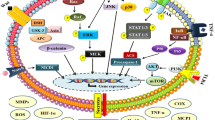
Abstract
Rheumatoid arthritis (RA), a chronic auto-immune disease, is often result of persistent and misdirectional inflammation and cannot be effectually resolved by single-target selective drugs. Present study attempted to uncover anti-arthritic efficacy and governing molecular mechanism of BLFE and its phytoconstituents berberine and rutin, with focus on dysregulated oxi-inflammation and structural integrity during articular damage using Collagen II–CFA-induced RA mice model. NMR-based phytometabolomic analysis revealed presence of phenolics and alkaloids such as berberine and rutin. BLFE, rutin and berberine remarkably mitigated Collagen II–CFA-induced disease severity index, articular damage, immune cells influx and pannus formation. An effective decrease in levels of TNF-α, IL-6, IL-1β, IFN-γ, IL-13, IL-17, MMPs, RORγt, Ob-cadherin, Cox-2, iNOS and enhancement in IL-10, IL-4 and IL-5, BMP-6/7 was observed in BLFE, rutin and berberine treatments. Molecular mechanistic analysis demonstrated reduction in expression of p-STAT-1/3, p-PI3K, p-Akt, p-JNK, p-p38, p-IκB, p-NF-κB and β-catenin via BLFE, rutin and berberine. Furthermore, reduced activation of p-ERK and p-GSK3β and enhanced splenic Tregs was only noticed in BLFE and berberine. Thus, the signifying presence of these phytoconstituents could contribute to the above-mentioned findings. These findings imply that BLFE could be beneficial for assuaging deleterious aspects of RA mediated via perturbed inflammation.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis is a multi-factorial, systemic auto-immune disease with complex etiology that predominantly characterized by inflammation and destruction of small diarthrodial joints of forelimbs as well as feet (Firestein 2003). RA is a one of the leading reasons of disability and affects approximately 1% of population worldwide and is mainly driven by non-resolving chronic inflammation (Guo et al. 2018). To date, several animal models of arthritis have been established to ascertain the complexity of pathogenesis and to delineate cellular as well as molecular mechanisms which promote joint inflammation, deformity and loss of function. Most commonly used mice model for evaluating anti-rheumatic therapeutics is CIA; as it shared multiple pathological features detected in human RA including mononuclear cell infiltration, pannus formation and destruction of cartilage and bone in the distal joints (Brand et al. 2007). Recent advances inferred that gradual accumulation of inflammatory mediators, activation of proteases (MMPs) and distortion in BMPs are the ultimate consequences of diverse arrays of interlinked signaling pathways such as MAPKs, PI3K/Akt/NF-κB, signal transducer and activators of transcription STATs that have been identified as pathogenic aspects in RA (Guo et al. 2016; Malemud 2013; Smolen and Steiner 2003).
Several symptomatic drugs such as corticosteroids, NSAIDs and DMARDs have proven efficient to relieve joint pain and inflammation (Smolen and Steiner 2003). However, due to disastrous adverse effects of these single-target drugs; plant-derived anti-inflammatory agents, particularly phenolics and alkaloids have gained much attention as a safe balanced treatment strategy for mitigation of joint inflammation and destruction (Alwarith et al. 2019; Khanna et al. 2017). Berberis lycium Royle of Berberidaceae family is one such high-value medicinal herb wildly dispersed in the Western Himalayan region at an altitude of 2000–2700 m (Sharma et al. 2018). In our previous studies, we reported a strong presence of different bioactive compounds (phenolics and alkaloids) in BLFE and its attributes in attenuation of LPS and Con-A-mediated oxi-inflammatory aggravation and DSS-induced ulcerative colitis by suppressing NF-κB/MAPKs activation. These finding conclusively suggested its potential as a suppressor of oxi-inflammatory mediators associated with inflammatory disorders (Sharma et al. 2018, 2019). To the best of our knowledge, despite presence of strong anti-inflammatory bioactive molecules, attributes as well as mechanistic molecular mechanism (S) of B. lycium Royle fruits for other inflammatory disorders including RA remains undiscovered. As, studies have also reported rheumatoid arthritis is a common complication of disease limited to colon such as ulcerative colitis (Atzeni et al. 2014). Therefore, present study aimed to decipher the efficacy and holistic molecular mechanism of BLFE in assuaging persistent inflammation and maintenance of articular structural homeostasis during prevalent RA in mice. Further, to know whether the presence of bioactive molecules of BLFE contributed for these effects, we also assessed in vivo efficacy of berberine and rutin. To test our hypothesis, we delineated effects of orally administered BLFE and its phytoconstituents rutin and berberine against CII–CFA-induced RA by examining DAI, Th1/Th2/Th17 axis, structural proteins, joint histopathology, inflammatory signaling cascades such as NF-κB, MAPKs, PI3K/Akt and STAT as well as splenic Tregs sub-population.
Materials and methods
Chemicals
Detailed description of chemicals used, and method is given in supplementary section File S1.
Extraction and characterization of BLFE
Berberis lycium fruits were extracted and quantified putative bioactive phytomolecules present in extract using UPLC–DAD–ESI/QTOF/MS/MS were reported in our previous study (Sharma et al. 2018).
NMR spectroscopy
Briefly, a final volume (600 μl) of BLFE reconstituted in D2O and methanol (80:20) was shifted to 5 mm NMR tube. NMR spectra were captured at 25 °C with frequency of 600.13 MHz on 600 MHz Bruker AVANCE III-600 spectrometer (Bruker, Germany). The detailed procedure was performed as mentioned by Kumar et al. (2017).
Animal ethics statement and induction of RA
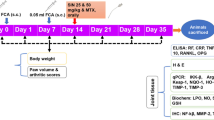
All animal experiments were approved by Institutional Animal Ethics Committee (Approval no. IAEC/IHBT/P-12/May 2018). RA was established in Balb/c mice as described by Xu et al. (2018) with minor modifications. Type II collagen was dissolved using 0.05 M acetic acid to a concentration of 2.5 mg/ml by rotating 1 h at 4 °C and mixed with equal volume of Freund’s complete adjuvant (1 mg/ml; sigma Aldrich). On day 0, mice were immunized with 100 μl emulsion containing 100 μg type II collagen and 50 μg CFA at the base of tail. The booster dose (50 μg type II collagen and 25 μg CFA) was injected on day 21st. Dex, BLFE, rutin and berberine were administered orally once a day for 42 consecutive days. The detailed experimental scheme illustrated in Fig. 1a and supplementary section File S1.
a Schematic study design for investigating the effect of BLFE (250 and 500 mg/kg b.w) and its phytoconstituents rutin (Rut) (50 mg/kg b.w) and berberine (BER) (50 mg/kg b.w) in CII and CFA-induced RA. b 1H NMR-based NMR metabolomics of BLFE. c–g BLFE and its phytoconstituents rutin and berberine ameliorate progression of CIA and paw joint architecture damage. c DAI score d Representative images of paw tissue e Haematoxylin and eosin stained of paw joint sections f Summary of the histological scores g Toluidine blue staining. Values are mean ± SD. (+ + + +) p ≤ 0.0001 versus control; (*) p ≤ 0.05, (**) p ≤ 0.01 and (****) p ≤ 0.0001 versus CIA group
Arthritis score index
Severity of arthritis was assessed in a blind fashion considering paw swelling, redness and extent of inflammation to determine the arthritis score. The severity of arthritis was recorded on the basis of scores assigned in supplementary section Table S1.
Cytokine estimation, gene expression and Western blot analysis
Briefly, each tissue sample (100 mg) was macerated in liquid nitrogen. Secreted inflammatory cytokines, gene expression of targeted inflammatory mediators and structural proteins and immunoblotting of oxi-inflammatory aggravation sensing pathways were performed as described previously (supplementary section File S1) (Sharma et al. 2019). Primer sequences used for gene expression analysis are listed in supplementary section Table S2 and list of antibodies used is depicted in supplementary section Table S3.
Histological, toluidine blue staining and IHC study
Formalin fixed paw joint tissues were decalcified in 8% formic acid solution for 72 h at 4 °C and then embedded into paraffin. Subsequently, 5 μm sections of tissue blocks were used for H & E staining as described previously (Sharma et al. 2019). The paw joint sections were examined under EVOS FL cell imaging system (Thermo Fisher Scientific, US) by a pathologist. For assessing severity of CIA, various parameters such as inflammatory cell infiltration, synovial cell proliferation, articular architectural damage and pannus formation were scored by a pathologist. Briefly, scores were assigned on basis of parameters given in supplementary section Table S4.
In order to assess articular integrity, paraffin embedded joint tissue sections were stained with toluidine blue. Briefly, sections were deparaffinised, rehydrated and stained with 0.1% toluidine blue for 10 min. Next, sections were washed with distilled water and incubated in alcohol for 2–3 min at 37 °C. Finally, sections were cleared using xylene and mounted. The images were captured using EVOS FL cell imaging system. To examine the β-catenin expression, IHC was carried out, as per protocol reported previously (Sharma et al. 2019; Tirpude et al. 2021).
Flow cytometry analysis of T cell sub-population in splenocytes
Splenocytes were isolated and flow cytometry was performed to gauze T cells sub-population as mentioned in our previous study (Sharma et al. 2019).
Statistical analyses
Resource equation method was used to calculate the group size needed for adequate statistical analysis. Statistical analyses were carried out using one-way ANOVA, followed by Tukey’s test to identify statistical significance amongst means. Differences with p value ≤ 0.05 were considered as statistically significant. Results are presented as mean ± SD.
Results
Phytochemical profiling of BLFE
To validate and corroborate chemical characterization of BLFE determined previously using UPLC–DAD–ESI/QTOF/MS/MS, NMR analysis was performed. Similar to our previous characterization, NMR analysis also revealed presence of different phytomolecules (phenolic acid, phenolics and alkaloids), amino acids, sugars, alcohol and vitamins in BLFE (Fig. 1b, supplementary section Table S5).
BLFE and its phytoconstituents rutin and berberine attenuate severity of CIA
The severity of CIA was evaluated by arthritic score, calculated as composite score of paw redness, swelling and extent of inflammation. Arthritis symptoms in CII–CFA group substantially enhanced from day 10th of CII–CFA injection and reached maximum on day 30th (Fig. 1c). However, BLFE treatment, particularly 500 mg significantly (P < 0.05) suppressed arthritic scores at all selected time points in comparison to CII–CFA group. Administration of berberine and rutin also showed marked reduction in arthritic score from day 10th onward. Gross pathological changes in CII–CFA group as shown in Fig. 1d, further proving establishment of arthritic conditions, which were also abolished on treatments with BLFE, rutin and berberine.
BLFE and its phytoconstituents rutin and berberine mitigate deteriorating joint damage
To corroborate protective and anti-inflammatory effect of BLFE and its bioactive constituents rutin and berberine in progression of RA, histopathological changes in paw joints of different animal groups were assessed. Compared to control group, CIA mice showed bone erosion, marked synovial tissue proliferation, loss of joint space, extensive inflammatory cell infiltration, pannus formation, and degradation of articular cartilage. Representative microscopic images (Fig. 1e, f) revealed administration of BLFE (250 and 500 mg), rutin (50 mg) and berberine (50 mg) appreciably attenuated apparent CII–CFA-induced pathological anomalies in paw joints. Interestingly, effect of BLFE and rutin was at par with dexamethasone, while berberine showed minimal effect in comparison to other treatments (Fig. 1e, f). Next, toluidine blue staining substantiated histopathological results. As shown in toluidine blue staining images (Fig. 1g), CII–CFA induction precedes marked articular cartilage degeneration whereas BLFE as well as rutin and berberine treatment prevented this scenario affirmed protective role of these treatments in maintaining articular integrity.
BLFE and its phytoconstituents rutin and berberine modulate Th1/Th2/Th17 cytokine axis
To further ascertain anti-inflammatory mechanisms of BLFE and its phytoconstituents rutin and berberine in rheumatic conditions, we analyzed expression of Th1 (TNF-α, IL-1β, IFN-γ, IL-6 and IL-2), Th2 (IL-4, IL-5, IL-13 and IL-10) and Th17 (IL-17) cytokines. Results demonstrated that CIA significantly enhanced transcription of TNF-α, IL-6, IL-1β and IFN-γ compared to control (Fig. 2a–d) whereas all treatments significantly (p ≤ 0.05) reversed enhanced expression of TNF-α, IL-6, IL-1β and IFN-γ. Moreover, inhibitory effects of BLFE, rutin and berberine was also observed on secretary levels of TNF-α, IL-6, IL-1β and IFN-γ (Fig. 2e–h).
BLFE and its phytoconstituents rutin (Rut) and berberine (BER) modify cytokines milieu as evident by a TNF-α mRNA b IL-6 mRNA c IL-1β mRNA d IFN-γ mRNA e TNF-α levels f IL-6 levels g IL-1β levels h IFN-γ levels i IL-5 mRNA j IL-4 mRNA k IL-10 mRNA l IL-13 mRNA m IL-5 levels n IL-4 levels o IL-10 level p IL-17 mRNA in paw joint tissue. Values are mean ± SD (n = 3–4). (+ +) p ≤ 0.01 and (+ + + +) p ≤ 0.0001 versus control; (*) p ≤ 0.05, (**) p ≤ 0.01, (***) p ≤ 0.001 and (****) p ≤ 0.0001 versus CIA group
Amongst Th2/Th17 cytokines, decrease in levels of IL-5, IL-4 and IL-10 and increases in IL-13 and IL-17 expression was observed with CII–CFA induction, which was appreciably reversed by BLFE (250 and 500 mg/kg) (Fig. 2i–p). However, rutin and berberine treatments significantly reversed levels of IL-10, IL-13 and IL-17 while statistically non-significant slight modification was also noticed for IL-5 and IL-4 expression (Fig. 2i–p).
BLFE and its phytoconstituents rutin and berberine modify structural proteins
Next, we analyzed the expression of matrix metalloproteinases (MMP-1, 2, 3, 9 and 13) and BMPs (BMP-2, 6, 7) to know whether BLFE and its phytoconstituents rutin and berberine could diminish articular cartilage degeneration. In contrast with control group, significant increase in expression of MMP-1, 2, 3, 9 and 13 was observed in CIA group which was remarkably attenuated in all treated groups (Fig. 3a–e). Furthermore, a slight reduction in expression of BMP-2, 6, 7 was noticed in CIA group. Conversely, only BMP-6 expression was considerably increased by all treatments whereas BMP-2 and BMP-7 expression was augmented via BLFE (albeit@500 mg) and berberine treatment in CIA mice (Fig. 3f–h).
BLFE and its phytoconstituents rutin (Rut) and berberine (BER) modulate structural proteins as evident from the expression of MMPs and BMPs. a MMP-1 b MMP-2 c MMP-3 and d MMP-9 e MMP-13 f BMP-2 g BMP-6 and h BMP-7. Values are mean ± SD (n = 3–4). ( +) p ≤ 0.05, (+ +) p ≤ 0.01, (+ + +) p ≤ 0.001 and (+ + + +) p ≤ 0.0001 versus control; (*) p ≤ 0.05, (**) p ≤ 0.01, (***) p ≤ 0.001 and (****) p ≤ 0.0001versus CIA group
BLFE and its phytoconstituents rutin and berberine modulate activation of IκB/NF-κB/MAPKs
To determine underlined molecular mechanism, expression and activation levels of NF-κB, IκBα and MAPKs in paw joint tissues was assessed. Immunoblotting analysis revealed that phosphorylation of NF-κB and IκBα was categorically (p ≤ 0.05) exaggerated in CIA group as compared to control (Fig. 4a, b). However, BLFE as well as rutin and berberine treatment down-regulated CIA induced phosphorylation of NF-κB and IκBα. Thus, it appears that inhibition of IκB dependent NF-κB activation could contribute to suppression of inflammatory responses in paw joint tissues. Additionally, to determine the effect of BLFE as well as rutin and berberine on MAPKs activation, phosphorylation status of p38, JNK and ERK was analyzed (Fig. 4c–e). It was noticed that enhanced activation of p38 and JNK in CIA group was reduced in all treated groups. However, ERK activation was only suppressed by BLFE and berberine (Fig. 4c).
BLFE and its phytoconstituents rutin (Rut) and berberine (BER) treatments mollify activation of IκB-NF-κB, and MAPKs in paw joint tissue of CIA. a p-IκB-α/ IκB-α b p-NF-κB/ NF-κB c p-ERK/ERK d p-JNK/JNK e p-p38/p38. Values are mean ± SD (n = 3). ( +) p ≤ 0.05, (+ +) p ≤ 0.01, (+ + +) p ≤ 0.001 and (+ + + +) p ≤ 0.0001 versus control; (*) p ≤ 0.05, (**) p ≤ 0.01, (***) p ≤ 0.001 and (****) p ≤ 0.0001 versus CIA group
BLFE and its phytoconstituents rutin and berberine modulate activation of PI3K/Akt/GSK3β/STAT-1/3 and β-catenin expression
Next, we determined effects of BLFE as well as rutin and berberine on expression and activation of PI3K, Akt and GSK3β. In comparison to control, activation of PI3K, Akt and GSK3β was considerably enhanced in CIA group (Fig. 5a–c). On the other hand, BLFE, rutin and berberine treatments showed varied decrease in activation of PI3K and Akt (Fig. 5a, b). Additionally, enhanced activation of GSK3β was also suppressed by BLFE and berberine treatment, whereas rutin treatment showed no obvious changes in its activation status. Effects of rutin on GSK3β activation was in line with dexamethasone (Fig. 5c). Additionally, to verify correlation between activation of STAT and NF-κB as well as cytokines production, expression and activation status of STAT-1 and STAT-3 was assessed. Results revealed massive increase in STAT-1 and STAT-3 expression and activation in CIA group (Fig. 5d, e). On the other hand, BLFE as well as rutin and berberine treatment group showed noticeable reduction in both STAT-1 and STAT-3 activation at all tested doses (Fig. 5d, e). Similarly, remarkably enhanced β-catenin expression in CIA group was reduced by BLFE and its phytoconstituents rutin and berberine (Fig. 5f).
BLFE and its phytoconstituents rutin (Rut) and berberine (BER) modulate P13K/Akt/GSK3β and STAT/β-catenin signaling axes. a p-PI3K/ PI3K b p-Akt/Akt c p-GSK3β/ GSK3β d p-STAT-1/STAT-1 e p-STAT-3/STAT-3 f β-catenin. Values are mean ± SD (n = 3). ( +) p ≤ 0.05, (++) p ≤ 0.01, versus control; (*) p ≤ 0.05, (**) p ≤ 0.01, (***) p ≤ 0.001 and (****) p ≤ 0.0001 versus CIA group
BLFE and its phytoconstituents rutin and berberine inhibit mediators of joint inflammation
It has proven that activation of PI3K/Akt and STAT-3 signaling triggers various factors such as RORγt, Ob-cadherin, Cox-2 and iNOS which are directly or indirectly involved in the progression of RA. Expression analysis demonstrated increase levels of RORγt, Ob-cadherin iNOS and Cox-2 in CIA mice (Fig. 6a–d) which were markedly reduced by BLFE, rutin and berberine treatments (Fig. 6a–d).
BLFE and its phytoconstituents rutin (Rut) and berberine (BER) attenuate paw joint inflammation as indicated by levels of a RORγt b OB-cadherin c iNOS d COX-2 in paw joint tissue. e–f modulation in splenic T cells proliferation as depicted by e CD4+CD25+ cells population were analyzed using flow cytometry f Representation of CD4+ vs CD25+ scatterplots. Values are mean ± SD (n = 3–4). (+ + +) p ≤ 0.001 and (+ + + +) p ≤ 0.0001 versus control; (*) p ≤ 0.05, (**) p ≤ 0.01, (***) p ≤ 0.001 and (****) p ≤ 0.0001 versus CIA group
BLFE and berberine treatment modulate splenic T cell proliferation
To assess effect of BLFE as well as rutin and berberine on adaptive immune response, we analyzed population of CD3+CD4+CD25+ Tregs in splenic tissues. The frequency of CD3+CD4+CD25+ cells was reduced in CIA mice (Fig. 6e, f). However, treatment with BLFE and berberine significantly enhanced CD3+CD4+CD25+ population in comparison to CIA mice. Rutin treatment showed no effects on Tregs cell population (Fig. 6e, f). These results strongly demonstrated role of BLFE and berberine in curbing systemic negative effects of progressive CIA.
Discussion
Plant based phytomedicines have been reported to play multi-faceted role in human health. In our previous work, we explored potential of BLFE in alleviating colitis by targeting activation of NF-κB/MAPKs (Sharma et al. 2019). Persistent and non-resolving inflammation is recognized as a major facet in perpetuating the structural damage in RA; therefore, it is prudent to accomplish mitigation of inflammation to control the progression and development of disease. Thus, present study was envisaged to decipher the underlying molecular mechanism governing anti-inflammatory potential of BLFE and its bioactive constituents rutin and berberine in context of RA. Our finding revealed that BLFE as well as rutin and berberine treatments ameliorate oxi-inflammation and other pathologies associated with RA via targeting activation of GSK-3β/STAT/PI3K/AKT/MAPKs/NF-κB signaling axes. Immunization with CII–CFA resulted in characteristic manifestations of RA which was apparently reduced via BLFE, berberine and rutin as evident from reduced DAI score and gross pathological changes. Histopathological assessment and toluidine O staining further substantiated these results. It was observed that BLFE as well as rutin and berberine treatments considerably thwart CII–CFA-induced robust joint inflammation, synovial tissue proliferation, pannus formation and articular cartilage degeneration, thereby indicating its potential in counteracting pathologies induced by CIA and associated manifestations. The present findings are in line with previous studies wherein it was observed that phytomolecules-enriched extracts and herbal formulations can efficiently impede CII induced joint inflammation (Balkrishna et al. 2019; Nho et al. 2019). Generally, cytokines imbalance induced by multiple signaling pathways is known to be critical and major meditator of RA pathogenesis (McInnes and Schett 2007). It has been proven that disruption in cytokine secretion reinforces joint inflammation and articular destruction eventually by altering regulation of structural proteins such as MMPs and BMPs that are evident as detectable hallmark and contribute to pernicious pathology of RA (Alunno et al. 2017). Reduction of matrix-degrading MMPs and maintenance of BMPs is likely to be an essential mechanism for effective anti-rheumatic regime. Thus, it is apparent that inhibition of matrix-degrading MMPs and enhancement of BMPs up to certain level would be inordinately beneficial in restoring articular bone architecture and reducing chronic inflammatory responses. Considering, these facts we next sought to assess the effect of BLFE, berberine and rutin on cytokines, proteases and bone morphogenic proteins milieu. Fascinatingly, similar to these claims, our finding also demonstrated a massive and significant increases in levels of TNF-α, IL-6, IL-1β, IFN-γ, IL-13, and IL-17 with concomitant reduction in anti-inflammatory IL-10 and Th2 cytokines IL-4 and IL-5, while BLFE, berberine and rutin treatments appeared to revere these changes impressively. Thus, supporting observed histopathological and DAI severity findings and signifies that maintenance of cytokine network with BLFE, berberine and rutin administration could be helpful in preventing joint inflammation and articular cartilage degeneration. Additionally, matrix-degrading MMPs and BMPs analysis further revealed counter effects of BLFE as well as rutin and berberine treatment on enhanced expression of MMPs and diminished levels of BMPs, particularly BMP-6 and 7. These observations are in agreement with studies where authors reported inhibition of MMPs and cytokines by phytomolecules during the progression of RA (Guo et al. 2016; Chen et al. 2019). The exact underlying molecular mechanism (s) of BLFE as well as rutin and berberine are not clear, but it is plausible to conclude that alleviations of RA and associated symptoms might occur by ameliorating pro-inflammatory cytokines and MMPs production.
To further ascertain governing molecular mechanism by which BLFE, berberine and rutin influence RA progression, expression and activation of principal inflammatory signaling cascade IκB/NF-κB/MAPKs and other interdependent regulatory cascade such as PI3K/Akt/GSK-3β/STAT/catenin, which are supposed to modulate redox homoeostasis and articular structural integrity in RA, were assessed (Wang et al. 2018; Jope et al. 2006; Tarner et al. 2007). Noteworthy, elevated activation of IκB-dependent NF-κB, and its master regulators ERK1/2, JNK and p38 MAPKs was observed in CII–CFA-challenged mice. However, BLFE as well as and berberine treatment effectively suppressed phosphorylation of IκBα-dependent NF-κB and MAPKs, while rutin was not able to inhibit activation of ERK. It is worth mentioning that dexamethasone also appeared to follow similar pattern as occurred with BLFE and berberine. These findings indicate the possible regulatory role of MAPKs in orchestrating NF-κB mediated inflammatory responses in RA. It has been evident that persistent oxi-inflammatory stress has its impact on PI3K activation and results into downstream activation of Akt and GSK3β which could be responsible for activation of NF-κB and MAPKs. Corroborating this, we also observed that CIA group is accompanied by a robust activation of PI3K, Akt and GSK3β signaling. On the other hand, attenuation of both PI3K and Akt activation was noticed in BLFE as well as rutin and berberine groups, while suppression in GSK3β activation was only observed with BLFE and berberine administered groups but not with rutin. Together, it is convincing to conclude that BLFE as well as rutin and berberine-mediated suppression of PI3K/Akt could have contributed to apparent inhibition of NF-κB/MAPKs that ultimately results in the amelioration of inflammation and articular matrix degradation induced by collagen and CFA. It is worth to mention that this is the first study implicating BLFE as well as rutin and berberine-mediated anti-rheumatic attributes could be linked to its inhibitory influences on PI3K/Akt/MAPKs/NF-κB signaling. Administration of rutin showed no effect on the activation of GSK3β, further suggesting the dual impact of ERK activation on GSKβ. Our work is in agreement with previous studies wherein it was evident that inhibition of inflammation associated with arthritis may be allied with PI3K/Akt/MAPKs/NF-κB pathways (Chen et al. 2019; Wu et al. 2017; Zhuang et al. 2017).
It has also proved that STAT signaling and cytokines balance is a vicious coalition in arthritis disease (Ivashkiv and Hu 2003). Particularly, constitutive activation of STAT-1 and STAT-3 plays an important role in the orchestration of cytokine and MMPs responses. Considering this, we sought to analyze the activation status of STAT-1 and STAT-3. It was observed that the activation of STAT-1 and STAT-3 was upregulated in CIA group which were suppressed by BLFE as well as rutin and berberine treatments. The activation of STAT-1 and STAT-3 also indicate the possibilities of enhanced expression of β-catenin in CIA group. As, studies revealed that ablation of STAT-1 and STAT-3 activation resulted in suppression of β-catenin (Ibrahem et al. 2014). Indeed, in present work we noticed enhanced expression of β-catenin in CIA group which was gradually and categorically suppressed by BLFE as well as rutin and berberine. Collectively, these outcomes justify our hypothesis and demonstrate that BLFE as well as rutin and berberine are strong anti-inflammatory agents with inhibitory effects on multi-faceted signaling involved in progression and severity of RA. It is also conceivable that presence of strong bioactive components including berberine and rutin has attributed in the observed effects of BLFE. As a result of PI3K/Akt/β-catenin/STAT3/MAPKs/NF-κB activation, induction of various other factors such as RORγt, Ob-cadherin, Cox-2 and iNOS would be imminent (Firestein 2003; Yang et al. 2007). As expected, enhanced expression of RORγt, Ob-cadherin, Cox-2 and iNOS was observed in CIA mice whereas a remarkable inhibition of these factors was observed with all used treatment. Taken together, results confirmed multi-dimensional potential of BLFE as well as rutin and berberine in inhibiting mediators that contribute to synovial inflammation.
Encouraged by apparent strong anti-arthritis attributes, we further evaluated whether BLFE as well as rutin and berberine exhibit splenic Tregs (CD3+CD4+CD25+) population modulatory attribute during RA. T cell analysis showed decrease in the CD3+CD4+CD25+ cell number in CIA group, while administration of BLFE and berberine enhanced the proliferation of CD3+CD4+CD25+ T cells. An inadequate immunoregulatory response of Tregs is considered a characteristic of auto-immune inflammatory disorders and any therapeutic strategy to revert this circumstance would present a major anti-inflammatory intervention for RA (Vyas et al. 2019). This result again highlights significance of BLFE and berberine in ameliorating systemic inflammatory disorder like RA by maintaining adaptive immune hemostasis. This signaling interplay induced by BLFE, rutin and berberine could have ultimately aided in inhibiting escalation of inflammatory and articular cartilage destructive mediators resulting in the RA abrogating attributes.
Although, the present work highlights promising effects of BLFE in RA, still certain limitations need to be addressed. For example, given deceptive anti-arthritis attributes of BLFE, it is prudent to ascertain its therapeutic potential on specific structural proteins that play main role articular repair process. Additionally, it would be fascinating to assess the synergetic effects of bioactive constitutes of BLFE on RA. Third, we only investigated anti-arthritis effects of BLFE using CIA in Balb/C mice and more specific model such as genetically engineered mouse models may be required to establish anti-arthritis efficacy of BLFE.
Conclusion
In the present work, we deciphered the effects and underlying molecular mechanism (s) of BLFE in mitigation of various aspects of joint inflammation and articular matrix destruction in CII and CFA-induced RA. Our results indicate that BLFE are strong modulators of RA which is evident by the facts that they maintain articular architectures, suppression of inflammatory mediators such as Th1/Th17 cytokines, iNOS and COX-2 and matrix-degrading MMPs in diseased animals. In particular, inhibition of inflammatory signaling mechanism NF-κB/MAPKs, PI3K/Akt and β-catenin/STAT along with enhanced proliferation of splenic Tregs represent multi-faceted protective attributes of BLFE. Further, we also assessed the potential of bioactive constitutes berberine and rutin for prevalent RA via assessing all above-mentioned parameters and observed same finding. Collectively, these findings provide convincing evidence that apparent anti-inflammatory and anti-arthritic attributes of BLFE could be ascribed to the presence of bioactive molecules, such as berberine and rutin. Thus, BLFE as well as its bioactive constituents rutin and berberine could be used as promising nutraceutical for management and prevention of RA.
Data availability
Manuscript has data included as electronic supplementary material.
Abbreviations
- BLFE:
-
Berberis lycium fruit extract
- BMPs:
-
Bone morphogenic proteins
- CII–CFA:
-
Collagen II and CFA
- CIA:
-
Collagen-induced arthritis
- Con-A:
-
Concanavalin A
- DAI:
-
Disease Activity Index
- CFA:
-
Freund’s complete adjuvant
- IHC:
-
Immunohistochemistry
- H & E:
-
Hematoxylin and Eosin
- MMPs:
-
Matrix metalloproteinases
- NMR:
-
Nuclear magnetic resonance
References
Alunno A, Carubbi F, Giacomelli R, Gerli R (2017) Cytokines in the pathogenesis of rheumatoid arthritis: new players and therapeutic targets. BMC Rheumatol. https://doi.org/10.1186/s41927-017-0001-8
Alwarith J, Kahleova H, Rembert E et al (2019) Nutrition interventions in rheumatoid arthritis: the potential use of plant-based diets. A review. Front Nutr. https://doi.org/10.3389/fnut.2019.00141
Atzeni F, Defendenti C, Ditto MC et al (2014) Rheumatic manifestations in inflammatory bowel disease. Autoimmun Rev 13:20–23. https://doi.org/10.1016/j.autrev.2013.06.006
Balkrishna A, Sakat SS, Joshi K et al (2019) Herbo-mineral formulation ‘Ashwashila’ attenuates rheumatoid arthritis symptoms in collagen-antibody-induced arthritis (CAIA) mice model. Sci Rep. https://doi.org/10.1038/s41598-019-44485-9
Brand DD, Latham KA, Rosloniec EF (2007) Collagen-induced arthritis. Nat Protoc 2:1269–1275. https://doi.org/10.1038/nprot.2007.173
Chen J, Wu W, Zhang M, Chen C (2019) Taraxasterol suppresses inflammation in IL-1β-induced rheumatoid arthritis fibroblast-like synoviocytes and rheumatoid arthritis progression in mice. Int Immunopharmacol 70:274–283. https://doi.org/10.1016/j.intimp.2019.02.029
Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423:356–361. https://doi.org/10.1038/nature01661
Guo Y, Xing E, Song H et al (2016) Therapeutic effect of dioscin on collagen-induced arthritis through reduction of Th1/Th2. Int Immunopharmacol 39:79–83. https://doi.org/10.1016/j.intimp.2016.06.029
Guo Q, Wang Y, Xu D et al (2018) Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. https://doi.org/10.1038/s41413-018-0016-9
Ibrahem S, Al-Ghamdi S, Baloch K et al (2014) STAT3 paradoxically stimulates β-catenin expression but inhibits β-catenin function. Int J Exp Pathol 95:392–400. https://doi.org/10.1111/iep.12102
Ivashkiv LB, Hu X (2003) The JAK/STAT pathway in rheumatoid arthritis: Pathogenic or protective? Arthritis Rheum 48:2092–2096. https://doi.org/10.1002/art.11095
Jope RS, Yuskaitis CJ, Beurel E (2006) Glycogen Synthase Kinase-3 (GSK3): Inflammation, Diseases, and Therapeutics. Neurochem Res 32:577–595. https://doi.org/10.1007/s11064-006-9128-5
Khanna S, Jaiswal KS, Gupta B (2017) Managing rheumatoid arthritis with dietary interventions. Front Nutr. https://doi.org/10.3389/fnut.2017.00052
Kumar S, Patial V, Soni S et al (2017) Picrorhiza kurroa enhances β-cell mass proliferation and insulin secretion in streptozotocin evoked β-cell damage in rats. Front Pharmacol. https://doi.org/10.3389/fphar.2017.00537
Malemud CJ (2013) Intracellular signaling pathways in rheumatoid arthritis. J Clin Cell Immunol. https://doi.org/10.4172/2155-9899.1000160
McInnes IB, Schett G (2007) Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 7:429–442. https://doi.org/10.1038/nri2094
Nho J-H, Lee H-J, Jung H-K et al (2019) Effect of Saururus chinensis leaves extract on type II collagen-induced arthritis mouse model. BMC Complement Altern Med. https://doi.org/10.1186/s12906-018-2418-z
Sharma A, Sharma R, Kumar D, Padwad Y (2018) Berberis lycium Royle fruit extract mitigates oxi-inflammatory stress by suppressing NF-κB/MAPK signalling cascade in activated macrophages and Treg proliferation in splenic lymphocytes. Inflammopharmacology. https://doi.org/10.1007/s10787-018-0548-z
Sharma A, Tirpude NV, Kulurkar PM et al (2019) Berberis lycium fruit extract attenuates oxi-inflammatory stress and promotes mucosal healing by mitigating NF-κB/c-Jun/MAPKs signalling and augmenting splenic Treg proliferation in a murine model of dextran sulphate sodium-induced ulcerative colitis. Eur J Nutr 59:2663–2681. https://doi.org/10.1007/s00394-019-02114-1
Smolen JS, Steiner G (2003) Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov 2:473–488. https://doi.org/10.1038/nrd1109
Tarner IH, Müller-Ladner U, Gay S (2007) Emerging targets of biologic therapies for rheumatoid arthritis. Nat Clin Pract Rheumatol 3:336–345. https://doi.org/10.1038/ncprheum0506
Tirpude NV, Sharma A, Joshi R et al (2021) Vitex negundo Linn extract alleviates inflammatory aggravation and lung injury by modulating AMPK/PI3K/Akt/p38-NF-κB and TGF-β/Smad/Bcl2/caspase/LC3 cascade and macrophages activation in murine model of OVA-LPS induced allergic asthma. J Ethnopharmacol 271:113894. https://doi.org/10.1016/j.jep.2021.113894
Vyas SP, Hansda AK, Goswami R (2019) Rheumatoid arthritis: ‘melting pot’ of T helper subsets. Int Rev Immunol 38:212–231. https://doi.org/10.1080/08830185.2019.1621865
Wang S, Wang L, Wu C et al (2018) E2F2 directly regulates the STAT1 and PI3K/AKT/NF-κB pathways to exacerbate the inflammatory phenotype in rheumatoid arthritis synovial fibroblasts and mouse embryonic fibroblasts. Arthritis Res Ther. https://doi.org/10.1186/s13075-018-1713-x
Wu X, Long L, Liu J et al (2017) Gambogic acid suppresses inflammation in rheumatoid arthritis rats via PI3K/Akt/mTOR signaling pathway. Mol Med Rep 16:7112–7118. https://doi.org/10.3892/mmr.2017.7459
Xu H, Cai L, Zhang L et al (2018) Paeoniflorin ameliorates collagen-induced arthritis via suppressing nuclear factor-κ B signalling pathway in osteoclast differentiation. Immunology 154:593–603. https://doi.org/10.1111/imm.12907
Yang XO, Panopoulos AD, Nurieva R et al (2007) STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem 282:9358–9363. https://doi.org/10.1074/jbc.c600321200
Zhuang Y, Liu J, Ma P et al (2017) Tamarixinin a alleviates joint destruction of rheumatoid arthritis by blockade of MAPK and NF-κB activation. Front Pharmacol. https://doi.org/10.3389/fphar.2017.00538
Acknowledgements
All authors express their gratitude to the Director, CSIR- IHBT Palampur, for constant support. We acknowledge CSIR for project MLP-0204 and MLP-0155 along with for funding source. AS is thankful to Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, India for Ph.D. registration and ICMR for SRF fellowship. CSIR-IHBT communication number for this manuscript is: 4829
Funding
This work was supported by Council of Scientific and Industrial Research, India (MLP0204, MLP0155).
Author information
Authors and Affiliations
Contributions
YP conceptualized and designed the study. AS and NVT developed animal model, performed bench work and analyzed the data. NVT was involved in immunization, gross examination of animals during study, histopathology and immunohistochemistry. NB performed ELISA. DK analyzed NMR data. AS and NVT designed and wrote the manuscript. YP edited and revised the manuscript. All authors corrected and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, A., Tirpude, N.V., Bhardwaj, N. et al. Berberis lycium fruit extract and its phytoconstituents berberine and rutin mitigate collagen–CFA-induced arthritis (CIA) via improving GSK3β/STAT/Akt/MAPKs/NF-κB signaling axis mediated oxi-inflammation and joint articular damage in murine model. Inflammopharmacol 30, 655–666 (2022). https://doi.org/10.1007/s10787-022-00941-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-00941-z