Abstract
Although Berberis plant species have been advocated as immune modulators, information regarding their mechanism(s) of action is limited. Therefore, in the present study we assessed the efficacy of Berberis lycium Royle fruit extract (BLFE) in the attenuation of lipopolysaccharide (LPS)-induced oxi-inflammatory aggravation and concanavalin A (Con-A)-induced proliferation in murine peritoneal macrophages and lymphocytes, respectively. BLFE strongly suppressed production of the oxidative and inflammatory effector molecules nitric oxide (NO), reactive oxygen species (ROS), inducible nitric oxide synthase (iNOS), inflammatory cytokines (TNF-α/IL-6/IL-1β/IFN-γ) as well as chemokines (MCP-1 and RANTES), with a concomitant enhancement in heme oxygenase-1 (HO-1) and IL-10 levels. Subsequent mechanistic analysis revealed that BLFE strongly inhibited the phosphorylation of IκBα as well as MAPKs such as extracellular signal-regulated kinase (ERK), p38 MAPK, and c-Jun NH2-terminal kinase (JNK), thereby directly resulting in the suppression of nuclear factor-κB (NF-ĸB) and c-Jun activation, ultimately culminating in the observed attenuation of inflammatory molecules. Additionally, BLFE appeared to mitigate Con-A-induced proliferation of Tregs (CD3+ CD4+ CD25+) thereby suggesting its modulatory effects on adaptive immune cells. UPLC–DAD–ESI-QTOF-MS/MS of BLFE revealed the presence of major bioactive phenolics and alkaloids including chlorogenic acid, rutin, catechin and quercetin 3-d-galactoside, berberine and magnoflorine, which could have synergistically contributed to the above findings. Overall, this work provides evidence that BLFE may be effective in the mitigation of inflammatory disorders, especially those associated with NF-κB/MAPK activation.
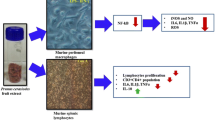
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is a natural immune response to invading pathogens and injury, but the process is also implicated in the pathogenesis of various chronic inflammatory disorders, such as rheumatoid arthritis, inflammatory bowel disease and neurodegenerative disorders (Jiang et al. 2017; Sharma et al. 2018). Inflammatory responses involve both innate and adaptive immune cells, as well as effector ROS molecules such as NO and various cytokines and chemokines including TNF-α, IL-1β, IL-6, MCP-1 and RANTES. These responses are under tight transcriptional regulation involving both NF-κB-dependent and -independent inflammatory pathways such as ERK, p38 MAPK, and JNK (Lee et al. 2017; Wang et al. 2017). Lipopolysaccharide-mediated activation of immune cells and initiation of inflammatory responses have been implicated in the pathological aggravation of various inflammatory disorders and considering this, inflammatory pathways are molecular targets of various steroidal and NSAID medications. However, due to known side effects and rising costs of traditional therapeutics, a global emphasis is focused on identification and development of much safer and affordable plant-based anti-inflammatory agents (Sharma et al. 2018). In this context, there are growing evidences that plant secondary metabolites and plant-based extracts can confer protection against inflammatory damage by suppressing the production of effector molecules as well as their mediatory pathways such as NF-κB and MAPKs (Bajpai et al. 2018; Kim et al. 2018).
Berberis lycium Royle, also known Indian berberry, is a valuable plant that is wildly distributed in the Himalayan region at 2000–2700 m altitude (Ali et al. 2015; Pradhan and Saha 2015). Traditionally, it has been utilized to treat various ailments such as diarrhoea, intestinal colic, jaundice, internal wounds, diabetes and rheumatism (Ali et al. 2015). Preliminary investigations have indicated that different parts of this plant (especially bark and roots) exhibit anti-microbial, anti-inflammatory, anti-carcinogenic and hepatoprotective activities which are thought to be due to the presence of various bioactive phytomolecules such as alkaloids, phenolics and anthocyanins (Andola et al. 2011; Chand et al. 2011; Ali et al. 2015). However, despite these emerging findings, there appears to be a distinct dearth of studies pertaining to understanding the molecular mechanism(s) governing the putative anti-inflammatory and anti-oxidant effect of this plant. Therefore, keeping the foregoing discussion in view, we hypothesized that B. lycium fruit extract (BLFE) could ameliorate inflammatory aggravation in innate immune cells by modulating NF-κB- and MAPK-mediated signalling pathways. To test our hypothesis, we assessed the influence of BLFE on LPS-mediated inflammation in murine macrophages as well as Con-A-mediated proliferation in splenic lymphocytes. Further, the BLFE was characterized by UPLC–DAD–ESI-QTOF-MS/MS so as to correlate and ascertain the diversity of prevalent bioactive phytomolecules. Therefore, the present study attempts to provide a holistic understanding of molecular mechanisms governing the immunomodulatory and anti-inflammatory attributes of BLFE.
Materials and methods
Preparation of BLFE
Berberis lycium fruits at maturation stage were collected from Kangra valley (Himachal Pradesh) located in western Himalayan region of India. BLFE was prepared using hydroalcoholic (50:50 water and ethanol) solvent system with minor modifications as described by Bhatt et al. (2018). Briefly, after separation of seeds from fruits (1000 g), pulp was used for the extraction of BLFE for 24 h at room temperature by percolation and the process was repeated twice. After extraction, samples were filtered using Whatman filter paper and concentrated using a rotary evaporator (Buchi, India) followed by lyophilisation (Labconco, USA). The total phenolic content (TPC) and total flavonoid content (TFC) of BLFE were estimated using Folin–Ciocalteu spectrometric and aluminium chloride colorimetric methods, respectively (Joshi et al. 2015), and are reported as gallic acid (Sigma-Aldrich, US) equivalent of BLFE (GAE/mg) on fresh weight (FW) basis. The antioxidant activity (AA) of BLFE was assessed using DPPH and ABTS assay as described by Joshi et al. (2015).
Animal husbandry
Six-to-eight-week-old Balb/C mice were obtained from animal house facility, CSIR-IHBT, Palampur. Mice were maintained under normal 12-h dark:12-h light cycle at 25 ± 2 °C temperature and 40–70% relative humidity in individual ventilated caging system (Citizen, India). Animals were fed with commercially available basal diet and water ad libitum. All experimental procedures were approved by the Institutional animal ethics committee (Approval no. IAEC/IHBTP4/Jan 2016) and carried out in accordance with guidelines prescribed by the National Regulatory Authority, CPCSEA, Government of India.
Macrophage culture and assessment of cellular viability
Peritoneal macrophages from mice were isolated as described previously (Sharma et al. 2018). For cellular assays, lyophilized BLFE was reconstituted completely Roswell Park Memorial Institute (RPMI)-1640 (HiMedia laboratories, Mumbai, India) (supplemented with 10% FBS and 1% antibiotic–antimycotic solution) while control group cells were treated with RPMI-1640 alone. The toxicity of different concentrations of BLFE (25, 50, 100, 200, and 400 µg/ml) on viability of peritoneal macrophages was estimated by measuring the reduction of MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (HiMedia laboratories, Mumbai, India) to formazan. Briefly, 0.35 × 106 cells/well were seeded into 96-well culture plate in 200 µl media for 48 h in the presence or absence of BLFE. After visual confirmation of cell morphology, MTT assay was used to determine and calculate cell viability as reported by Sharma et al. (2018).
Inflammatory stimulation and assessment of nitric oxide production
Briefly, peritoneal macrophages (0.35 × 106) were seeded in 96-well plates for 6 h in RPMI-1640 supplemented with 10% FBS (HiMedia laboratories, Mumbai, India). The cells were treated with different concentrations of reconstituted BLFE (50, 100 and 200 µg/ml) for 12 h in a CO2 incubator, followed by stimulation with LPS (Sigma-Aldrich, US) (1.5 μg/ml) for another 16 h. Finally, NO production was measured using the Griess reagent as per the manufacturer’s instruction (Promega, USA). Dexamethasone (Dex, 2 μg/ml) was used as positive control for all experiments.
Assay for intracellular ROS determination in stimulated macrophage
Briefly, macrophages (1.5 × 106/well) were seeded on 24-well plates and treated with various concentrations of BLFE. At the end of experiment, ROS accumulation was assessed using carboxy-2,7-dichlorohydrofluorescein diacetate (DCFDA); (Sigma-Aldrich, US) dye as described previously by Sharma et al. (2018). Finally, fluorescence images were captured with EVOS FL cell imaging system (Thermo Fisher Scientific, US) for comparative analysis.
Measurement of cytokines in culture supernatants
Macrophages (1.5 × 106) were treated with three concentrations (50, 100 and 200 μg/ml) of BLFE for 12 h, as described in the previous section. Estimation of TNF-α, IL-6, IL-1β, IFN-γ and IL-10 cytokines in cell culture supernatants was performed using commercially available sandwich ELISA kits following the manufacturer’s protocol (eBioscience, San Diego, CA, USA). All the samples were assessed in triplicates.
Total RNA isolation and qRT-PCR
At the end of the experiment, total RNA was isolated and qRT-PCR for TNF-α, IL-1β, IL-6, IFN-γ, IL-10, MCP-1 and RANTES was performed as reported previously (Sharma et al. 2018). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression was used as an internal control to normalize mRNA expression. The primer sequences used for mRNA analysis were as presented below:
Primers | Primer sequence (5′ end–3′ end) |
|---|---|
GAPDH-F | TCA CTC AAG ATT GTC AGC AATGC |
GADPH-R | TCA TGA GCC CTT CCA CAA TG |
IL1B-F | CGT TCC CAT TAG ACA ACTGCA CTA |
IL1B-R | TCC ATT GAG GTG GAG AGCTTTC |
IL6-F | CTT CCA TCC AGT TGC CTT CTTG |
IL6-R | GGG AGT GGT ATC CTC TGTGAA GTC |
iNOS-F | AGC GAG GAG CAG GTG GAA |
iNOS-R | CCT TTT TTG CCC CAT AGG AAA |
TNF-α-F | GAA ACA CAA GAT GCT GGGACAGT |
TNF-α-R | CAT TCG AGG CTC CAG TGA ATTC |
IFN-γ-F | CTC CGG CCT AGC TCT GAGA |
IFN-γ-R | TGA CTG TGC CGT GGC AGT AA |
IL-10-F | ATT TGA ATT CCC TGG GTG AGAA |
IL-10-R | TGC TCC ACT GCC CTT GCT CTT CTC |
MCP-1-F | CTG AAG CCA GCT CTC TCT CTC TCT TCCT |
MCP-1-R | GAG CCA ACA CGT GGA TGCT |
RANTES-F | TCC CTG TCA TCG CTT GCT CTA |
RANTES-R | CGG ATG GAG ATG CCG ATTT |
Protein extraction and immunoblotting
Total proteins were isolated and quantified as described earlier (Sharma et al. 2018). The primary antibodies anti-iNOS, anti-IκBα, anti-p-IκBα, anti-NF-κB, anti-p-NF-κB, anti-Nrf-2, anti-p38, anti-p-p38, anti-c-Jun, anti-p–c-Jun, anti-ERK, anti-p-ERK, anti-JKN and anti-p-JNK were obtained from Cell Signalling Technologies, USA. Anti-β-tubulin antibodies were purchased from Santa Cruz Biotechnology, USA, and used as an internal control. Western blot images were quantified using ImageJ software.
Splenic immunophenotyping
Briefly, the mouse spleen was aseptically removed, washed in PBS and then dissociated into a single-cell suspension using syringe plunger, followed by centrifugation at 400 g for 10 min. The single-cell suspension was then treated with RBC lysis buffer for 10 min to rupture any erythrocytes present. The remaining splenocytes were then washed twice in PBS by centrifugation at 400 g for 5 min. This final pellet was then re-suspended in RPMI 1640 supplemented with 10% FBS and 1% antibiotic–antimycotic solution (i.e. Complete RPMI), and counted in a haemocytometer. The isolated splenocytes (1 × 106) were seeded in six-well plates and incubated with BLFE (50, 100, and 200 μg/ml) for 12 h followed by stimulation with Con-A (7 μg/ml) (Sigma-Aldrich, US) for 36 h. After incubation, phenotypic characterization of splenocytes was performed by flow cytometer analysis using AMNIS ImageStream®X Mark II Imaging Flow Cytometer (Merck Millipore, Germany). Splenocytes were suspended in FACS buffer (PBS, pH 7.4, with 0.5% BSA and 0.1% sodium azide) and incubated at 4 °C for 15 min with FITC-anti-mouse CD3, PE-anti-mouse CD4, and APC-anti-mouse CD25 antibodies (eBioscience, San Diego, CA) as previously described by Sharma et al. (2017). The cells were washed twice with PBS and re-suspended in FACS buffer. CD3+, CD4+ and CD4+ CD25+ sub-populations were analysed by the IDEAS software, Germany.
UPLC–DAD–ESI-QTOF-MS/MS analysis
To assess, the individual phenolics and alkaloids of BLFE UPLC–ESI-QTOF-MS/MS (Waters, Milford, MA, USA) were performed. Briefly, a mixture of methanol:water (50:50 v/v) was used to prepare the sample (10 mg/ml). Prior to injection into UPLC system (BEH C18 column, 2.1 mm x 100 mm, 1.7 μm particle size), the sample was filtered using a 0.22-μm Millex GV syringe filter (Millipore, MA, USA). Briefly, for phenolic and alkaloid characterizations, 0.1% formic acid in water as solvent A and 0.1% formic acid in acetonitrile as solvent B were used as mobile phase, with 0.25 ml/min flow rate at 25 °C column temperature. For chromatographic separations of phenolics and quercetin 3-d-galactoside, the used mobile phase consisted of 0–0.3 min (10% B), 0.3–7 min (45% B), 7–8 min (95% B), 8–9 min (95% B), 9–10 min (10% B) and 10–12 min (10% B). For analysis of alkaloids, the used mobile phase (solvent A 0.05% formic acid in water and acetonitrile as solvent B with 0.3 ml/min flow rate) consisted of 0–0.2 min (13% B), 0.2–3.5 min (30% B), 3.5–4 min (13% B), and 4–5 min (13% B) (Bala et al. 2015). Additionally, the mobile phase used for the characterization of berberine consisted of 0–0.5 min (10% B), 0.5–5 min (35% B), 5–7 min (90% B), 7–8.5 min (90% B), 8.5–9 min (10% B) and 9–12 min (10% B). Standard stock solutions (1 mg/ml) of gallic acid, pro-catechuic acid, chlorogenic acid, catechin, caffeic acid, p-coumaric acid, naringin, cinnamic acid, kaempferol and quercetin 3-d-galactoside were prepared in HPLC-grade methanol (Sigma-Aldrich, USA). The quantity of the detected compounds was reported as mg/g of fruit extract. The MS/MS experiment was performed on a Q-TOF mass spectrometer equipped with an electron spray ionization source (Micromass, Manchester, UK) and software Masslynx v4.1. The MS parameters in positive mode were as follows: capillary voltage 3.2 kV, cone voltage 30 V, source block temperature 80 °C, scan range, m/z 100–1000, source block temperature 80 °C, RF lens1 45, aperture 0.5, RF lens 20.6, cone gas 50 l/h, desolvation temperature 220 °C, desolvation gas 400 l/h, argon collision gas pressure 3.2 × 10 − 3 mbar and ion energy 23.0 eV.
Bioactivity validation of phytomolecules characterized in BLFE
In an attempt to ascertain and validate the apparent biological effects of BLFE, two of the characterized compounds, berberine (Sigma-Aldrich, USA) and rutin (Sigma-Aldrich, USA) in BLFE were analysed for the inhibition of LPS-stimulated inflammatory responses in macrophages. Briefly, cells were pre-exposed to different concentrations (25, 50 and 100 µM/ml) of berberine and rutin, respectively, for 12 h followed by LPS induction for another 16 h and subsequent analysis of NO production and iNOS expression by RT-PCR and western blotting as detailed in previous sections.
Statistical analyses
Results are presented as the mean ± SD. One-way ANOVA, followed by Tukey’s test was used to perform the statistical analyses. Differences with p value ≤ 0.05 were considered as statistically significant.
Results
Characterization and cytocompatibility of BLFE
Analysis of TPC revealed that BLFE contained 46.7 ± 4.3 µg gallic acid equivalent per mg fresh weight, while 14.1 ± 1.72 µg/mg gallic acid equivalent of TFC was also observed. Moreover, the anti-oxidant potential of BLFE assessed by DPPH and ABTS assay showed an IC50 of 101.7 ± 2.6 µg/ml and 26.9 ± 1.2 µg/ml, respectively. These results suggest that BLFE could be useful in ameliorating oxi-inflammatory stress in a biological system. Pursuing this, we initially evaluated cytotoxicity of BLFE in macrophages. As shown in Fig. 1a, BLFE treatment even up to a range of 400 μg/ml did not result in any apparent cytotoxic effects. Based on these results, BLFE concentrations of 50, 100 and 200 μg/ml were selected for further experiments.
BLFE attenuates NO production and iNOS expression in LPS-stimulated murine peritoneal macrophages. a Percentage cellular viability of macrophages treated at different concentrations of BLFE for 48 h. Cells pre-treated with BLFE (50, 100, 200 μg/ml) for 12 h followed by stimulation with LPS (1.5 μg/ml) for another 16 h. b NO production. iNOS mRNA (c) and protein expression (d). Values are mean ± SD of three independent experiments; values with different letters are significantly different at p < 0.05
BLFE ameliorates NO production and iNOS expression in activated macrophages
LPS stimulation to macrophages increases NO production, which is regulated by iNOS. Therefore, to analyse the effect of BLFE on NO production, the cellular nitrite level (an indicator of NO production) of activated macrophages treated with different concentration of BLFE was measured. As presented in Fig. 1b, stimulation with LPS resulted in significant (p ≤ 0.05) enhancement in NO levels (20.9 μM) as compared to control (5.7 μM; vehicle alone) while BLFE treatment (50, 100 and 200 μg/ml) exerted a significant (p ≤ 0.05) yet concentration-independent inhibition in NO production (up to 15.5 μM) compared to LPS-stimulated macrophages (Fig. 1b). Subsequent analysis of iNOS mRNA expression revealed a significant (p ≤ 0.05) increase in LPS-stimulated macrophages in comparison with control. On the other hand, BLFE at 200 μg/ml significantly inhibited (p ≤ 0.05) the expression of iNOS by 61% in comparison with LPS-stimulated cells (Fig. 1c). Further, protein analysis showed a strong accumulation in iNOS in LPS-treated cells which were significantly (p ≤ 0.05) attenuated by BLFE treatment at 100 and 200 μg/ml BLFE (Fig. 1d). Here, the results indicate that the attenuation of NO by BLFE may be associated with inhibition of the iNOS expression. The anti-inflammatory drug dexamethasone (Dex) also showed a strong attenuation in iNOS at both transcriptional and translational levels.
BLFE augments redox homeostasis by reducing ROS accumulation and Nrf-2 expression while enhancing HO-1 expression in LPS-stimulated macrophages
As illustrated in Fig. 2a, BLFE treatment to LPS-stimulated cells inhibited the accumulation of ROS, which was in accordance with NO production assay. Analysis of mRNA expression of the anti-oxidant enzyme HO-1 demonstrated a non-significant reduction of 44.2% in LPS-treated cells as compared to control cells, which was significantly (p ≤ 0.05) increased in BLFE-treated cells (up to 84.8%) in comparison with LPS-stimulated cells (Fig. 2b). Immunoblotting for the analysis of Nrf-2 expression showed a significant (p ≤ 0.05) increase (32.4%) in LPS-stimulated macrophages which were also significantly attenuated in the presence of BLFE (up to 53.1%) albeit in a dose-independent manner. Interestingly, dexamethasone treatment did not appear to significantly enhance HO-1 (as compared to BLFE) while both dexamethasone and BLFE showed similar effects in modulating Nrf-2 expression in LPS-stimulated cells (Fig. 2c).
BLFE ameliorates redox stress in peritoneal macrophages. Cells pre-treated with BLFE (50, 100, 200 μg/ml) for 12 h followed by stimulation with LPS (1.5 μg/ml) for another 16 h. a ROS accumulation by DCFDA assay. b mRNA expression of HO-I and c Nrf2 expression by immunoblotting. Values are mean ± SD of three independent experiments; values with different letters are significantly different at p < 0.05
BLFE modulates key cytokine and chemokine production in LPS-stimulated macrophages
To address the effect of BLFE on pro- and anti-inflammatory mediators, we next investigated the mRNA expression level of the inflammation regulatory effector cytokines TNF-α, IL-1β, INF-γ, IL-6 and IL-10 in LPS-stimulated macrophages by qRT-PCR. The results indicated that LPS insult to macrophages significantly (p ≤ 0.05) increased the expression of TNF-α, IL-1β, INF-γ and IL-6 by 4.3-, 153.2-, 3.1- and 22.8-folds, respectively in comparison to control (Fig. 3a–d). In contrast, treatment with different concentrations (50, 100 and 200 μg/ml) of BLFE significantly (p ≤ 0.05) inhibited the mRNA expression of TNF-α, IL-1β, and IL-6 by 3.8-, 6.9- and 4-folds, respectively, in comparison to LPS-stimulated cells. However, BLFE-mediated suppression of INF-γ could only be observed at the highest concentration of 200 μg/ml (Fig. 3d). No change in IL-10 levels could be observed in LPS-treated cells, whereas a significant (p ≤ 0.05) increase of 10.1-fold was noted in BLFE-treated cells (Fig. 3e). Analysis of cytokine levels in culture supernatants is presented in Fig. 4a–e. On induction with LPS, a significant (p ≤ 0.05) enhancement in the level of TNF-α, IL-1β and IL-6 by 206.9-, 4.9- and 487.7-folds was observed, but had no significant effect on INF-γ (1.2-fold) production. On the other hand, 100 and 200 μg/ml BLFE exposure to stimulated cells strongly inhibited the production of these cytokines. In particular, a significant decrease in all measured pro-inflammatory cytokines with a concomitant increase in IL-10 levels was observed specifically at 200 μg/ml of BLFE treatment (Fig. 4e). Furthermore, it was observed that the inhibition of pro-inflammatory cytokines at 200 μg/ml was either equal or greater than the inhibition by dexamethasone. Similarly, analysis of chemokines RANTES and MCP-1 revealed significant upregulation in LPS-stimulated cells, which was again significantly (p ≤ 0.05) attenuated by the presence of BLFE at all tested concentrations (Fig. 5a, b).
BLFE augments inflammtory homesostsis in LPS-stimulated macrophages. Cells pre-treated with BLFE (50, 100, 200 μg/ml) for 12 h followed by stimulation with LPS (1.5 μg/ml) for another 16 h. a TNF-α, b IL-1β, c IFN-γ, d IL-6 and e IL-10 mRNA fold change was measured using qRT-PCR. Values are mean ± SD of three independent experiments; values with different letters are significantly different at p < 0.05
BLFE modulates interleukin production in LPS-stimulated macrophages. Cells pre-treated with BLFE (50, 100, 200 μg/ml) for 12 h followed by stimulation with LPS (1.5 μg/ml) for another 16 h. a TNF-α, b IL-1β, c IFN-γ, d IL-6 and e IL-10 production was measured using ELISA. Values are mean ± SD of three independent experiments; values with different letters are significantly different at p < 0.05
BLFE alleviates chemokine production in LPS-stimulated murine peritoneal macrophages. Cells pre-treated with BLFE (50, 100, 200 μg/ml) for 12 h followed by stimulation with LPS (1.5 μg/ml) for another 16 h. a RANTES and b MCP-1 fold change was measured using qRT-PCR. Values are mean ± SD of three independent experiments; values with different letters are significantly different at p < 0.05
BLFE suppresses the activation of IκBα, NF-κB and c-Jun transcription factors
To investigate whether attenuation of inflammatory responses by BLFE are mediated through the NF-κB pathway, we examined the effect of BLFE on LPS-induced phosphorylation of IκBα and NF-κB in stimulated cells (Fig. 6a, b). Compared with un-stimulated (control) cells, the phosphorylation of IκBα significantly (p ≤ 0.05) increased in LPS-stimulated cells (Fig. 6a). In contrast, phosphorylation in BLFE-treated cells was significantly (p ≤ 0.05) decreased for all tested concentrations. Interestingly, the inhibitory effect of BLFE on IκBα phosphorylation was significantly (p ≤ 0.05) higher than dexamethasone. Furthermore, it was observed that all tested concentrations of BLFE significantly (p ≤ 0.05) suppressed the activation of NF-κB in a concentration-independent manner (Fig. 6b). These results suggest that inhibition of inflammatory effector molecules by BLFE is meditated via attenuation of NF-κB phosphorylation. Next, we examined the potential role of BLFE on LPS-induced c-Jun expression in murine macrophages (Fig. 6c). The results revealed that in the presence of BLFE, LPS-induced phosphorylation of c-Jun was markedly suppressed by all the tested concentrations.
BLFE inhibits activation of a p-IĸBα/IĸBα, b p-NF-κB/NF-κB and c p-c-Jun/c-Jun expression in LPS-stimulated murine peritoneal macrophages. Cells pre-treated with BLFE (50, 100, 200 μg/ml) for 12 h followed by stimulation with LPS (1.5 μg/ml) for another 16 h. Values are mean ± SD of three independent experiments; values with different letters are significantly different at p < 0.05
BLFE suppresses the activation of MAPKs (ERK, JNK and p38) in LPS-stimulated macrophages
The regulatory role of MAPKs in LPS-induced activation of NF-κB and other inflammatory markers was analysed through the phosphorylation of ERK, JNK and p38 in stimulated macrophages. As shown in Fig. 7a–c, LPS stimulation significantly (p ≤ 0.05) enhanced the phosphorylation of ERK1/2, JNK and p38 in comparison to the control (un-stimulated) cells. Conversely, BLFE pre-treatment significantly (p ≤ 0.05) downregulated the phosphorylation of ERK1/2, JNK and p38 (albeit at 100 and 200 μg/ml only). These results confirmed that the suppressive effect of BLFE on NF-κB and inflammatory effector molecules is regulated by inhibition of MAPKs.
BLFE decreases phosphorylation of a p-ERK/ERK b p-JNK/JNK and c p-p38/p38 expression in LPS-stimulated peritoneal macrophages. Cells pre-treated with BLFE (50, 100, 200 μg/ml) for 12 h followed by stimulation with LPS (1.5 μg/ml) for another 16 h. Values are mean ± SD of three independent experiments; values with different letters are significantly different at p < 0.05
BLFE treatment dysregulates CD3+ CD4+ CD25+ Treg homeostasis in Con-A-stimulated splenocytes
Con-A-treated splenocytes showed a non-significant 22.4% decrease in CD3+ cells as compared to control (Fig. 8a). On the other hand, analysis of BLFE (200 μg/ml) treatment on splenocytes revealed a significant (p ≤ 0.05) increase (95.2%) in CD3+ population in comparison to Con-A-stimulated cells (Fig. 8a). However, we noticed a significant decrease in CD3+ CD4+ cell population in Con-A-stimulated cells as compared to the control although BLFE treatment did not result in any significant changes compared to Con-A-treated cells (data not shown). Additionally, compared with un-stimulated cells, a significant increase of 88.5% was observed in CD3+ CD4+ CD25+ cell population in Con-A-treated cells, while pre-treatment with BLFE (200 μg/ml) significantly decreased CD3+ CD4+ CD25+ numbers by 31.8% as compared with stimulated cells (Fig. 8b).
Effect of BLFE on T-cell population in Con A-stimulated murine splenocytes. Cells pre-treated with BLFE (50, 100, 200 μg/ml) for 12 h followed by stimulation with Con A (7 μg/ml) for another 36 h. a CD3+ b CD3+ CD4+ CD25+ cell populations were analyed using flow cytometry and c CD4+ vs CD25+ scatterplots. Values are mean ± SD of three independent experiments; values with different letters are significantly different at p < 0.05
Characterization of BLFE by UPLC–DAD–ESI-QTOF-MS/MS
Table 1 shows the retention time and MS/MS spectral data of the bioactive compounds present in the BLFE characterized using UPLC–ESI-QTOF-MS/MS. A total of 16 compounds from different classes of phytomolecules including eight alkaloids and eight phenolics (seven flavonoids and one phenolic acid) were identified by comparing mass fragmentation patterns (Table 1). The LC/MS chromatogram (total ion chromatogram) in positive ion mode and mass spectrum of BLFE are presented in Fig. 9a and supplementary file S1, respectively. On the basis of retention time, UVλmax and mass fragmentation patterns with respect to standards, peaks 3, 4, 6, 7, 8 and 16 were identified as chlorogenic acid, catechin, magnoflorine, rutin and quercetin 3-d-galactoside and berberine, respectively (Table 1; Supplementary files S1 and S2). Additionally, peaks 1, 2, 5 and 9–14 in Fig. 9a were identified as cyanidin-3-O-galactoside, cyanidin-3-O-glucoside, demethylated product of magnoflorine, berbamine, myricetin-3-glucoside, petunidin-3-O-glucoside, jatrorrhizine, dihydroxy derivative of jatrorrhizine, protopine and palmatine, respectively, by comparison of their mass fragmentation patterns (Table 1 and Supplementary file S1). Further, a total of four phenolics, namely chlorogenic acid (10.0 ± 0.9 mg/g), catechin (1.8 ± 3.2 mg/g), rutin (0.62 ± 0.7 mg/g) and quercetin 3-d-galactoside (2.51 ± 1.2 mg/g) as well as two alkaloids, namely magnoflorine (17.22 ± 3.8 mg/g) and berberine (0.58 ± 0.1 mg/g) in BLFE were quantified by UPLC–DAD method (Table 2 and Fig. 9b–e).
Characterization of BLFE using a UPLC–ESI-QTOF-MS/MS (TIC spectra). 1—cyanidin-3-O-galactoside, 2—cyanidin-3-O-glucoside, 3—chlorogenic acid,4—catechin, 5—demethylated product of magnoflorine, 6—magnoflorine, 7—rutin, 8—quercetin 3-d-galactoside, 9—berbamine, 10—myricetin-3-glucoside, 11—petunidin-3-O-glucoside, 12—jatrorrhizine, 13—dihydroxy derivative of jatrorrhizine, 14—protopine, 15—palmatine, 16—berberine. b–e UPLC–DAD chromatogram of b phenolic mixture: 1—gallic acid, 2—pro-catechuic acid, 3—chlorogenic acid, 4—catechin, 5—caffeic acid, 6—rutin, 7—p-coumaric acid, 8—naringin, 9—cinnamic acid and 10- kaempferol; c 11-quercetin 3-d-galactoside; d alkaloid mixture: 1—syringin, 2—cardifolioside 3—magnoflorine 4—tinocardiside and e 5—Berberine
Berberine and rutin corroborate the anti-inflammatory effects of BLFE
The phytomolecules berberine and rutin were characterized in BLFE and preliminary assessment of their biological effects corroborated the apparent anti-inflammatory attributes of BLFE. We observed that treatment with berberine (at 50 and 100 µM/ml) and rutin (at 100 µM/ml) caused a significant (p ≤ 0.05) decrease in NO production in LPS-induced macrophages (Fig. 10a). Further assessment of iNOS expression, both at transcriptional as well as translational levels, indicated its robust inhibition in the presence of berberine and rutin in LPS-stimulated macrophages (Fig. 10b, c). These observations suggest that the presence of these compounds could have been at least partially responsible for observed cellular effects of BLFE in the present study.
BLFE constituents berberine and rutin ameliorate NO production and iNOS expression in stimulated peritoneal macrophages. Cells pre-treated with (25, 50 and 100 μM/ml) for 12 h followed by stimulation with LPS (1.5 μg/ml) for another 16 h. a NO production. b mRNA expression of iNOS and c iNOS expression by immunoblotting. Values are mean ± SD of three independent experiments; values with different letters are significantly different at p < 0.05
Discussion
Plant species and products enriched with bioactive secondary metabolites are considered potent cytoprotective agents against various oxidative and inflammatory disorders. Indeed, in the present study, analysis of TPC, TFC and AA indicated a strong presence of bioactive entities in BLFE, which is consistent with previous studies using different parts of Berberis species (Campisi et al. 2014; Pradhan and Saha 2015). On subsequent UPLC–DAD–ESI-QTOF-MS/MS analysis, we observed that BLFE had a high content of secondary metabolites chlorogenic acid, rutin, catechin, quercetin 3-d-galactoside, berberine and magnoflorine, which are generally recognized for their anti-oxidant and anti-inflammatory potential (Kuo et al. 2004; Nakanishi et al. 2010; Hwang et al. 2013; Guo et al. 2018; Xianchu et al. 2018). It is thus evident that fruits of Berberis species are an important source of bioactive compounds that could be useful in developing an alternate therapeutic strategy for the alleviation of inflammatory and oxidative cellular stressors. To test this possibility, ex vivo anti-inflammatory efficacy of BLFE using LPS-stimulated macrophages and Con-A-activated splenic T cells was investigated. The unifying hallmark of LPS-induced inflammation is the abnormal and excessive production of various effector molecules through the activation of inflammatory pathways such as NF-κB and MAPKs. Here, in the present study, we observed that pre-treatment with BLFE strongly suppressed the majority of inflammatory mediators through the inhibition of NF-κB, c-Jun and MAPKs (ERK, JNK and p38) in stimulated macrophages and thus exhibited excellent anti-inflammatory activity. BLFE treatment attenuated NO production as well as suppressed iNOS expression compared to LPS-stimulated cells. Additionally, BLFE at both 100 and 200 μg/ml inhibited protein expression of iNOS, while iNOS mRNA expression could only be significantly influenced at the concentration of 200 μg/ml. These results suggest that interference with iNOS protein expression may be the primary contributing factor for the apparent inhibitory effects of BLFE on NO production in activated macrophages. These results are in agreement with studies performed by Chiou et al. (2000) and Shin et al. (2016) using andrographolide and berberine, respectively, in stimulated Raw 264.7 cells. Analysis of characterized phytomolecules (berberine and rutin) in BLFE also validated these observations wherein a strong attenuation of NO production and iNOS expression was observed. This also suggests that the presence of a milieu of such characterized bioactive components in BLFE could be responsible for the diverse anti-inflammatory effects as observed in the present study.
To further substantiate the anti-inflammatory attributes of BLFE, various inflammatory cytokines and chemokines were assessed. The results revealed that LPS treatment significantly augmented the levels of TNF-α, IL-1β, IL-6 and INF-γ, while pre-treatment with BLFE (100 and 200 μg/ml) significantly reduced the levels of all the tested cytokines. In contrast, significantly increased IL-10 production in the presence of BLFE was also observed suggesting immunomodulatory attributes of BLFE. These results suggest that BLFE modulated the production of effector inflammatory molecules as well as regulatory molecules, thereby suggesting its efficacy in maintaining inflammatory homeostasis. The present findings are similar to previous studies using different plant extracts wherein secondary metabolite-enriched plant fractions exhibited anti-inflammatory effects (Sharma et al. 2018; Kumar et al. 2016; Kang et al. 2007). In addition, studies on isolated chlorogenic acid and catechin have also shown significant inhibitory effects on inflammation and cytokine levels (Noll et al. 2012; Hwang et al. 2013). The increased levels of MCP-1 and RANTES are also known to attract and recruit immune cells at the site of infection that may further augment inflammatory damage. We observed that BLFE treatment attenuated the production of these chemokines, thereby further supporting its potency in the amelioration of inflammation (Qin et al. 2016; Park et al. 2017).
To explore the molecular mechanism(s) governing the apparent anti-inflammatory attributes of BLFE, we assessed the activation of inflammatory regulators, NF-κB and MAPKs (ERK, JNK and p38). It was observed that LPS treatment significantly upregulated NF-κB activation, which was strongly inhibited by pre-treatment with BLFE. Similarly, phosphorylation of IκBα, an upstream regulator of NF-κB, was also equally suppressed by BLFE suggesting that the apparent BLFE-mediated inhibitory effect on NF-κB phosphorylation could be attributed to the downregulation of IκBα phosphorylation. Overall, it appears that suppression of NF-ĸB induced by IκBα inhibition on account of BLFE treatment would have contributed to the observed attenuation of inflammatory effector molecules in LPS-stimulated macrophages. In agreement with our results, a study on LPS-stimulated THP-1 cells showed IκBα-dependent inhibition of NF-κB activation with Angelica dahuricae Radix extract treatment (Kang et al. 2007). Similarly, another finding showed that Apo-9-fucoxanthinone exposure reduced the activation of NF-κB by inhibiting the IκB phosphorylation in LPS-stimulated zebra fish model (Kim et al. 2018). In addition to plant extracts, it has been shown that pure magnoflorine and rutin are also potent NF-κB modulators against extracellular stressors (Lee et al. 2012; Guo et al. 2018). LPS-induced activation of NF-κB and c-Jun by MAPKs such as ERK, JNK and p38 pathways is also known to promote inflammatory responses (Wang et al. 2017; Bajpai et al. 2018). As such, in the present study, it was observed that phosphorylation of MAPKs (ERK, JNK and p38) was significantly increased with LPS treatment while BLFE exposure significantly decreased the expression of ERK and JNK at all the test concentrations. However, p38 as well as c-Jun phosphorylation could only be attenuated at 100 and 200 μg/ml BLFE. Overall, inhibition of these MAPKs signalling pathways suggested multi-faceted molecular targets of BLFE-mediated inflammatory suppression. These findings are consistent with previous studies using resveratrol, magnoflorine, blueberries, and Angelica dahuricae Radix extract (Kang et al. 2007; Wang et al. 2017; Guo et al. 2018).
The inflammatory microenvironment can also disturb the cellular redox homeostasis, thereby causing enhanced ROS production and accumulation, which in turn can further stimulate NF-κB-mediated inflammation resulting in a vicious oxi-inflammatory cycle. Considering the observed potent anti-inflammatory attributes of BLFE in the present study, we next sought to determine the influence of BLFE on ROS production and related mechanism. It was observed that LPS treatment increased ROS accumulation in macrophages which was strongly attenuated by BLFE treatment. On the contrary, a decrease in gene expression of HO-1 in LPS-stimulated macrophages was observed which was significantly enhanced by BLFE treatment. The anti-oxidant enzyme HO-1 plays a crucial role in mitigating inflammation and oxidative stress (Pae and Chung 2009; Och et al. 2014) and thus it appears that increased HO-1 production could have contributed to observed attenuation of ROS in BLFE-treated cells. These observations are consistent with other studies which illustrated that enhanced HO-1 expression plays a significant role in mediating the anti-inflammatory effects of various natural compounds in LPS-stimulated macrophages (Li et al. 2011; Och et al. 2014). When under significant oxidative stress, the Nrf-2 orchestrates and augments antioxidant response elements (such as SOD/catalase/HO-1) for mitigative response. In the present study, we observed that LPS-treated cells strongly upregulated the protein expression of Nrf-2, possibly due to prevalent cellular oxidative stress, which the cells tried to counter (albeit unsuccessfully). In contrast, Nrf-2 expression in BLFE-treated cells was on par with the control. This suggests that BLFE treatment would have attenuated cellular ROS accumulation before it could reach a threshold so as to cause an activation of Nrf-2. HO-1 is also known to be activated by Nrf-2 independent of the mechanism involving NF-κB and MAPKs (p38, ERK and JNK) that may have contributed to its observed activation in BLFE-treated cells (Wijayanti et al. 2004; Och et al. 2014). Overall, the apparent BLFE-mediated attenuation of the inflammatory pathways of NF-κB, c-Jun with MAPKs could have directly contributed in the alleviation of ROS accumulation and subsequent inhibition of the oxi-inflammatory vicious cycle accompanied, in part, by the activation of HO-1 pathway. Assessment of T cell proliferation revealed that Con-A treatment induced the development of CD3+ CD4+ CD25+ Treg cells as compared to control which was significantly attenuated by BLFE treatment, albeit only at a higher concentration (200 μg/ml). Interestingly, the same concentration of BLFE also appeared to upregulate total CD3+ cells compared to stimulated control. It has been shown that Con-A can encourage the maturation and activation of Treg cells which contributes to immune-related disorders such as during inflammation. Overall it appears that BLFE could exert beneficial effects on adaptive immune cells as well by controlling Con-A-mediated proliferation of Treg cells (Shinohara and Tsukimoto 2018).
Conclusion
In the present study, we observed that BLFE treatment exerted anti-oxidant and anti-inflammatory attributes by inhibiting LPS-mediated induction of pro-inflammatory effector molecules. Based on the observed results, we speculate that BLFE may suppress NF-κB- and c-Jun-mediated inflammatory responses via IĸBα-dependent as well as MAPK (ERK, JNK and p38)-dependent pathways (Fig. 11). Overall, it is reasonable to conclude that the apparent inhibitory effects of BLFE on LPS-induced NF-κB, c-Jun, ERK, JNK and p38 activation resulted in the attenuation of subsequent production of NO, pro-inflammatory cytokines and chemokines. The apparent diverse immunosuppressive attributes of BLFE could be ascribed to the presence of different bioactive molecules, such as berberine and rutin, as well as their mutual synergism in influencing the inflammatory pathways. Taken together, these findings provide compelling evidence that B. lycium fruits could be a new addition in the development of anti-inflammatory functional foods. Future research directions may include in vivo assessment of preventive as well as therapeutic attributes of BLFE against various inflammatory disorders.
Schematic diagram of probable signalling cascade mediating BLFE-induced anti-inflammatory effects. LPS stimulation augments inflammatory response by enhancing effector molecules such as NO, ROS, cytokines and chemokines through the activation of NF-κB, c-Jun and MAPK (ERK, JNK and p38) signalling pathways. BLFE suppresses LPS-induced IĸBα as well as MAPK-dependent NF-κB activation, which culminates in the suppression of inflammatory effector molecules. Notwithstanding this, the inhibitory effect of BLFE on c-Jun, ERK, JNK and p38 activation could also directly result in inhibition of iNOS, cytokine and chemokine levels. Red “dotted inverted-T” symbols signify the possible inhibitory effects of BLFE
References
Ali H, Uddin S, Jalal S (2015) Chemistry and biological activities of Berberis lycium Royle. J Biol Act Prod Nat 5:295–312. https://doi.org/10.1080/22311866.2015.1073627
Andola HC, Rawal RS, Bhatt ID (2011) Comparative studies on the nutritive and anti-nutritive properties of fruits in selected Berberis species of West Himalaya, India. Food Res Int 44:2352–2356. https://doi.org/10.1016/j.foodres.2010.07.017
Bajpai VK, Alam MB, Quan KT, Ju MK, Majumder R, Shukla S, Huh YS, Na M, Lee SH, Han YK (2018) Attenuation of inflammatory responses by (+)-syringaresinol via MAP-Kinase-mediated suppression of NF-κB signaling in vitro and in vivo. Sci Rep. https://doi.org/10.1038/s41598-018-27585-w
Bala M, Verma PK, Awasthi S, Kumar N, Lal B, Singh B (2015) Chemical prospection of important ayurvedic plant Tinospora cordifolia by UPLC-DAD-ESI-QTOF-MS/MS and NMR. Nat Prod Commun 10:43–48
Bhatt V, Sharma S, Kumar N, Sharma U, Singh B (2017) Simultaneous quantification and identification of flavonoids, lignans, coumarin and amides in leaves of Zanthoxylum armatum using UPLC-DAD-ESI-QTOF–MS/MS. J Pharm Biomed Anal 132:46–55. https://doi.org/10.1016/j.jpba.2016.09.035
Bhatt V, Kumar N, Sharma U, Singh B (2018) Comprehensive metabolic profiling of Zanthoxylum armatum and Zanthoxylum acanthopodium leaves, bark, flowers and fruits using ultra high performance liquid chromatography. Sep Sci Plus 1:311–324. https://doi.org/10.1002/sscp.201800004
Campisi A, Acquaviva R, Bonfanti R, Raciti G, Amodeo A, Mastrojeni S, Ragusa S, Iauk L (2014) Antioxidant properties of Berberis aetnensis C. Presl (Berberidaceae) roots extract and protective effects on Astroglial cell cultures. Sci World J 2014:1–7. https://doi.org/10.1155/2014/315473
Chand N, Durrani FR, Ahmad S, Khan A (2011) Immunomodulatory and hepatoprotective role of feed-added Berberis lycium in broiler chicks. J Sci Food Agric 91:1737–1745. https://doi.org/10.1002/jsfa.4399
Chiou W-F, Chen C-F, Lin J-J (2000) Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells by andrographolide. Br J Pharmacol 129:1553–1560. https://doi.org/10.1038/sj.bjp.0703191
Dou J, Lee VSY, Tzen JTC, Lee M-R (2007) Identification and comparison of phenolic compounds in the preparation of Oolong tea manufactured by semi fermentation and drying processes. J Agric Food Chem 55:7462–7468. https://doi.org/10.1021/jf0718603
Guo S, Jiang K, Yang C, Yang Y, Yang J, Zhao G, Wu H, Deng G (2018) Magnoflorine ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-κB and MAPK activation. Front Pharmacol. https://doi.org/10.3389/fphar.2018.00982
Hwang SJ, Kim YW, Park Y, Lee HJ, Kim KW (2013) Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm Res 63:81–90. https://doi.org/10.1007/s00011-013-0674-4
Jiang F, Guan H, Liu D, Wu X, Fan M, Han J (2017) Flavonoids from sea buckthorn inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through the MAPK and NF-κB pathways. Food Funct 8:1313–1322. https://doi.org/10.1039/c6fo01873d
Joshi R, Rana A, Gulati A (2015) Studies on quality of orthodox teas made from anthocyanin-rich tea clones growing in Kangra valley, India. Food Chem 176:357–366. https://doi.org/10.1016/j.foodchem.2014.12.067
Junqueira-Gonçalves MP, Yáñez L, Morales C, Navarro M, Contreras AR, Zúñiga GE (2015) Isolation and Characterization of Phenolic Compounds and Anthocyanins from Murta (Ugni molinae Turcz.) Fruits. Assessment of antioxidant and antibacterial activity. Molecules 20:5698–5713. https://doi.org/10.3390/molecules20045698
Kang OH, Lee GH, Choi HJ, Park PS, Chae HS, Jeong SI, Kim YC, Sohn DH, Park H, Lee JH, Kwon DY (2007) Ethyl acetate extract from Angelica Dahuricae Radix inhibits lipopolysaccharide-induced production of nitric oxide, prostaglandin E2 and tumor necrosis factor-α via mitogen-activated protein kinases and nuclear factor-κB in macrophages. Pharmacol Res 55:263–270. https://doi.org/10.1016/j.phrs.2006.12.001
Kim EA, Kim SY, Ye BR, Kim J, Ko SC, Lee WW, Kim KN, Choi IW, Jung WK, Heo SJ (2018) Anti-inflammatory effect of Apo-9′-fucoxanthinone via inhibition of MAPKs and NF-κB signaling pathway in LPS-stimulated RAW 264.7 macrophages and zebrafish model. Int Immunopharmacol 59:339–346. https://doi.org/10.1016/j.intimp.2018.03.034
Kukula-Koch W (2017) The elevation of {LC}-ESI-Q-TOF-MS response in the analysis of isoquinoline alkaloids from some Papaveraceae and Berberidaceae representatives. J Anal Methods Chem 2017:1–9. https://doi.org/10.1155/2017/8384107
Kumar R, Nair V, Gupta YK, Singh S, Arunraja S (2016) Berberis aristata ameliorates adjuvant-induced arthritis by inhibition of NF-κB and activating nuclear factor-E2-related factor 2/hem oxygenase (HO)-1 signaling pathway. Immunol Investig 45:473–489. https://doi.org/10.3109/08820139.2016.1172638
Kuo C-L, Chi C-W, Liu T-Y (2004) The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett 203:127–137. https://doi.org/10.1016/j.canlet.2003.09.002
Lee W, Ku S-K, Bae J-S (2012) Barrier protective effects of rutin in LPS-induced inflammation in vitro and in vivo. Food Chem Toxicol 50:3048–3055. https://doi.org/10.1016/j.fct.2012.06.013
Lee HJ, Shin JS, Lee KG, Park SC, Jang YP, Nam JH, Lee KT (2017) Ethanol extract of Potentilla supine Linne suppresses LPS-induced inflammatory responses through NF-κB and AP-1 inactivation in macrophages and in endotoxic mice. Phyther Res 31:475–487. https://doi.org/10.1002/ptr.5773
Li B, Lee DS, Choi HG, Kim KS, Kang DG, Lee HS, Jeong GS, Kim YC (2011) Sauchinone suppresses pro-inflammatory mediators by inducing heme oxygenase-1 in RAW264.7 macrophages. Biol Pharm Bull 34:1566–1571. https://doi.org/10.1248/bpb.34.1566
Nakanishi T, Mukai K, Yumoto H, Hirao K, Hosokawa Y, Matsuo T (2010) Anti-inflammatory effect of catechin on cultured human dental pulp cells affected by bacteria-derived factors. Eur J Oral Sci 118:145–150. https://doi.org/10.1111/j.1600-0722.2010.00714.x
Noll C, Lameth J, Paul J-L, Janel N (2012) Effect of catechin/epicatechin dietary intake on endothelial dysfunction biomarkers and proinflammatory cytokines in aorta of hyperhomocysteinemic mice. Eur J Nutr 52:1243–1250. https://doi.org/10.1007/s00394-012-0435-0
Och A, Szewczyk K, Pecio Ł, Stochmal A, Załuski D, Bogucka-Kocka A (2014) Oryeongsan inhibits LPS-induced production of inflammatory mediators via blockade of the NF-kappaB, MAPK pathways and leads to HO-1 induction in macrophage cells. BMC Complement Altern Med. https://doi.org/10.1186/1472-6882-14-242
Och A, Szewczyk K, Pecio Ł et al (2017) UPLC-MS/MS profile of alkaloids with cytotoxic properties of selected medicinal plants of the Berberidaceae and Papaveraceae families. Oxid Med Cell Longev 2017:1–7. https://doi.org/10.1155/2017/9369872
Pae H-O, Chung H-T (2009) Heme oxygenase-1: its therapeutic roles in inflammatory diseases. Immune Netw 9:12. https://doi.org/10.4110/in.2009.9.1.12
Park C-Y, Heo J-N, Suk K, Lee W-H (2017) Sodium azide suppresses LPS-induced expression MCP-1 through regulating IκBζ and STAT1 activities in macrophages. Cell Immunol 315:64–70. https://doi.org/10.1016/j.cellimm.2017.02.007
Plazonić A, Bucar F, Maleš Ž et al (2009) Identification and quantification of flavonoids and phenolic acids in Burr Parsley (Caucalis platycarpos L.), using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Molecules 14:2466–2490. https://doi.org/10.3390/molecules14072466
Pradhan PC, Saha S (2015) Anthocyanin profiling of Berberis lycium Royle berry and its bioactivity evaluation for its nutraceutical potential. J Food Sci Technol 53:1205–1213. https://doi.org/10.1007/s13197-015-2117-4
Qin X, Jiang X, Jiang X, Wang Y, Miao Z, He W, Yang G, Lv Z, Yu Y, Zheng Y (2016) Micheliolide inhibits LPS-induced inflammatory response and protects mice from LPS challenge. Sci Rep 6:6. https://doi.org/10.1038/srep23240
Ruiz A, Hermosin-Gutierrez I, Mardones C, Vergara C, Herlitz E, Vega M, Dorau C, Winterhalter P, von Baer D (2010) Polyphenols and antioxidant activity of calafate (fucoxanthinone) fruits and other native berries from Southern Chile. J Agric Food Chem 58:6081–6089. https://doi.org/10.1021/jf100173x
Sharma R, Sharma A, Kumari A, Kulurkar PM, Raj R, Gulati A, Padwad YS (2017) Consumption of green tea epigallocatechin-3-gallate enhances systemic immune response, antioxidative capacity and HPA axis functions in aged male swiss albino mice. Biogerontology 18:367–382. https://doi.org/10.1007/s10522-017-9696-6
Sharma A, Joshi R, Kumar S, Sharma R, Padwad Y, Gupta M (2018) Prunus cerasoides fruit extract ameliorates inflammatory stress by modulation of iNOS pathway and Th1/Th2 immune homeostasis in activated murine macrophages and lymphocytes. Inflammopharmacology. https://doi.org/10.1007/s10787-018-0448-2
Shin JS, Choi HE, Seo S, Choi JH, Baek NI, Lee KT (2016) Berberine decreased inducible nitric oxide synthase mRNA stability through negative regulation of human antigen R in lipopolysaccharide-induced macrophages. J Pharmacol Exp Ther 358:3–13. https://doi.org/10.1124/jpet.115.231043
Shinohara Y, Tsukimoto M (2018) Adenine nucleotides attenuate murine T cell activation induced by concanavalin A or T cell receptor stimulation. Front Pharmacol. https://doi.org/10.3389/fphar.2017.00986
Shuang-lai ZH, Sheng-shan DO, Xin-ru LI, Run-Hui L, Wei-dong ZH, Hong-lin H, Yi Z, Yao-hua H, Shu-ping W (2011) Qualitative and quantitative analysis of alkaloids in Cortex Phellodendri by HPLC-ESI-MS/MS and HPLC-DAD. Chem Res Chin Univ 27:38–44
Sun M, Liu J, Lin C, Miao L, Lin L (2014) Alkaloid profiling of the traditional Chinese medicine Rhizoma corydalis using high performance liquid chromatography-tandem quadrupole time-of-flight mass spectrometry. Acta Pharmaceutica Sinica B 4(3):208–216. https://doi.org/10.1016/j.apsb.2014.04.003
Wang G, Hu Z, Fu Q, Song X, Cui Q, Jia R, Zou Y, He C, Li L, Yin Z (2017) Resveratrol mitigates lipopolysaccharide-mediated acute inflammation in rats by inhibiting the TLR4/NF-κBp65/MAPKs signaling cascade. Sci Rep. https://doi.org/10.1038/srep45006
Wijayanti N, Huber S, Samoylenko A, Kietzmann T, Immenschuh S (2004) Role of NF-kB and p38 MAP kinase signaling pathways in the lipopolysaccharide-dependent activation of heme oxygenase-1 gene expression. Antioxid Redox Signal 6:802–810. https://doi.org/10.1089/ars.2004.6.802
Xianchu L, Lan Z, Ming L, Yanzhi M (2018) Protective effects of rutin on lipopolysaccharide-induced heart injury in mice. J Toxicol Sci 43:329–337. https://doi.org/10.2131/jts.43.329
Zhang Y, Wu W, Han F, Chen Y (2008) LC/MS/MS for identification of in vivo and in vitro metabolites of jatrorrhizine. Biomed Chromatogr 22:1360–1367. https://doi.org/10.1002/bmc.1066
Acknowledgements
We express our gratitude to the Director, CSIR-IHBT Palampur, for his support and encouragement. We acknowledge SEED, Department of Science and Technology, for providing financial assistance through project GAP-0193 and CSIR for project MLP-0204, respectively, along with Academy of Scientific and Innovative Research (AcSIR), Chennai, India, for Ph.D. registration. We are thankful to Mr. Pawan Kumar for his technical support. CSIR manuscript number for this work is 4327.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, A., Sharma, R., Kumar, D. et al. Berberis lycium Royle fruit extract mitigates oxi-inflammatory stress by suppressing NF-κB/MAPK signalling cascade in activated macrophages and Treg proliferation in splenic lymphocytes. Inflammopharmacol 28, 1053–1072 (2020). https://doi.org/10.1007/s10787-018-0548-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-018-0548-z