Abstract
Cyanidin and chlorogenic acid are polyphenols from plant origin that are present in many common fruits, particularly in berries. To corroborate the protective or detrimental effects of both compounds from a neuro-inflammatory perspective, in vitro experiments were carried out in human astrocytes (U-373). Astrocytes were pre-treated with a range of concentrations of either cyanidin, chlorogenic acid or a combined treatment for a period of 30 min, before exposure to Escherichia coli lipopolysaccharide (LPS) challenge for 23.5 h, after which cytotoxicity (propidium iodide exclusion assay), cytoprotective effects (XTT assay) and effects on functional capacity (secretion of pro-inflammatory cytokines IL-6 and MCP-1) were evaluated. No treatment resulted in cytotoxicity, but high dose (20 µg/mL) LPS significantly reduced mitochondrial reductive capacity (p < 0.001). This effect was prevented in a dose-dependent manner by both cyanidin and chlorogenic acid, as well as by the combination treatment. However, in the absence of LPS, IL-6 secretion was significantly increased in response to 2 µM of either cyanidin or chlorogenic acid (both p < 0.0001), as well as the combination treatment (p < 0.01). MCP-1 secretion followed a similar trend, but did not reach statistical significance. Although we acknowledge the requirement for in vivo investigations to validate our interpretations, current data highlight the potential risk for antioxidant toxicity that is linked to high dose supplementation with single compound antioxidants. Research focused at elucidating synergistic effects between different antioxidants is required to minimise risk of adverse effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The natural antioxidant market is probably one of the most thriving niches in the supplement industry today (Ghosh 2015; Olivo 2016). People are living longer, but unfortunately not healthy, with high incidence of disease from the sixth decade of life (WHO 2015). A major focus for the modern consumer is the use of supplements to counter the harmful effects of a fast-paced lifestyle. In particular, oxidant damage is implicated in ageing and many ageing-related diseases, such as neurodegeneration and others (Ames et al. 1993; Jones et al. 2002; MacNee et al. 2014). Plant polyphenol antioxidants in particular have been shown to be of particular benefit in this regard (Petersen and Smith 2016; Smith 2018; Kodali et al. 2018).
An interesting phenomenon in this niche is the disparity between approaches, especially to antioxidant supplement development between ethnopharmacologists and mainstream pharmacologists: while the norm in traditional pharmacology practise is to isolate a compound and then assess its biological activity or efficacy in particular health or disease scenarios, ethnopharmacologists (as well as physiologists and even botanists) are more often assessing the efficacy and safety of more intact plant-derived extracts, i.e. a less processed product with more constituents.
There are of course merits in both approaches: on one hand, the effect of an isolated compound, as well as potential drug-supplement interactions, may be easier to accurately predict when compared to that of a cocktail of ingredients which may not all be known and scientifically tested. On the other, antioxidant capacity of plant-derived extracts has been shown to be lost during harsh solvent extraction processed when compared to milder (water) extraction (Tobwala et al. 2014). This may suggest that many ingredients at low concentration may contribute to the net antioxidant effect of a plant, or that these actives have synergistic action, as suggested previously (Verpoorte et al. 2005), and argues against purification of single compounds. More comparative research on individual plant products is probably required before a reconciliation of opposing ideas will be possible.
Another obvious consideration, and the focus of the current study, is consumer safety and risk of overdose, as the lay public seems to believe that anything natural is necessarily beneficial to health, independent of dose. In terms of plant-derived extracts, a large portion of scientific investigations has been focusing on undesired effects by comparing large dose ranges to elucidate safe and effective levels for consumption in many pre-clinical and rodent studies (Smith 2011; Bennett et al. 2018). In addition, in the sphere of polyphenol antioxidants, a recent review argued that a more complex supplement does not necessarily hold more risk to consumers, given that the average human diet entails ingestion of several thousand substances daily (Khakimov and Engelsen 2017). Furthermore, while the assumption is made that ingestion of an isolated compound carries less risk, not many isolated polyphenols have been subjected to the same rigorous testing to confirm this. Given the reports of adverse effects of commonly used single-ingredient antioxidants such as vitamins C and E and melatonin (Kim et al. 2018; Colliou et al. 2017; Hart et al. 2012; Miller and Guallar 2009; Zhang and Zhang 2014), this is perhaps an oversight that should be revisited.
Thus, the aim of the current study was to determine the relative safety of two widely consumed antioxidant polyphenols—cyanidin and chlorogenic acid—by testing the response of human astrocytes to a range of single compound doses, as well as a combination dose. Astrocytes (comprising 20–40% of all glial cells) are known to produce cytokines capable of either neuronal protection, or functional impairment (Kaushal et al. 2015). Astrocytes have an established capacity to secrete low levels of specifically IL-6 and MCP-1 under normal physiological conditions, in response to, e.g. normal byproducts of metabolic processes, to maintain homeostasis (Balkwill and Burke 1989). IL-6 in particular has been reported to modulate secretory pathways for other cytokines, which may be involved in neurodegenerative processes (Bolin et al. 2005). In addition, we have previously shown astrocytes—and their secretion of IL-6 and MCP-1 in particular—to be sufficiently sensitive to reflect in vitro cellular responses to LPS stimulus as well as potential benefits of plant-derived products (e.g. Bennett et al. 2018). Thus, astrocyte viability and functional responses of these glial cells in terms of their capacity for MCP-1 and IL-6 secretion were chosen as model for neuro-inflammatory risk in the current study.
Methods
Cell culture propagation
Human astrocytes (N7805-100, Life Technologies) of low passage (< 10) were thawed and cultured in complete Dulbecco’s Modified Eagle Medium (DMEM), containing 10% foetal bovine serum (FBS) and 1% penicillin–streptomycin, and subsequently seeded into a 48-well culture plate at a density of 1 × 104 cells/well in serum-free DMEM, and incubated (37 °C, 5% CO2) for 24 h to fully adhere to the plate.
Preparation of antioxidant pre-treatments
A 10 µM stock solution of chlorogenic acid and cyanidin was made up by dissolving in DMEM (1% FBS and penicillin–streptomycin). The mixtures were vortexed until the samples were completely solved, then they were filtered using a 0.22 µm sterile syringe filter. Finally, serial dilutions of each treatment were carried out to get 2 µM, 1 µM and 0.5 µM solutions.
The purity of cyanidin was determined by Extrasynthese using a HPLC and reaching ≥ 96%. On the other hand, chlorogenic acid was acquired by Sigma-Aldrich and its purity was assessed by titration with NaOH (≥ 96%). Oxygen radical absorption capacity (ORAC) was performed for both antioxidants as validation of their activity: chlorogenic acid and cyanidin had an ORAC of 6.74 µM Trolox equivalents (TE) and 1.32 µM TE, respectively.
Preparation of LPS
A 2 mg/mL E. coli LPS (L4391, Sigma-Aldrich) stock solution was prepared in Hank’s Buffered Salt Solution (HBSS) and added to wells at a final concentration of 15 µg/mL and 20 µg/mL for astrocyte inflammatory stimulation.
Treatment intervention
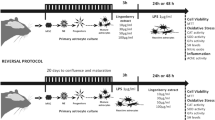
The supernatant was aspirated from each well, and the cell monolayer washed once with Dulbecco’s phosphate-buffered saline (DPBS) to remove remaining media residue. A 30-min pre-treatment period was then initiated, which involved the addition of the different treatments (0.5, 1.0 or 2.0 µM cyanidin, or 0.5, 1.0 or 2.0 µM chlorogenic acid, or a combination of 0.5 µM cyanidin and 0.5 µM chlorogenic acid) to the respective wells. For this period, serum-free media was added to the control and LPS-control groups.
After the pre-incubation period, LPS was added to the LPS-control wells and LPS-treatment combination groups, to achieve two different final LPS concentrations of 15 µg/mL and 20 µg/mL. The LPS vehicle was added to all control wells. The cells were incubated for a further 23.5 h. At the end of the incubation period, the supernatant was removed and stored at − 80 °C for subsequent cytokine quantification, while viability assays were performed on the cells.
Propidium iodide exclusion assay
A 1 mg/mL propidium iodide (PI) (P1304MP, Thermofisher Scientific) stock solution was prepared according to manufacturer’s instructions. For a 3 µM working solution, the stock solution was diluted 1:500 in phosphate-buffered saline (PBS).
Following the 24-h treatment intervention, supernatant was aspirated from each well. The cell monolayer was washed with DPBS before the cells were trypsinized, neutralised and centrifuged at 1500 rpm for 5 min at room temperature. The resulting supernatant was discarded, and each astrocyte pellet was resuspended in 1 mL PI working solution. The samples were incubated at room temperature for 10 min in the dark before flow cytometric analysis on BD FACSAria IIu (with BD FACSDiva v8.1 Software). Live stained, dead stained, and live unstained controls were also used.
XTT mitochondrial reductive capacity
Following the 23.5-h treatment period, the supernatant was removed from each well. The astrocyte monolayer was washed twice with DPBS to remove residual treatment, following which XTT (X4626, Sigma-Aldrich) solution (1 mg/mL) containing 0.5% phenazine methosulphate (P9625, Sigma-Aldrich) was added to each well, and a 4-h incubation period was initiated in a shaking incubator at 37 °C. Following incubation, optical densities were determined at 490-nm using a Universal Microplate Reader (Bio-Tek Instruments, Inc. EL800) and analysed using KCjunior for Windows Data Reduction Software (v1.41.3).
Cytokine measurement
Cell-free culture supernatant was analysed using a commercial magnetic bead panel assay (custom-designed Milliplex MAP Human Soluble Cytokine Receptor Panel, Merck Millipore) for concentrations of interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1). The fluorescent signals were analysed with a Bio-Plex 200 instrument, in conjunction with Bio-Plex Manager 6.1 software. Cytokine responses were expressed as absolute concentrations in cell culture supernatant.
Quantification of cytokine concentrations was performed based on a standard curve, derived from linear dilution of the manufacturer-supplied cytokine standards. The detection limit was 0.9 pg/mL for IL-6 and 1.6 pg/mL for MCP-1.
Statistical analysis
Results are represented as average of triplicates and standard error meanings (SEMs). Data distribution was established following a one-way analysis of variance (ANOVA) and Dunnett post hoc tests for three or more groups and t test student analyses for two groups. Statistical differences were considered significant for p < 0.05. Statistical analyses and figures were prepared in GraphPad Prism v.6.
Results
Cellular viability
Absolute cell survival was assessed using the Propidium Iodide (PI) exclusion assay. Neither dose of LPS, nor any of the doses of antioxidant compounds used, had any effect on human astrocyte cellular survival (Fig. 1). Given the significant drop in mitochondrial reductive capacity after exposure to high-dose LPS seen with the XTT data, it is possible that a longer incubation protocol may have reflected changes in cell survival which is not evident here after 24 h. A longer duration protocol may provide more information on ultimate cell death/survival. However, in the current context, the fact that no cell death is evident can be seen as a strength of the study, as cytokine concentrations do not need to be adjusted for potential effects of cell death.
Taken together, the XTT and PI data suggest that the current protocol did not induce cell death at the time points assessed, but did affect changes in mitochondrial reductive capacity. To further explore the oxidative stress—inflammation link, pro-inflammatory cytokine secretion was assessed for cells exposed to either 15 or 20 µg/mL LPS after pre-treatment with different doses of either chlorogenic acid, cyanidin, or a combination treatment containing 0.5 µM of each.
Mitochondrial reductive capacity
The mitochondrial reductive capacity of human astrocytes was evaluated by the XTT assay. Cells were pre-treated with a range of concentrations from 0.5 to 2 μM of chlorogenic acid, cyanidin or chlorogenic/cyanidin (both 0.5 µM) combination for 30 min. After that time, cells were challenged with E. coli LPS (15 and 20 µg/mL) for 23.5 h in the presence of the antioxidant treatments. As shown in Fig. 2, in the absence of LPS, no concentration of any compound seemed to affect the mitochondrial reductive capacity of the cells. Exposure to 20 µg/mL LPS, but not the lower dose, caused a significant reduction of mitochondrial reductive capacity (more than 35%; p < 0.001 vs. control).
Mitochondrial viability of human astrocytes determined by XTT assay, following treatment with chlorogenic acid (a), cyanidin (b) or chlorogenic acid + cyanidin (c) with or without LPS stimulation. Results are expressed as percentage of mitochondrial activity over control cells. #p < 0.001 versus control; **p < 0.01 versus LPS (20 µg/mL); ***p < 0.001 versus LPS (20 µg/mL). Statistical differences were detected through one-way ANOVA Dunnett post hoc test
This decrease in mitochondrial reductive capacity was countered in a dose-dependent manner by both chlorogenic acid and cyanidin, as well as by the combination treatment containing a relatively low dose (0.5 µM) of each (Fig. 2).
Inflammatory cytokine secretion
The response of astrocytes to the compounds, in the presence and absence of an inflammatory stimulus (LPS), was assessed in terms of two major pro-inflammatory cytokines associated with both neuroinflammation and systemic inflammation in chronic disease—IL-6 and MCP-1.
When the astrocytes were treated only with different concentrations of LPS, the lower dose of toxin elicited secretion of a greater amount of IL-6 with respect to the control cells. However, at the higher dose of LPS (20 µg/mL), where mitochondrial reductive capacity was already compromised at the end of the relatively short 24 h incubation period (refer to XTT data, Fig. 2), cellular capacity for cytokine secretion was also compromised (Fig. 3). ANOVA indicated significant main effect of treatment on IL-6 secretion (p < 0.0001). Although a similar trend was observed for MCP-1, with secretion levels almost double after low-dose LPS exposure, this effect did not quite reach statistical significance.
Pro-inflammatory responses of untreated control cells exposure to increasing concentrations of LPS. Results are expressed as absolute concentration of cytokines (pg/mL) in culture supernatants: a IL-6 and (B) MCP-1. ***p < 0.001 versus LPS (15 µg/mL); ****p < 0.0001 versus LPS (15 µg/mL). Statistical differences were detected through one-way ANOVA Dunnett post hoc test
When considering cellular functional response capacity in terms of inflammatory cytokines, the lower doses of chlorogenic acid resulted in a somewhat dampened IL-6 and MCP-1 response to low-dose LPS, but did not limit the response to high-dose LPS (Fig. 4a, b). Similar results were observed for both the IL-6 and MCP-1 response after cyanidin pre-treatment (Fig. 4c, d). However, after high dose pre-treatment (2 μM), although both cyanidin and chlorogenic acid treatments enabled a cytokine response to high-dose LPS, the dampening of the response seen at low-level LPS exposure, was largely lost. This may be the result of the antioxidant treatments themselves, since cytokine responses were also significant in the absence of LPS at the highest antioxidant treatment levels (Fig. 4a–d). In addition, in astrocytes pre-treated with a combination of low-dose chlorogenic acid and cyanidin, a significantly increased IL-6 and MCP-1 response was evident, again independent of LPS exposure (Fig. 4e, f).
Effect of chlorogenic acid (a, b), cyanidin (c, d) and chlorogenic acid + cyanidin (e, f) with or without LPS stimulation on pro-inflammatory cytokine production by astrocytes. Results are expressed as absolute concentration of cytokines (pg/mL) in culture supernatants. #p < 0.01 versus control; ####p < 0.0001 versus control; *p < 0.05 versus LPS (20 µg/mL); **p < 0.01 versus LPS (20 µg/mL); ***p < 0.001 versus LPS (20 µg/mL); ****p < 0.0001 versus LPS (20 µg/mL); +p < 0.05 versus LPS (15 µg/mL); ++p < 0.01 versus LPS (15 µg/mL); +++p < 0.001 versus LPS (15 µg/mL). Statistical differences were detected through one-way ANOVA Dunnett post hoc test and Student’s t test
Discussion
Current data contribute to the antioxidant literature by assessing the effects of different doses of antioxidant compounds on the inflammatory response, both basally and in response to an inflammatory stimulus, in a physiologically relevant context.
In our astrocyte model, neither the antioxidant treatments assessed, nor the LPS doses employed as inflammatory stimulus, resulted in cell death, as evidenced by the propidium exclusion assay results. However, using a range of doses of LPS, we could simulate conditions comparable with chronic disease-related low-grade inflammation, where deficits in mitochondrial function have indeed been observed (Baker et al. 2014; De Felice and Farreira 2014). The fact that current data suggest mitochondrial dysfunction or impaired function at the highest dose of LPS but not at the lower dose, further enables interpretation of the effect of antioxidant treatment both in the absence and presence of LPS-induced mitochondrial pathology.
After low-dose, single compound antioxidant treatment, cellular responses are in line with a picture of oxygen radical quenching, as has previously been demonstrated for the cyanidin employed here (Cásedas et al. 2018). This antioxidant effect would enable cells to maintain functional capacity—i.e. the cytokine response to LPS challenge—but will at the same time decrease LPS-associated oxidant-induced damage to cells, so that a dampened inflammatory response is required in response to astrocyte damage. (To fully interpret the cytokine results, it is important to remember that the cytokine levels seen are the sum of the astrocyte cytokine response to the LPS stimulus through activation of toll-like receptor 4 on the cell surface (Li et al. 2016; Tarassisshin et al. 2014) and the cytokines released from the cell in response to oxidant damage (Karve et al. 2016).)
After high-dose antioxidant treatment however, the inflammatory response seems to be exacerbated, even in the absence of LPS, which may be interpreted as a pro-oxidant response (Bennett et al. 2018). Although current data may be insufficient for firm conclusions, given that data collection was performed at one time point only, in our opinion, there is sufficient published literature to support this interpretation of damage induced/exacerbated by antioxidants under certain conditions.
Firstly, the class of anthocyanidins is known to have their antioxidant effects via two mechanisms: firstly, by chelating metal ions (such as copper or iron) to prevent lipid damage by interrupting the lipid peroxidation reaction chain and secondly, by scavenging free radicals (Nimse and Pal 2015). In the process, the polyphenolic antioxidant is converted to a somewhat more stable radical. However, both the accumulation of altered antioxidants themselves and other characteristics of the extracellular matrix may result in even these stable radicals becoming capable of inducing cellular damage (Liu 2014). For example, quercetin—another natural polyphenol with the capacity for metal chelation—was shown to exert opposing effects under different conditions. While quercetin was reported to prevent DNA damage by metal chelation in the presence of low cupric ion levels, at high levels of cupric ions, quercetin seemed to exarcerbate DNA damage via both increased reactive oxygen species (ROS) production and altered NF-kB signalling (Jun et al. 2007; Galaris and Evangelou 2002). Furthermore, in the context of vitamin E (α-tocopherol), an α-tocopheroxyl radical was shown to induce lipid oxidation in the absence of other antioxidants (Bowry and Stocker 1993). Thus, considering the demonstrated high potency of cyanidin in comparison to other antioxidants (Cásedas et al. 2018; Amorini et al. 2001), these reports lend more credibility to an interpretation for high risk of pro-oxidant function at high dose. Although similar studies could not be found for chlorogenic acid, this antioxidant was recently reported to induce apoptosis-like cell death in bacteria after depletion of reactive oxygen species (Lee and Lee 2018), which also suggests mechanisms and potency sufficiently high to exert pro-oxidant effect. Further in-depth investigations are required to fully elucidate the mechanisms at play and the conditions under which these antioxidants may become pro-oxidant.
Of interest in this context, more levels of complexity can be added to understanding how different antioxidants may compliment or oppose each other: disparate neuroprotective effects have been reported for the anthocyanins kuromanin and callistephin, which was ascribed to the fact that of the two, only kuromanin had the capacity to generate superoxide which could act as nitric oxide (NO) scavenger (Winter et al. 2017). Our discussion of results here was centred around oxygen radical quenching, as that is the known mechanism for LPS-induced cell damage. However, chlorogenic acid has similarly been shown to have anti-inflammatory effects in murine macrophages in a NO-dependent manner (Hwang et al. 2014), which also implicates interaction of nitrogen radicals in the interactions between antioxidants and radicals they aim to neutralise. In addition, astrocytes themselves have been shown to undergo a phenotype switch upon activation, enabling them to secrete antioxidants of their own (Barres, 2008; Hernandez et al. 2008; Parpura et al. 2012). The nature and potency of these antioxidants—and their ultimate fate in terms of stabilisation—also have to be considered to arrive at a comprehensive picture of events at cellular level.
In conclusion, clearly much more in-depth molecular research is required to achieve a clear picture of the effects of antioxidants, both in isolation and in combination, at cellular level. In addition, in vivo models are required to investigate interactions between (single or multiple) exogenous and endogenous antioxidants and situations modulating the cellular environment. Nevertheless, current data points out the risk of high-dose supplementation and the need for a better understanding of how different antioxidants may interact for maximal benefit and minimal risk. Further research in this context may inform on natural product compounding practises for increased consumer safety.
References
Ames BN, Shigenaga MK, Gold LS (1993) DNA lesions, inducible DNA repair, and cell division: three key factors in mutagenesis and carcinogenesis. Environ Health Perspect 101 (Suppl 5):35–44
Amorini AM, Fazzina G, Lazzarino G, Tavazzi B, Di Pierro D, Santucci R, Sinibaldi F, Galvano F, Galvano G (2001) Acitivity and mechanisms of the antioxidant properties of cyanidin-3-O-beta-glucopyranoside. Free Radic Res 35(6):953–966
Baker B, Maitra U, Geng S, Li L (2014) Molecular and cellular mechanisms responsible for cellular stress and low-grade inflammation induced by a super-low dose of endotoxin. J Biol Chem 289(23):16262–16269. https://doi.org/10.1074/jbc.M114.569210
Balkwill FR, Burke F (1989) The cytokine network. Immunol Today 10:299–304. https://doi.org/10.1016/0167-5699(89)90085-6
Barres BA (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60:430–440
Bennett AC, Van Camp A, Lopez V, Smith C (2018) Sceletium tortuosum may delay chronic disease progression via alkaloid-dependant antioxidant or anti-inflammatory action. J Physiol Biochem. https://doi.org/10.1007/s13105-018-0620-6
Bolin LM, Zhaung A, Strychkarska-Orczyk I, Nelson E, Huang I, Nguyen MQ (2005) Differential inflammatory activation of IL-6 (−/−) astrocytes. Cytokine 30(2):47–55
Bowry VW, Stocker R (1993) Tocopherol-mediated peroxidation: the pro-oxidation effect of vitamin E on the radical-initiated oxidation of human low-density lipoprotein. J Am Chem Soc 115:6029–6044
Cásedas G, González-Burgos E, Smith C, López V, Gómez-Serranillos MP (2018) Regulation of redox status in neuronal SH-SY5Y cells by blueberry (Vaccinium myrtillus L.) juice, cranberry (Vaccinium macrocarpon A.) juice and cyanidin. Food Chem Toxicol 118:572–580
Colliou E, Mari A, Delas A, Delarche A, Faguer S (2017) Oxalate nephropathy following vitamin C intake within intensive care unit. Clin Nephrol 88(12):354–358
De Felice FG, Farreira ST (2014) Inflammation, defective insulin signalling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes 63(7):2262–2272. https://doi.org/10.2337/db13-1954
Galaris D, Evangelou A (2002) The role of oxidative stress in mechanisms of metal-induced carcinogenesis. Crit Rev Oncol Hematol 42(1):93–103
Ghosh D (2015) Nutrition’s growing role in fighting inflammation. Neutraceuticals World, 1. http://www.nutraceuticalsworld.com/issues/2015-2001/view_features/ nutritions-growing-role-in-fighting-inflammation
Hart C, Cohen R, Norwood M, Stebbing J (2012) The emerging harm of antioxidants in carcinogenesis. Future Oncol 8:535–548
Hernandez MR, Miao H, Lukas T (2008) Astrocytes in glaucomatous optic neuropathy. Prog Brain Res 173:353–373
Hwang SJ, Kim Y, Park Y, Lee H, Kim K (2014) Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm Res 63:81–90
Jones DP, Mody VC Jr, Carlson JL et al (2002) Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med 33(9):1290–1300
Jun T, Liancai Z, Bochu W (2007) Effects of quercetin on DNA damage induced by copper ion. Int J Pharmacol 3(1):19–26
Karve IP, Taylor JM, Crack PJ (2016) The contribution of astrocytes and microglia to traumatic brain injury. Br J Pharmacol 173(4):692–702
Kaushal V, Dye R, Pakavathkumar P, Foveau B, Flores J, Hyman B, Ghetti B, Koller BH, LeBlanc AC (2015) Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ 22:1676–1686. https://doi.org/10.1038/cdd.2015.16
Khakimov B, Engelsen SB (2017) Resveratrol in the foodomics era: 1:25,000. Ann N Y Acad Sci 1403(1):48–58
Kim TJ, Buyn JS, Kwn HS, Kim DY (2018) Cellular toxicity driven by high-dose vitamin C on normal and cancer stem cells. Biochem Biophys Res Comm 497(1):347–353
Kodali M, Hattiangady B, Shetty GA, Bates A, Shuai B, Shetty AK (2018) Curcumin treatment leads to better cognitive and mood function in a model of Gulf War Illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus. Brain Behav Immun 69:499–514
Lee B, Lee DG (2018) Depletion of reactive oxygen species induced by chlorogenic acid triggers apoptosis-like death in Escherichia coli. Free Radic Res 52(5):605–615
Li N, Zhang X, Dong H, Zhang S, Sun J, Qian Y (2016) Litium amerliorates LPS-induced astrocyte activation partly via inhibition of toll-like receptor 4 expression. Cell Physiol Biochem 38:714–725
Liu Z (2014) Antioxidants may not always be beneficial to health. Nutrition 30:131–133
MacNee W, Rabinovich RA, Choudhury G (2014) Ageing and the border between health and disease. Eur Respir Soc 44:1055–1068
Miller ER III, Guallar E (2009) Vitamin E supplementation: what’s the harm in that? Clin Trials 6:47–49
Nimse SB, Pal D (2015) Free radicals, natural antioxidants and their reactions mechanisms. RSC Adv 5:27986. https://doi.org/10.1039/c4ra13315c
Olivo L (2016) Insights on antioxidants: keys to future development. Neutraceuticals World, 3. www.nutraceuticalsworld.com/issues/2016-2003/view_features/insights-on-antioxidants-keys-to-future-development
Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A et al (2012) Glial cells in (patho)physiology. J Neurochem 121:4–27
Petersen KS, Smith C (2016) Ageing-associated oxidative stress and inflammation are alleviated by products from grapes. Oxidative Medicine and Cellular Longevity (Special issue: Medicinal Plants in Therapy: antioxidant activities) 2016:4. https://doi.org/10.1155/2016/6236309
Smith C (2011) The effects of Sceletium tortuosum in an in vivo model of psychological stress. J Ethnopharmacol 133:31–36
Smith C (2018) Natural antioxidants in prevention of accelerated ageing: a departure from conventional paradigms required. J Physiol Biochem. https://doi.org/10.1007/s13105-018-0621-5
Tarassisshin L, Suh HS, Lee SC (2014) LPS and IL-1 differentially activate mouse and human astrocytes: role of CD14. Glia 62(6):999–1013
Tobwala S, Fan W, Hines CJ, Folk WR (2014) Antioxidant potential of Sutherlandia frutescens and its protective effects against oxidative stress in various cell cultures. BMC Complement Altern Med 14:271 http://www.biomedcentral.com/1472-6882/14/271
Verpoorte R, Choi YH, Kim HK (2005) Ethnopharmacology and systems biology: a perfect holistic match. J Ethnopharmacol 100(12):53–56
WHO (2015) WHO Fact sheet. http://www.who.int. Accessed 15 Oct 2015
Winter AN, Ross EK, Khatter S, Miller K, Linseman DA (2017) Chemical basis for he disparate neuroprotective effects of the anthocyanins, callistephin and kuromanin, against nitrosative stress. Free Rad Biol Med 103:23–34
Zhang H, Zhang Y (2014) Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res 57:131–146
Acknowledgements
The South African National Research Foundation (NRF) is acknowledged for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cásedas, G., Bennett, A.C., González-Burgos, E. et al. Polyphenol-associated oxidative stress and inflammation in a model of LPS-induced inflammation in glial cells: do we know enough for responsible compounding?. Inflammopharmacol 27, 189–197 (2019). https://doi.org/10.1007/s10787-018-0549-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-018-0549-y