Abstract
Naringenin (Nar) is a flavonoid derived from plant foods. It has been shown to have anti-inflammatory properties. Many studies have shown that overexpression of reactive oxygen species (ROS) and nuclear factor-κB (NF-κB) leads to increased mucin (MUC) 5AC expression in chronic inflammation of the airway. In addition, some studies have reported that naringenin inhibits NF-κB activity in a murine model of asthma. We speculated that naringenin might be associated with mucous hypersecretion, but the molecular mechanisms remain to be defined. Our study has also investigated whether naringenin could inhibit production of ROS and the activity of NF-κB on the inflammatory pulmonary diseases induced by human neutrophil elastase (HNE) and reduce the mRNA and protein levels of MUC5AC as shown by reverse transcriptase-polymerase chain reaction and real-time PCR (RT-PCR). Serum total MUC5AC protein was detected by enzyme-linked immunosorbent assay (ELISA), the protein morphological changes of MUC5AC were also observed by immunofluorescence and confocal laser technology. Hyperactivation of epidermal growth factor receptor (EGFR) signaling is commonly involved in the mucous hypersecretion process and initiates both the activation of extracellular signal-related kinases 1/2 (ERK1/2) and of phosphatidylinositol 3-kinase (PI3K) and Akt kinase. NF-κB is a key factor downstream of PI3K/Akt signaling, which induces overexpression of the MUC5AC gene. Our data revealed that naringenin inhibited the activation of EGFR resulting in the downregulation of the enzyme activities. Naringenin also reduced the protein expressions of p-EGFR, PI3K, p-Akt, p-ERK1/2, and NF-κB as shown by western blotting. Furthermore, naringenin significantly inhibited PI3K/Akt and ERK MAPKinase signaling with a concurrent reduction in production of ROS and NF-κB activities. These results suggest that naringenin may play a protective role by minimizing mucous production during airway inflammation by down-regulating ROS production and inhibiting the NF-κB activity via EGFR-PI3K-Akt/ERK MAPKinase signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucous hypersecretion is an important manifestation of chronic inflammatory airway diseases, including severe asthma, cystic fibrosis, chronic obstructive pulmonary disease, and bronchiectasis [1, 2]. Mucous obstruction of the airway is the major cause of morbidity and mortality in patients with these diseases [3]. MUC5AC mucin is a major component of airway mucous [4, 5], and its gene expression involves numerous signaling pathways.
Human neutrophil elastase (HNE) is a serine protease secreted by neutrophils into airways, and can be present in high concentrations [6, 7] in the airway secretions of subjects with chronic inflammatory airway diseases. HNE is the most potent mucous agonist, inducing the production of MUC5AC, ROS generation, and epidermal growth factor receptor (EGFR) activation in human airway epithelial cells [8–10]. ROS represents a group of ubiquitous molecules that regulate important steps in signal transduction cascades and many critical cellular events. ROS have long been known to be generated by NADPH oxidase in inflammatory cells [11], which subsequently stimulate EGFR activation through both ligand-dependent and ligand-independent mechanisms [12]. Activation of EGFR plays a central regulatory role in the synthesis of mucin. Binding of EGFR to its ligand to form homo or heterodimers induces autophosphorylation of EGFR. The activation of phospho-EGFR (p-EGFR) is combined with the relevant signaling protein, triggering downstream signaling cascades, including MAPKs p38, c-jun N-terminal kinase (JNK), extracellular signal-related kinases1/2 (ERK1/2), and big MAPK (BMK, ERK5). When this information is transferred to the nucleus, nuclear factor-κB (NF-κB) and other cis-acting elements are activated to start stricky protein synthesis [13, 14] of pro-inflammatory factors and mucin. Among the MAPKs, only ERK1/2 is involved in the secretion of MUC5AC mucin by neutrophil elastase [15]. EGFR activation leads to downstream activation of the phosphatidylinositol 3-kinase (PI3K) transduction pathway [16]. ERK1/2 and PI3K are involved in the activation of NF-κB [17], all of which cause the synthesis of MUC5AC mucin.
Naringenin (Nar), is found in grapefruit extract, is a naturally occurring flavanone which has anti-inflammatory and antioxidant properties [18, 19]. Du et al. [20] reported that naringenin could improve the immunosuppressive environment by down-regulating TGF-β1 and reducing regulatory T cells in mice with pulmonary fibrosis. TGF-β1 also induces survival signals through transactivation of EGFR, which is required for Akt phosphorylation [21]. Thus, naringenin may attenuate the levels of EGFR transcription directly. Previous studies have shown that HNE induces MUC5AC mucin expression via a cascade involving PKC-ROS-TACE in human airway epithelial cells [22]. Therefore, the naringenin flavanone inhibits TGF-α-dependent EGFR activation by attenuating the generation of ROS indirectly [23]. Furthermore, some studies have shown naringenin has anti-inflammatory functions in vitro and inhibits NF-κB in macrophages [24]. Shi et al. reported that naringenin can suppress mucous production by inhibiting NF-κB activity in a murine model of asthma [25].
We have hypothesized that naringenin may have inhibitory effects on mucous hypersecretion by modulating ROS production and inhibiting NF-κB activity and these molecular mechanisms depend on both the PI3K/Akt and ERK MAPKinase signaling pathways.
Materials and methods
Cell culture
HBE16 human airway epithelial cells were obtained from segmental human airway and immortalized using an origin-defective SV40 [26]. These cells were purchased from Bio-Engineering Company, Guangzhou Yasunobu (China). The cells were cultured at 37°C in RPMI 1640 medium supplemented with 10% fetal bovine serum (Hyclone) and 100 units/ml of penicillin/streptomycin.
Materials and antibodies
Naringenin was obtained from Sigma-Aldrich (St. Louis, MO) (N5893-1G, 038K1039). This was dissolved in dimethylsulfoxide (DMSO) to make a stock solution of 0.1 mg/μl (400 mM) [27]. The Reactive Oxygen kit was purchased from Molecular Probes, the electrophoresis reagents and protein assay kit were acquired from Bio-Rad (Hercules, CA). HNE was purchased from United States EPC EPC (Elastin Products Company, Owensville, MO). Active Oxygen Scavenger DMTU and AG 1478 were obtained from Calbio Chemical Inc (Germany). Triton-X100 was obtained from Sigma-Aldrich. Mouse anti-human mucin (mucin, MUC) 5AC monoclonal antibody was obtained from Chemicon Inc (Temecula, CA). Anti-IκBα, anti-NF-κB (p65), and anti-PI3K antibodies were generously provided by Santa Cruz Biotechnology Inc (Santa Cruz, CA). The phospho-specific antibodies for EGFR, ERK1/2 and Akt, anti-EGFR and anti-Akt antibodies were obtained from Cell Signaling Inc (Danvers, MA). Isothiocyanate (FITC)-labeled goat anti-fluorescein mouse IgG, HRP-labeled goat anti-rabbit IgG, and HRP-labeled goat anti-mouse IgG were purchased from T-shirt, Beijing Jinqiao Biotechnology Co, Ltd (China).
Cell viability analysis
HBE16 cells were plated at a density of 1–2 × 104 cells per well in 200 μL RPMI 1640 medium in 96-well plates for 24 h. At this point, serum-free culture medium was added in place of RPMI 1640 and cells were maintained for 24 h. Cells were exposed to different concentrations of concentrations of naringenin for 24 h. The viability of cells was evaluated using a conventional 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction assay, as previously described. Briefly, MTT solution (final concentration, 0.5 mg/ml) was added to each well. The plates were then incubated for 4 h at 37°C. After incubation, 150 μl of DMSO was added to each well for 15 min at room temperature and absorbance measured at 570 nm using an ELISA reader. (TECAN SunriseTM Remote, Austria). The mean percentage of cell survival relative to that of untreated cells was estimated using data from three individual experiments.
Measurement of intracellular ROS levels
Cells were plated at a density of 1 × 106 per well in wells pretreated with naringenin (6-well plates) and held for 24 h. Cells were then exposed to various concentrations of HNE for 30 min. H2DCFDA solution (final concentration, 10 μmol/l) was subsequently added to each well. The plates were incubated for 20 min at 37°C. After incubation, the relative content of ROS in cells was measured by using Fluorescence microplate reader (Becton–Dickinson, San Jose, CA, USA).
Analysis of gene expression
Total RNA was isolated from HBE16 cell using Trizol reagent according to the manufacturer’s instructions (Takara Bio Inc, Japan) and was verified by 1.5% agarose gel electrophoresis. The absorbance (A260/280 nm) was in the range of 1.8–2.0. Reverse-transcriptase (RT) was carried out with oligodeoxythymidylate primer using 3 μg of total RNA from each sample for complementary DNA synthesis. According to Genebank NM_002638 (67–420 bp), the following primers designed with Primer Premier 5.0 software were used: MUC5AC forward primer: 5′-CAG-CCA-CGT-CCC-CTT-CAA-TA-3′ and MUC5AC reverse primer: 5′-ACC-GCA-TTT-GGG-CAT-CC-3′; EGFR forward primer: 5′-CTC-ACG-CAG-TTG-GGC-ACT-TT-3′ and EGFR reverse primer: 5′-TCA-TGG-GCA-GCT-CCT-TCA-GT-3′. Total RNA was incubated with 1 μl of oligo (dT), 1 μl of 10× RT Buffer, 1 μl of MgCl2, 0.5 μl of AMV Reverse Transcriptase (Takara Bio Inc, Japan), 3.75 μl of RNase Free dH2O, and 0.25 μl of RNase Inhibitor in a reaction mixture (total volume: 10 μl). The reaction mixture was incubated at 30°C for 10 min, 42°C for 30 min, followed by incubation for 5 min at 99°C. Then, 10 μl of 5× PCR, 0.5 μl each of the forward and reverse MUC5AC or EGFR primers, 0.25 μl of TaKaRa Ex TaqTMHS (Takara Bio Inc, Japan) and enough H2O to bring the total volume to 50 μl were added to the reaction products. PCR was conducted on these in an automated research thermocycler for 30 cycles. PCR conditions of MUC5AC included: initial denaturation for 2 min at 94°C; denaturation for 30 s at 94°C; annealing for 30 s at 59°C; extension for 1.5 min at 72°C, and a final extension at 72°C for 5 min. PCR conditions of EGFR was the same as MUC5AC. PCR products were analyzed by 1.5% agarose gels electrophoresis. Quantitative data normalized to beta-actin were obtained using computerized densitometry and ImageQuant software (Amersham Biosciences Inc).
The samples of cDNA were then subjected to real-time PCR analysis on an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, USA) using standard conditions. The primers and the TaqMan probes for the detection of MUC5AC and EGFR were purchased from Applied Biosystems. Each reaction consisted of 12.5 μl 2× QuantiTect Probe PCR master mix, 300 nM forward and 300 nM reverse primers, and 175 nM probe and 1 μl of cDNA made up to 25 μl with nuclease-free water. The reaction parameters were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The relative MUC5AC or EGFR mRNA quantity was obtained using a comparative cycle threshold method and was normalized to their own total protein as an endogenous control.
Determination of MUC5AC protein in cell supernatant by ELISA
Production of MUC5AC protein in cell lysates and in cell culture supernatants was measured by ELISA with mouse anti-human MUC5AC. In brief, cell lysates were prepared with PBS at multiple dilutions. One hundred microliter of each sample with bicarbonate-carbonate buffer was incubated in a 96-well plate at 37°C, then held overnight at 4°C. After blocking, plates were washed three times with PBS containing 0.05% Tween 20 (PBST), and blocked with 100 μl of 1% BSA/PBST for 1 h at 37°C. Plates were again washed three times with PBST, and then incubated with 50 μl of mouse monoclonal MUC5AC antibody (1:500), diluted with 1% BSA/PBST. Plates were gently shaken for 1 h at 37°C. After 1 h, 100 μl of horseradish peroxidase-goat anti-mouse IgG conjugate (1:1,000) was dispensed into each well for 1 h then washed three times. Color reaction was developed with 3,3′,5,5′-tetramethylbenzidine (TMB) peroxidase solution and stopped with 2 N H2SO4. Absorbance was read at 450 nm with a microplate reader. Absorbance was compared with standard samples and content of MUC5AC was calculated.
Western blot analysis
Cells were washed three times with ice-cold PBS, and harvested with ice-cold lysis buffer containing 1 ml of RIPA lysis buffer (Pik-day Bio Co, Ltd, China), 10 μl of PMSF (1000 mM), 10 μl of serine/threonine phosphatase inhibitors, and 10 μl of tyrosine phosphatase inhibitor. Cell lysates were precleared by centrifugation at 12,000 rpm during for 5 min at 4°C. The supernatants were collected for the total protein samples. Protein concentrations were determined using the bicinchoninic acid-based BCA protein assay kit (Pierce Chemical, Rockford, IL). The proteins from various groups were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 8–12% running gel and transferred into polyvinylidene difluoride (PVDF) membrane (Millipore, USA). The membranes were incubated in blocking solution (5% dried non-fat milk in PBST) for 1 h with gentle shaking at room temperature, and then probed with first antibody to anti-IκBα (1:200), anti-NF-κB (p65) (1:200), anti-EGFR (1:200), anti-pEGFR (1:200), anti-PI3K (1:1000), anti-pAkt (1:1000), anti-pERK1/2 (1:1000), or β-actin (1:2000) overnight at 4°C. Membranes were washed with PBST three times for 15 min. On the next day, the membranes were incubated with peroxidase conjugated secondary antibody (anti-rabbit or mouse IgG, 1:2000) for 1 h with gentle shaking at 37°C. Filters were rinsed and washed three times in PBST and then the immunoreactive bands were visualized by enhanced chemiluminescence (ECL).
Cell-immunochemistry and laser confocal microscope
The cell-immunochemistry and laser confocal microscopy technology provided direct observation of MUC5AC and EGFR protein expression. Glass coverslips were put in 24-well plates and cells in RPMI 1640 were plated at a density of 1 × 105 cells per well for 24 h, and kept maintenance in serum-free culture medium for 24 h. After washing three times with PBS for 15 min, cells were fixed with 4% paraformaldehyde for 30 min at room temperature, washing again with PBS, permeabilized with 0.1% Triton X-100 in PBS for 30 min and washed with PBS for 15 min. The cells were then rinsed, blocked in 1% BSA plus 1% normal goat serum and incubated with mouse anti-MUC5AC (1:500) or rabbit anti-EGFR (1:50) overnight at 4°C. After three 15 min washes in PBS, slides were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-fluorescein mouse or rabbit IgG (1:50) for 30 min at room temperature. Staining with diamidino-phenyl-indole (DAPI) (5 μg/ml) for 5 min, and then the cells were rapidly washed with PBS three times for 15 min. Coverslips were embedded in 50% glycerol. Cells were visualized using laser confocal microscope (TCS-SP2, Leica, Germany). Representative images were taken with a Spot 4.3 digital camera and software, and edited using Adobe Photoshop.
Statistical analysis
All in vitro experiments were carried out at least three times with three independent samples. Results are presented as means ± SD. A significant difference from the respective controls was assessed using Student’s t-test for each paired experiment. Differences between the control and the experimental groups (HNE alone, pretreatment of naringenin and DMTU pretreatment or AG1478 pretreatment) were performed by one-way analysis of variance (ANOVA) using the SPSS17.0 statistical package (SPSS Inc, USA). A P-value of <0.05 or <0.01 was regarded as significant.
Results
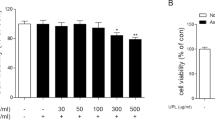
No significant effect on cell viability of low concentrations of Naringenin (20–100 μM) or HNE (5–50 nM)
Cells were incubated with 20–400 μM naringenin or 5–50 nM HNE to assess their viability after different exposure levels. Cell proliferation was assessed using the same concentrations of naringenin or HNE at different time (3–48 h). Results demonstrated that, with 24 h incubations, a low concentration of naringenin (20–100 μM) or HNE (5–50 nM) had no cytotoxic effect on cell proliferation. Growth rates were 84–98% and 75–99%, respectively. However, after 12 h, only 40–50% of the cells were viable after 200 μM naringenin or 100 nM HNE. Higher concentrations of naringenin (200–400 μM) or HNE (100 nM) tended to restrain cell proliferation (Fig. 1a–b). Cells were incubated with 100 μM naringenin or 50 nM HNE for different time (3–48 h), the cells were viable after treatment for 3–24 h, whereas after 48 h, the mortality of cells increased significantly (Fig. 1c). Naringenin and HNE appeared to cause the cells to lose cell viability in a dose- and time-dependent manner.
Naringenin attenuates MUC5AC mucin expression in HBE16 cells by modulating ROS production
To examine whether naringenin attenuates the expression of MUC5AC gene and protein by modulating the production of ROS, the relative content of ROS in cells stimulated by various concentrations of HNE (5, 25, 50 nM) was measured using a Reactive Oxygen Species assay kit. Results showed that HNE induced ROS generation in a dose-dependent manner. The levels of ROS were clearly higher than the normal control group (Fig. 2, HNE). In previous reports, HNE has been shown to induce MUC5AC mucin production involving ROS generation in chronic inflammatory airway diseases [22, 28]. Naringenin appears to have particular antioxidant activities and anti-inflammatory properties [29, 30] and exerts protective effects on mucous hypersecretion in airway inflammation in bronchial asthma [25]. Therefore, we speculated that the attenuation of mucin generation by naringenin may be related to its antioxidant activities and anti-inflammatory properties.
Naringenin (Nar) attenuated production of intracellular ROS, the relative content of ROS was measured by a Reactive Oxygen Species assay kit. Control: cells were cultured in serum medium alone. Data are presented as mean ± SD (n = 3) (# P < 0.01 when compared with control; *,**P < 0.05 when compared with HNE alone)
To further verify the possibility of antioxidant activities, cells were treated with naringenin (100 μM) and ROS scavengers (DMTU) (20 μM) prior to stimulation by different concentrations of HNE. As previously described, the levels of ROS were assayed in naringenin and DMTU pretreatment groups. In both cases, the relative content of ROS decreased significantly compared to the HNE only treated cells (Fig. 2, Nar and DMTU). These results confirm that naringenin decreased the production of ROS.
To confirm the effects of naringenin on MUC5AC generation, MUC5AC gene expression was assayed by reverse transcriptase PCR and real-time PCR. Total serum MUC5AC protein was detected by ELISA, while confocal laser microscopy technology provided for direct observation of MUC5AC protein expression changes. HNE induced MUC5AC gene expression (Fig. 3a) and mucin protein production (Figs. 4a, 5b) in a dose-dependent manner, and had maximal effect on mucin expression at a concentration of 50 nM. Pre-incubation of cells with naringenin (20–100 μM) showed that naringenin reduced HNE-induced MUC5AC gene expression (Fig. 3c) and mucin protein production (Figs. 4b, 5c) in a dose-dependent manner. DMTU also reduced the levels of MUC5AC gene and protein expression in the same manner (Figs. 3b, 4c). In cells treated with both naringenin and DMTU, the levels of these factors were greatly decreased, compared with naringenin or DMTU only incubation group (Figs. 3d, 4c). Consistent with our previous speculation, naringenin reduced mucous hypersecretion by decreasing ROS production. It is possible, however, that the reduction in mucin generation after exposure to naringenin may involve pathways beyond its effects on ROS production.
Effects of HNE and naringenin on MUC5AC mucin protein production. We analyzed for MUC5AC mucin protein in the cell lysate (dark areas) and in the supernatant (light areas) by ELISA. Data are presented as mean ± SD (n = 3) (a *P < 0.05, compared with control (without HNE); b *P < 0.05, compared with HNE alone; c # P < 0.05, compared with control; *P < 0.05, **P < 0.01 compared with HNE alone)
Naringenin inhibited NF-κB (n-NF-κB and c-NF-κB) activation
NF-κB plays important roles in regulating mucous hypersecretion in inflammatory diseases [31], which has been considered as a potential target for anti-inflammatory treatments [32]. Riina Nieminern et al. partially explained the pharmacological efficacy of flavonoids as anti-inflammatory compounds that inhibit inducible nitric oxide synthase (iNOS) expression and NO production by inhibiting activation of NF-κB in activated macrophages [24]. Naringenin also inhibited pulmonary NF-κB activation in airway inflammation and responsiveness [25]. Together, these studies showing that naringenin tended to restrain NF-κB activation and production, and may have some connection with mucous hypersecretion in response to inflammatory stimulation, but no more precise mechanisms of action are known in detail.
To evaluate the effect of naringenin on transcription and protein levels of MUC5AC mucin, cells pretreated with naringenin (100 μM) were stimulated by HNE (50 nM). Nuclear NF-κB protein and cytoplasmic NF-κB protein were isolated from HBE16 cells and assayed by western blot (Fig. 6a). Densitometric analysis of NF-κB protein expression was carried out and total NF-κB protein ratios were expressed as the fold increase relative to the control (Fig. 6b–c). Consistent with our investigation, in pretreated cells, the total levels of NF-κB proteins were significantly reduced after naringenin pre-treatment. From these results, we conclude that naringenin had a moderate inhibitory effect.
Inhibitory effect of naringenin on the activation of NF-κB in HBE16 cells. Cells were pretreated with naringenin (100 μM) for 24 h or DMTU (20 μM) for 30 min prior to HNE (50 nM) stimulation. Data are presented as mean ± SD (n = 3) (# P < 0.05, compared with control (without HNE); *P < 0.05, compared with HNE alone)
Naringenin inhibited NF-κB pathway, which was required for activation of the EGFR
Activation of NF-κB has been shown to be responsible for ROS induction in response to multiple stimuli [33]. To examine whether NF-κB activation requires HNE-induced release of ROS, we pretreated HBE16 cells with the ROS scavenger DMTU. Pretreatment with DMTU reduced ROS production (Fig. 2) and NF-κB activation (Fig. 6a–c) by HNE. It was interesting that naringenin had a similar effect as DMTU (Fig. 6a–c), suggesting that naringenin may act as a free radical scavenger. EGFR phosphorylation is known to play a critical role in mediating the generation of mucin in response to ROS induced by a variety of stimulation. NF-κB is a downstream activator of EGFR and can play an important role in EGFR stimulation. Our results clearly show that inhibition of ROS production can suppress EGFR phosphorylation and reduce the levels of NF-κB.
As expected, treatment of cells with naringenin markedly suppressed activation of the EGFR by HNE stimulation. Reverse-transcriptase PCR and real-time PCR analysis revealed that EGFR gene expression increased in cells exposed to HNE only (50 nM) (Fig. 7), whereas the levels were markedly decreased in cells co-incubated with naringenin and HNE. These results are similar to those seen with the EGFR inhibitor AG1478 (Fig. 7). We also measured the changes in EGFR protein by western blot. It is interesting that both nonphosphorylated and phosphorylated EGFR were markedly attenuated when cells were pretreated with either naringenin or AG1478, followed by HNE exposure (Fig. 9). Laser confocal microscopy also demonstrates that EGFR expression in the cytosol of naringenin and HNE co-incubated cells was clearly reduced when compared with that of cells primed only with HNE (Fig. 8b–c).
Naringenin down-regulates EGFR mRNA transcription in HBE16 cells. Cells were pretreated with naringenin (100 μM) for 24 h or the EGFR inhibitor AG1478 (5 μM) for 30 min prior to HNE (50 nM) stimulation. Data are presented as mean ± SD (n = 3) (# P < 0.01, compared with control (without HNE); *P < 0.05, compared with HNE alone)
Effects of HNE and naringenin on phosphorylated-EGFR (p-EGFR) protein production. Intracellular p-EGFR was stained by immunofluorescence and observed by laser confocal microscopy. As previously noted, results show that p-EGFR protein expression was decreased with green fluorescence that is lower than that seen with HNE stimulation without pretreatment. Immunofluorescence procedures are described in “Materials and methods” section
The EGFR controls the activation of the PI3K pathway and ERK1/2 pathways
The PI3K/Akt and ERK1/2 pathways are other mediators which are required for production of mucin and cell growth [34, 35]. Akt is a major downstream target of PI3K and is activated in response to various stimuli such as growth factors and hormones. It phosphorylates many cytosolic and nuclear substrates that are involved in the generation of activation signaling molecules, such as NF-κB [36]. The activation of the PI3K/Akt and ERK1/2 pathways were mediated by EGFR activation and ROS production [37]. To address whether the activation of NF-κB is involved with the ROS-EGFR-PI3K/Akt and ERK1/2 pathways and the cascades that may be modulated by naringenin in HBE16 cells, we examined the role of naringenin on the molecular mechanisms.
Our studies show that the EGFR specific inhibitor AG1478 completely blocked HNE-induced p-ERK1/2, PI3K, and p-Akt protein expression, all of which also showed obvious changes when cells were pre-incubated with naringenin (Fig. 10a–c). Densitometric analysis of the protein expression was done with total protein ratios expressed as a fold increase relative to the experimental group (Fig. 10d–f). From these results, we conclude that naringenin markedly suppressed activation of the PI3K/Akt and ERK1/2 pathways by blocking phosphorylation of EGFR and the generation of proteins in the cytoplasm of human bronchial epithelial cell.
Discussion
Mucous hypersecretion and accumulation in the airway during inflammation are pathological symptoms associated with various chronic airway diseases [38, 39]. It is an independent risk factor that has an important impact on the occurrence, development, and prognosis of the disease [40]. The risk for chronic diseases of the airway is lower with a diet that is high in dietary flavonoids [41]. Several reports have shown that naringenin, a flavanone, has beneficial effects that have been attributed to its antioxidant and anti-inflammatory functions. In acute allergic asthma, for example, naringenin appears to play a protective role through clarification and reduction of mucous levels. However, its potential role in reducing mucous hypersecretion in inflammatory airway disease has not been fully investigated. Naringenin displays inhibitory effects on ROS generation and the activation of NF-κB in HBE16 cells stimulated by human neutrophil elastae (HNE). We have investigated possible mechanisms of action for naringenin during airway inflammation through an exploration of the changes seen in the levels of ROS, NF-κB, EGFR, p-EGFR, p-ERK1/2, PI3K, and p-Akt expression. By comparing these parameters in cells stimulated by HNE and cells pretreated by naringenin before HNE exposure, we hoped to gain a theoretical basis for the treatment of these diseases.
The production of mucin is the result of many signal transduction mechanisms in addition to various inflammatory proteins and chemokines. Patients with chronic obstructive pulmonary disease (COPD) are characterized by chronic mucous obstruction and severe neutrophilic inflammation in the airways. HNE is secreted by neutrophils in inflamed airways leading to a significant increase in ROS production [42] and EGFR activation. This activity is mediated by inflammatory factors and results in mucin production by human epithelial cells.
Reactive oxygen species (ROS), an inflammatory chemokine, regulates important steps in the signal transduction cascades as well as other critical cellular events [43]. In this study, we investigated the effects of the ROS scavenger DMTU and showed that it reduces both mucin gene expression and protein production in response to HNE. Naringenin appears to have similar pharmacological effects as DMTU. The scavenging of ROS has been considered to be a useful treatment to minimize inflammation and modulate the generation of mucin. Our data clearly show that both DMTU and naringenin can markedly suppress MUC5AC gene and protein expression and may be acting through their ability to scavenge ROS molecules.
NF-κB is a ubiquitous protein transcription factor that induces the transcription of a variety of genes. Many of these genes encode molecules important in inflammatory processes. The role of NF-κB activation and its regulation of cytokine production have been characterized [44]. As anticipated, the development of inflammation and mucous hypersecretion were attenuated by inhibiting NF-κB activation. According to the current findings, naringenin has inhibitory activity on NF-κB activation and downstream proinflammatory cytokine production [24]. We pretreated HBE16 cells with naringenin and measured the levels of nuclear and cytoplasmic-NF-κB in the culture supernatants of naringenin pretreated cells. These factors were decreased significantly when compared with HNE-stimulated cells that had not been pretreated with naringenin. While these results were consistent with previous reports, confirmation of these observations will be important for future treatment of chronic airway inflammation.
Based on our current findings, naringenin attenuated overexpression of mucin by modulating ROS generation and inhibiting NF-κB activation. However, it is not completely clear how naringenin may be playing its possible protective role during chronic inflammatory of the airway. Papaiahgari et al. and others have reported that transcription factors act as one of the important downstream effectors of the PI3K pathway, and could regulate the expression of genes which contribute to cell survival following hyperoxic insult. In addition, hyperoxia-induced transcription factor transcriptional response was contributed to ERK signaling in pulmonary epithelial cells under oxidative stress [45–47]. It is likely that the both the PI3K pathway and ERK signaling controlled activation of transcription factors in cellular responses in hyperoxic states. NF-κB is one of the most important transcription factors, thus we can deduce that both the PI3K pathway and ERK signaling participate in the progress of mucous hypersecretion during airway inflammation and this is linked to the activation of NF-κB.
HNE-induced ROS generation triggers cellular dysfunction and matrix remodeling, resulting in mucous cell metaplasia and goblet cell hyperplasia by activating various signaling pathways [48–51]. The intracellular signal pathways responsible for the response to ROS have been established with relation to EGFR and ERK1/2 MAP kinase in NCI-H292 cells [48, 50]. In particular, in normal human bronchial epithelial cells, the activation of EGFR is necessary for ROS signal transduction [49, 50]. ROS activates EGFR phosphorylation by ligand-dependent transactivation and ligand-independent activation. The activation of EGFR initiates both the activation of ERK1/2 and of PI3K/Akt kinase [16], which induces the production and activation NF-κB. This ultimately stimulates mucin gene and proinflammatory factor gene transcription.
Based on previous and current reports, we proposed that naringenin attenuated mucous hypersecretion by blocking the EGFR-PI3K-Akt/ERK MAP kinase signaling pathway. In our study, naringenin inhibited HNE-induced EGFR activation (Figs. 7, 8, 9). Subsequently, the downstream effectors were suppressed. A more detailed study has shown that p-Akt, PI3K, and p-ERK1/2 protein expression in the cells pretreated with naringenin were lower than HNE stimulation group without the pretreatment. This inhibitory effect of naringenin was similar to that for the EGFR inhibitor AG1478 (Fig. 10). We also found that the cells which were pretreated with both DMTU and naringenin prior to exposure to HNE demonstrated an even greater inhibition of MUC5AC gene and protein expression (Figs. 3d, 4c). This suggests naringenin may attenuate other signal molecules (such as EGFR) directly, however, this needs to be investigated further.
Naringenin down-regulates the activation of EGFR and phosphorylated-EGFR (p-EGFR) in HBE16 cells. Cells were pretreated with naringenin (100 μM) for 24 h or AG1478 (5 μM) for 30 min prior to HNE (50 nM) stimulation. Total EGFR ratios expressed as fold increase relative to control. Data are presented as mean ± SD (n = 3) (# P < 0.01, compared with control (without HNE); *P < 0.05, compared with HNE alone)
Inhibition of the EGFR pathway suppresses HNE stimulated PI3K activation and ERK1/2 and Akt phosphorylation. Total protein ratios expressed as fold increase relative to control. Data are presented as mean ± SD (n = 3) (# P < 0.01, compared with control (without HNE); *P < 0.05, compared with HNE alone)
In conclusion, our study demonstrates in vivo that the beneficial effects of naringenin on HNE induced mucous hypersecretion are specifically mediated by inhibiting ROS production and the activation of NF-κB. These molecular mechanisms are involved with the EGFR-PI3K-Akt/ERK MAP kinase signaling pathway. These studies show that naringenin could be used as a useful probe for studying the clinical pharmacology in inflammatory airway disease. Additionally, since naringenin comes from a variety of natural sources, it has the potential to be a very cost effective treatment for these types of lung diseases.
Abbreviations
- BSA:
-
Bovine serum albumin
- Nar:
-
Naringenin
- ROS:
-
Reactive oxygen species
- HNE:
-
Human neutrophil elastase
- MUC5AC:
-
Mucin 5AC
- EGFR:
-
Epidermal growth factor receptor
- NF-κB:
-
Nuclear factor-κB
- MAPK:
-
Mitogen-activated protein kinase
- ERK1/2:
-
Extracellular signal-related kinases1/2
- PI3K:
-
Phosphatidylinositol 3-kinase
- FITC:
-
Fluorescein isothiocyanate
- PBS:
-
Phosphate-buffered saline
- DMTU:
-
Active oxygen scavenger
References
Nadel JA (2002) In: Voelkel NF, MacNee W (eds) Chronic obstructive lung diseases. BC Decker Inc., Hamelton, p 161
Hogg JCF, Chu S, Utokaparch R, Woods WM, Elliott L, Buzatu RM, Cherniack RM, Rogers FC, Sciurba HO (2004) The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350:2645–2653
Wang J, Lory S, Ramphal R, Jin S (1996) Isolation and characterization of Pseudomonas aeruginosa genes inducible by respiratory mucus derived from cystic fibrosis patients. Mol Microbiol 22:1005–1012
Hovenberg HW, Davies JR, Carlstedt I (1996) Different mucins are produced by the surface epithelium and the submucosa in human trachea: identification of MUC5AC as a major mucin from the goblet cells. Biochem J 318:319–324
Hovenberg HW, Davies JR, Carlstedt I (1996) MUC5AC, but not MUC2, is a prominent mucin in respiratory secretions. Glycoconj J 13:839–847
Vandivier RW, Fadok PR, Hoffmann DL, Bratton C, Penvari KK, Brown JD, Brain FJ, Accurso PM (2002) Elastasemediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest 109:661–670
Liu H, Lazarus SC, Caughey GH, Fahy JV (1999) Neutrophil elastase and elastase-rich cystic fibrosis sputum degranulate human eosinophils in vitro. Am J Physiol 276:L28–L34
Breuer RC, Christensen TG, Lucey EC, Stone PJ, Snider GL (1987) An ultrastructural morphometric analysis of elastase-treated hamster bronchi shows discharge followed by progressive accumulation of secretory granules. Am Rev Respir Dis 136:698–703
Breuer RC, Christensen TG, Niles RM, Stone PJ, Snider GL (1989) Human neutrophil elastase causes glycoconjugate release from the epithelial cell surface of hamster trachea in organ culture. Am Rev Respir Dis 139:779–782
Babior BM (1995) The respiratory burst oxidase. Curr Opin Hematol 2:55–60
Ingram JL, Rice AB, Santos J, Van Houten B, Bonner JC (2003) Vanadium-induced HB-EGF expression in human lung fibroblasts is oxidant dependent and requires MAP kinases. Am J Physiol Lung Cell Mol Physiol 284:774–782
Fischer OM, Hart S, Gschwind A, Ullrich A (2003) EGFR signal transactivation in cancer cells. Biochem Soc Trans 31:1203–1208
Mendelsohn J, Baselga J (2003) Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol 21:2787–2799
Song J-S, Cho K-S, Yoon H-K, Moon H-S, Park S-H (2005) Neutrophil elastase causes MUC5AC mucin synthesis via EGF receptor, ERK and NF-kB pathways in A549 cells. Korean J Intern Med 20:275–283
Papaiahgari S, Qin Z, Klefberger SR, Cho HY, Reddy SP (2005) Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid Redox Signal 8:43–52
Thornton DJ, Sheehan JK (2004) From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc 1:54–61
Pietta AG (2000) Flavonoids as antioxidants. J Nat Prod 63(7):1035–1942
Birt DF, Hendrich S, Wang W (2001) Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther 90(2–3):157–177
Hamalainen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E (2007) Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only nf-κb activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm 2007:45673
Du G, Jin L, Han X, Song Z, Zhang H, Liang W (2009) Naringenin: a potential immunomodulator for inhibiting lung fibrosis and metastasis. Cancer Res 69(7):3205–3212
Valdes F, Murillo MM, Valverde AM, Herrera B, Sanchez A, Benito M, Fernandez M, Fabregat I (2004) Transforming growth factor-β activates both pro-apoptotic and survival signals in foetal rat hepatocytes. Exp Cell Res 292:209–218
Matt X, Shao G, Nadel JA (2005) Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-α-converting enzyme. J Immunol 6:4009–4016
Higdon JV, Frei B (2003) Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr 43:89–143
Chiaki I, Kenta S, Mineka Y, Yukiko W, Akio O, Toshinori N (2010) Naringenin chalcone suppresses allergic asthma by inhibiting the Type-2 function of CD4 T cells. Allergol Int 59:67–73
Ying SH, Jian D, Hua L, RuoRan L, PeiLi S, Lingling P, Zhen C, KaiSheng Y (2009) Naringenin inhibits induced airway inflammation and airway responsiveness NF-κB activity in a murine model of asthma. Can J Physiol. 87:729–735
Gruenert DC, Finkbeiner WE, Widdicombe JH (1995) Culture and transformation of human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 268:L347–L360
Lee ER, Kang YJ, Choi HY, Kang GH, Kim JH, Kim BW, Han YS, Nah SY, Paik HD, Park YS, Cho SG (2007) Induction of apoptotic cell death by synthetic naringenin derivatives in human lung epithelial carcinoma A549 cells. Biol Pharm Bull 30(12):2394–2398
Kim HJ, Park YD, Moon UY, Kim JH, Jeon JG, Lee JG, Bae YS, Yoon JH (2008) The role of Nox4 in oxidative stress induced MUC5AC overexpression in human airway epithelial cells. Am J Respir Cell Mol Biol 39:598–609
Birt DF, Hendrich S, Wang W (2001) Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther 90:157–177
Bodet C, La VD, Epifano F, Grenier D (2008) Naringenin has anti-inflammatory properties in macrophage and ex vivo human whole-blood models. J Periodont Res 43:400–407
Heyninck K, Krike MM, Beyaert R (2003) Structure-function analysis of the A20-binding inhibitor of NF-κB activation. FEBS Lett 536:135–140
Roth M, Black JL (2006) Transcription factors in asthma: are transcription factors a new target for asthma therapy? Curr Drug Targets 7(5):589–595
Murillo MM, Carmona-Cuenca I, Del Castillo G, Ortiz C, Roncero C, Sánchez A, Fernández M, Fabregat I (2007) Activation of NADPH oxidase by transforming growth factor-β in hepatocytes mediates up-regulation of epidermal growth factor receptor ligands through a nuclear factor-κB-dependent mechanism. Biochem J 405:251–259
Bjorge JD, Jakymiw A, Fufita DJ (2000) Selected glimpses into the activation and function of src kinase. Oncogene 19(49):5620–5635
Gervais M, Dugourd C, Muller L, Ardidie C, Canton B, Loviconi L, Corvol P, Chneiweiss H, Monnot C (2006) Akt down-regulates ERK1/2 nuclear localization and angiotensin II-induced cell proliferation through PEA-15. Mol Biol Cell 17:3940–3951
Hirsch E, Katanaev VL, Garlanda C (2000) Central role of for G protein coupled phosphoinositide 3-kinase gamma in inflammation. Science 287(5455):1049–1053
Mehta PK, Griendling KK (2007) Angiotensin II cell signalling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292:C82–C97
Vestbo J, Prescott E, Lange P (1996) Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity: Copenhagen City Heart Study Group. Am J Respir Crit Care Med 153:1530–1535
Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T (1992) Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 10:916–921
Psarras S, Caramori G, Contoli M et al (2005) Oxidants in asthma and in chronic obstructive pulmonary disease(COPD). Curt Pharm Des 11:2053–2062
Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A (2002) Flavonoid intake and risk of chronic diseases. Am J Clin Nutr 76(3):560–568
Fischer BM, Voynow JA (2002) Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species. Am J Respir Cell Mol Biol 26:447–452
Tzipora G, Tommer R, Elaine M (2005) Life and death decisions: ceramide generation and EGF receptor trafficking are modulated by oxidative stress. Antioxid Redox Signal 7:119–128
Min YD, Choi CH, Bark H, Son HY, Park HH, Lee S, Park JW, Park EK, Shin HI, Kim SH (2007) Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-kB and p38 MAPK in HMC-1 human mast cell line. Inflamm Res 56:210–215
Cho HY, Reddy SP, Yamamoto M, Kleeberger SR (2004) The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J 18:1258–1260
Papaiahgari S, Kleeberger SR, Cho HY, Kalvakolanu DV, Reddy SP (2004) NADPH oxidase and ERK signaling regulates hyperoxia-induced Nrf2-ARE transcriptional response in pulmonary epithelial cells. J Biol Chem 279:42302–42312
Roper JM, Mazzatti DJ, Watkins RH, Maniscalco WM, Keng PC, Reilly MA (2004) In vivo exposure to hyperoxiainduces DNA damage in a population of alveolar type II epithelial cells. Am J Physiol Lung Cell Mol Physiol 286:1045–1054
Takeyama K, Dabbagh K, Shim JJ, Pick TD, Ueki IF, Nadel JA (2000) Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol 164:1546–1552
Nabeyrat E, Jones GE, Fenwick PS, Barnes PJ, Donnelly LE (2003) Mitogen activated protein kinases mediate peroxynitrite-induced cell death in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 284:1112–1120
Shao MXG, Nadel JA (2005) Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA 102:767–772
Abe MK, Kartha S, Karpova AY, Liu JLPT, Kuo WL, Hershenson MB (1998) Hydrogen peroxide activates extracellular signal-regulated kinase viaprotein kinase C, Raf-1, and MEK1. Am J Respir Cell Mol Biol 18:562–569
Acknowledgments
This work was supported by grant from the National Nature Science Foundation of China (No.30770951), and China-Russia Cooperation Research Foundation (No.81011120108). There are no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, J., Li, Q., Zhou, X.D. et al. Naringenin attenuates mucous hypersecretion by modulating reactive oxygen species production and inhibiting NF-κB activity via EGFR-PI3K-Akt/ERK MAPKinase signaling in human airway epithelial cells. Mol Cell Biochem 351, 29–40 (2011). https://doi.org/10.1007/s11010-010-0708-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-010-0708-y