Abstract
Background
Osthole is a bioactive component reported in medicinal plants such as Angelica pubescens and Cnidium monnieri, known for analgesic activity. However, the toxicity, median effective dose (ED50), and dual modulation of nitric oxide and cyclooxygenase pathways along with inflammatory cytokines of osthole are yet to be determined.
Methods
The animals (mice) were assessed for general behaviour and mortality in varying doses (50, 300, and 2000 mg kg−1) of osthole for acute toxicity over 14 days. The analgesic activity was investigated using acetic acid and formalin-induced hyperalgesia, and anti-inflammatory activity was explored in carrageenan-induced paw oedema. ED50 of osthole was calculated using Design Expert software. Involvement of nitric oxide and cyclooxygenase pathways was investigated by agonist challenges with l-arginine and substance P, respectively. The expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) was determined in spinal sections by immunohistochemical analysis. Lipopolysaccharide (LPS) challenge was used to assess in vivo effect on inflammatory cytokines (TNFα and IL-6).
Results
Acute toxicity studies revealed no behavioural abnormality or mortality on osthole treatment and unremarkable histological findings. Osthole was found to significantly decrease acetic acid and formalin-induced hyperalgesia (ED50 = 5.43 mg kg−1) and carrageenan-induced paw oedema with no toxicity symptoms. Osthole produced a marked decrease in iNOS and COX-2 expression as well as TNFα and IL-6. The findings corroborate to modulation of iNOS and COX-2 and inflammatory cytokines by osthole. This study provides promising insights and prospects for application of osthole in pain management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain has assumed the form of a global epidemic with numerous pathogenic mechanisms responsible for it. Despite of the large number of patients suffering from pain syndromes, the availability of analgesics is insufficient especially for pain involving complicated pathways originating from spinal as well as central brain regions. These complex pathways are not localized rather, which involve many regions of brain which form the pain matrix (Farrar 2010; Bushnell et al. 2015; Martin et al. 2017). The inefficiency of analgesics such as paracetamol and NSAIDS in treating spinal pain (Cashman 1996) and the high incidence of gastrointestinal adverse with the use of NSAIDS have necessitated the search for newer analgesics (Machado et al. 2016). Pain reduces the quality of life and increases the economic burden by affecting the productivity of individuals (Crofford 2010), and is often accompanied by inflammation which is essentially the host defence response, involving the cell and soluble factors released in response to tissue injury (Wikel 2013). Studies have revealed the participation of prostaglandins, nitric oxide (NO), amino acid neurotransmitters, and pro-inflammatory cytokines such as tumour necrosis factor (TNFα), and interleukins (ILs) in pain and inflammation (Goudet et al. 2008; Ibana et al. 2015; Carballo-Villalobos et al. 2017). The classical analgesics act by inhibiting the inflammatory mediators generated byarachidonic acid metabolism that is initiated by COX, lipoxygenase, and epoxygenase (Basbaum et al. 2009; Smith and Murphy 2002). Furthermore, studies have highlighted the role of resident macrophages which on being stimulated by lipopolysaccharides produce NO, prostaglandins, and pro-inflammatory cytokines including IL-1β, IL-6, and TNFα (Ibana et al. 2015).
Natural products have emerged as appealing sources of leads for the development of new drugs (Wandji et al. 2018). Osthole is a naturally occurring coumarin isolated from Peucedanum ostruthium, Cnidium monnieri, Angelica pubescens, etc. (Zhang et al. 2015), and has been reported to possess a number of pharmacological activities such as anti-apoptotic, in vitro anti-inflammatory, nootropic, and neuroprotective (Liu et al. 2005; Ji et al. 2010; Liao et al. 2010; Li et al. 2002; Yang et al. 2014, 2010). However, comprehensive preclinical in vivo studies for toxicity evaluation, modulation of inflammatory and neurogenic pain, and the pharmacological mechanisms involved in the mode of action of osthole are lacking. Therefore, keeping in mind the medicinal potential of osthole, the current investigation is focused on investigating the acute toxicity of osthole and its effect on neurogenic and inflammatory hyperalgesia. Furthermore, calculation of ED50 of osthole and modulation of NO and COX and inflammatory cytokines were also studied.
Materials and methods
Chemicals
Indomethacin, substance P, and osthole were procured from Sigma-Aldrich. Mouse cytokine (TNFα and IL-6) kits were supplied by Krishgen Biotech, Mumbai, India. Other reagents and chemicals were of analytical grade and purchased from registered suppliers. Anti-iNOS antibody was purchased from Santacruz Biotech.
Animals
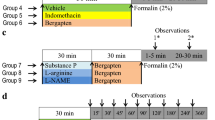
Mice of Swiss albino strain with body weight ranging from 30–40 g were procured from Indian Institute of Integrative Medicine (IIIM), Jammu, and kept in central animal house of GNDU maintained in air-conditioned room (25 ± 5 °C) with 12 h light–dark cycle. Water was provided ad libitum. All the animal studies were duly approved by the Institutional Animal Ethics Committee and the procedures performed in accordance with ethical guidelines (226/CPSCEA2015/11). The schematic representation of protocol is given in Fig. 1.
Acute toxicity studies
For acute toxicity studies, female mice were used and procedure was in accordance with OECD guidelines (OECD 2001). Briefly, four groups of animals (n = 3) were used. The first group was vehicle-treated control. The second, third, and fourth groups were treated with osthole in doses of 50, 300, and 2000 mg kg−1. Animals were observed for 14 days. At the end of 14 days, the animals were sacrificed and histological studies performed on heart, kidney, and liver of the animals using hematoxylin–eosin (H&E) staining. Photomicrographs were taken with light microscope (Lab vision I-3000) at 200×.
Acetic acid-induced abdominal writhing
Effect of osthole on chemical hyperalgesia was investigated using acetic acid-induced writhes. Intraperitoneal injection of 0.6% v/v acetic acid produces pain reaction quantified as the number of writhes in mice (Kulkarni 2005; Tadiwos et al. 2017). The number of abdominal contractions, trunk twist responses, and extension of hind legs were counted as indicators of pain. Animals were observed for a period of 30 min.
Determination of ED50 of osthole
For calculating median effective dose (ED50), the experiments were performed with osthole dose varied between 2.5 to 20 mg kg−1 in a randomized set of four animals in each group. Percentage anti-nociceptive activity was recorded. The experimental data were fitted using analysis of variance (ANOVA) for linear, quadratic, and cubic models. The best-fitted model was used for data plotting using regression equation. Then, ED50 of osthole was calculated from the graph.
Formalin-induced nociception
Hyperalgesia was induced by subcutaneous injection of 20 µL of 2% v/v formalin into the intraplantar region of the right hind paw. Thereafter, the animals were observed for the number of flinchings for a period of 60 min. Formalin is known to produce a biphasic response comprising of an early neurogenic phase that appears in the first 5 min and a late-inflammatory phase appearing in 25–30 min (Hunskaar and Hole 1987; Hajhashemi et al. 2011).
Mechanistic studies
Three groups of animals were taken to explore the involvement of cyclooxygenase and NO. Substance P is known to stimulate COX-2 (Koon et al. 2006), pre-treatment was used to study the involvement of COX-2; l-arginine (NO precursor) and L-NAME (NO synthase inhibitor) pre-treatment was used to investigate the involvement of NO.
Immunohistochemistry (IHC)
Mice were anesthetized with ketamine (50 mg kg−1, i.p) after 2 h of formalin administration. Blood was withdrawn and animals were sacrificed. Lumber region of spinal cord was removed and post-fixated with 4% paraformaldehyde in phosphate buffer for 4 h, and then immersed in 30% v/v sucrose solution for overnight at 4 °C. 3 µm sections of spinal-fixed tissue were obtained with cryomicrotome (Leica CM1950) and then incubated with polyclonal anti-iNOS and COX-2 antibodies diluted 200 times with blocking buffer PBS for 1 h and then washed with phosphate buffer was done. The sections were then incubated with HRP anti-mouse secondary antibody (1:1000) for 1 h followed by repeated washings with PBS. Finally, the sections were developed in 3,3-diaminobenzidine (DAB) and hydrogen peroxide solution and then counter stained with xylene.
Carrageenan-induced paw oedema
Carrageenan-induced paw oedema was used to investigate the anti-inflammatory effect of osthole. Briefly, 0.1 ml of (1% w/v) carrageenan was injected into the plantar surface of right hind paw. The paw thickness was measured at 30′, 60′, 120′, 180′, 240′, 300′, and 360′ after carrageenan injection. Increase in paw thickness was taken as an indicator of inflammation (Winter et al. 1962).
Measurement of plasma cytokine level in LPS-treated mice
Animals were divided into five groups. The first group was normal control group and received intraperitoneal injection of the vehicle, i.e., carboxymethyl cellulose (CMC 0.1% w/v); the second group was lipopolysaccharide (LPS)-treated control group and was treated with LPS at dose 1 mg kg−1. In third, fourth, and fifth groups, mice were pre-treated with osthole at dose 5, 10, and 20 mg kg−1, respectively, 30 min before LPS treatment. Blood samples were collected after 2 h of LPS treatment by retro-orbital puncture under anesthesia for the estimation of TNFα. For the estimation of IL-6, blood samples were withdrawn after 6 h of LPS treatment. Samples were centrifuged at 5000 rpm at 4 °C for 10 min; plasma was collected and frozen at −20 °C until further estimations. TNFα and IL-6 concentrations were determined using ELISA-based kits for TNFα and IL-6, respectively. Results were expressed as picogram per millilitre (pg/mL). IC50 of osthole for TNFα and IL-6 was also determined. IC50 calculations were done using osthole in dose range 5–20 mg kg−1 (in triplicates) using Design Expert. The response data in terms of % inhibition of TNFα and IL-6 were noted.
Statistical analysis
The data were presented as mean ± standard error mean (SEM). Statistical analysis was done by one-way ANOVA followed by post hoc analysis using Tukey’s test using Instat software (version 3.5). The ED50 and IC50 calculations were carried out using Design Expert software v 9.0 (Stat-Ease Inc, Minneapolis, USA).
Results
Acute toxicity studies
The animals did not exhibit any signs of behavioural toxicity in the 14 day acute toxicity study. The post-mortem analysis revealed that the renal sections were composed of glomeruli, tubules, interstitium, and blood vessels. The cardiac section revealed pericardium with normal underlying myocardium. No disarray, increase in fibrosis, or any myopathic effect was recorded. The sections of hepatic tissue showed hepatic parenchyma with intact architecture having unremarkable central vein, peripheral portal triad (composed normal looking of bile duct, portal vein, and hepatic artery), and hepatocytes. However, mid ballooning and cholestasis was evident in the peri-venular region. Focal spotty necrosis was also identified occasionally. Prominence of stellate cells in the liver sinusoids was also appreciable (Fig. 2).
Photomicrographs of hematoxylin–eosin stained sections of control myocardium (a), kidney (b), and liver (c); osthole (300 mg kg−1)-treated myocardium (d), kidney (e), and liver (f), and osthole (2000 mg kg−1)-treated myocardium (g), kidney (h), and liver (i). All pictures were taken with light microscope at ×200 magnification
Effect of osthole treatment in acetic acid-induced pain model
Intraperitoneal administration of acetic acid was found to induce abdominal contraction, trunk twisting, and extension of hind legs (writhes) in mice. Treatment with indomethacin and osthole at a dose of 10 mg kg−1 was found to significantly decrease writhes by 53 and 72.2%, respectively, as compared to acetic acid-treated mice (Fig. 3a).
Osthole dose optimization for anti-nociceptive activity
Percentage anti-nociceptive activity was 31.18% for 2.5 mg kg−1 dose and 78.84% for 20 mg kg−1. Best-fitted ANOVA model was highly significant at 99.99% (p = 0.001, F-value = 72.448 at DF = 2). There was a significant increase in anti-nociceptive activity up to 10 mg kg−1. Beyond 10 mg kg−1 of dose, the increase in effect was minimal. Regression equation (Eq. 1) is numerically optimized to yield a 50% increase in anti-nociceptive activity and optimum dose was found to be 5.43 mg kg−1 (Fig. 3b). However, for mechanistic studies, the dose-producing maximum response, i.e., 10 mg kg−1 was used.
Effect of osthole treatment on formalin-induced pain
Intraplantar injection of formalin was found to produce biphasic hyperalgesia in mice. The first phase was the neurogenic phase (1–5 min) and the second phase was the inflammatory hyperalgesia (20–30 min) as evidenced by increase in number of flinchings in both phases (Fig. 4a). Treatment with standard drug indomethacin did not significantly decrease the hyperalgesia in the neurogenic phase, whereas in the inflammatory phase, hyperalgesia was significantly deceased (59.8%) as compared to untreated control. Osthole treatment significantly attenuated hyperalgesia in both neurogenic (43.44%) and inflammatory phases (76.67%) as compared to formalin-treated mice (Fig. 4b).
a Effect of osthole on formalin-induced hyperalgesia, b effect of osthole on neurogenic and inflammatory phases in formalin-induced hyperalgesia in mice, c effect of substance P on analgesic effect of osthole in formalin-induced hyperalgesia, and d effect of L-NAME and l-arginine on analgesic effect of osthole in formalin-induced hyperalgesia. All values are expressed as mean ± SEM. Statistical differences were determined by one-way analysis of variance (ANOVA) followed by Tukey’s test ap < 0.05 vs. formalin control, bp < 0.05 vs. indomethacin, cp < 0.05 vs. osthole, dp < 0.05 vs. l-arginine
Substance P treatment for studying the involvement of neurokinins in the analgesic effect of osthole
Pre-treatment with substance P, a neurokinin receptor agonist, and stimulator of COX-2 significantly reversed the effect of osthole in both phases (Fig. 4c).
L-NAME and l-arginine pre-treatment for studying the involvement of NO in the analgesic effect of osthole
Pre-treatment with l-arginine, a NO precursor, reversed the analgesic effect of osthole as evidenced by a significant increase in the number of flinching in both neurogenic and inflammatory phases in formalin-induced pain as compared to osthole-treated group. However, pre-treatment with L-NAME, non-selective NO synthase inhibitor did not alter the effect of osthole in either phase in formalin-induced hyperalgesia (Fig. 4d).
Immunohistochemistry
Immunohistochemistry using iNOS antibodies revealed negligible expression of iNOS in the astrocytes of lumbar region in normal control mice, whereas the astrocytes in the lumbar region of formalin-treated mice showed enhanced expression of iNOS. Osthole-treated animals showed only marginal expression of iNOS (Fig. 4a–c). Immunohistological staining using COX-2 antibody revealed an enhanced expression as indicated by increased staining intensity in the spinal sections of formalin-treated mice as compared to normal- and osthole-treated mice (Fig. 5d–f).
Effect of osthole treatment in carrageenan-induced paw oedema
Intraplantar injection of carrageenan increased the paw volume steadily over a period of 360 min. The peak of oedema was evident at 120 min of observation. The maximum percent inhibition of paw oedema was observed after 3 h of carrageenan injection in osthole-treated group (Fig. 6a, b).
Effect of osthole on plasma cytokines level
LPS treatment was found to significantly increase the serum TNFα and IL-6. Pre-treatment with osthole reduced the level of both TNFα and IL-6 in dose-dependent manner as compared to untreated control group. The treatment with osthole at dose 5, 10, and 20 mg kg−1 reduced the level of TNFα by 51.91, 78.61, and 82.35% and IL-6 by 32.83, 58.53, and 72.25% (Fig. 7a, b).
Effect of osthole on plasma levels of TNFα (a) and IL-6 (b) in mice. All values are expressed as mean ± SEM. Statistical differences were determined by one-way analysis of variance (ANOVA) followed by Tukey’s test ap < 0.05 vs. control group and bp < 0.05 vs. LPS-treated group. Graph indicates second-order fitted regression equation for IC50 inhibition of TNFα (c) and IL-6 (d) by varying osthole doses
IC50 of osthole for TNFα and IL-6 inhibition
Experiments on mice were run in triplicates by varying the doses of osthole from 5 to 20 mg kg−1. The response data in terms of % inhibition of IL-6 and TNFα were noted. ANOVA modelling of the experimental data suggested quadratic model for both IL-6 and TNFα. The one factor plot of osthole vs. % inhibition of IL-6 gave IC50 dose of 8.02 mg kg−1 body weight (Fig. 6d). The best-fitted regression equation to find percentage inhibition is given as Eq. 2:
The one factor plot of osthole vs. % inhibition of TNFα is given in (Fig. 7c). IC50 of osthole for TNFα is 4.68 mg kg−1. The best-fitted regression equation to find percentage inhibition is give as Eq. 3:
Discussion
Formalin-induced pain is characterized by a biphasic response. The first phase is neurogenic pain which appears immediately after formalin injection, whereas the second phase is the inflammatory phase which appears later. Classical analgesics such as NSAIDS inhibit only the second phase of hyperalgesia and are ineffective in the neurogenic phase, whereas the opioid analgesics inhibit both the phases, but more prominent effect is evident in the neurogenic hyperalgesia (McNamara et al. 2007). Osthole was found to attenuate both the neurogenic as well as inflammatory phases of formalin-induced hyperalgesia. In acetic acid-induced hyperalgesia model, osthole treatment was found to markedly decrease the acetic acid-induced writhes. Algesia produced by acetic acid is known to be caused by the involvement of many complex aetiopathogenic factors such as neuropeptides, leukotrienes, histamine, serotonin, and other biogenic amines (Andrade et al. 2007; Duarte et al. 1988). Therefore, to explore the mechanism of osthole-induced analgesia, formalin-induced hyperalgesia was used, since it provided the effect on neurogenic and inflammatory pain.
The cross talk between NO and COX is well documented in several studies (Bhat et al. 2008). NO is known to upregulate COX-2 in cancer cells (Cha et al. 2017). Studies have also revealed that NOS and COX inhibitors have synergistic analgesic effect in formalin-induced nociceptive behaviour (Lam et al. 1996). Furthermore, excess NO production is documented to enhance the production of reactive oxygen species including peroxynitrile and superoxide (Janes et al. 2012). It has been demonstrated that inhibition of NO synthase by administration of Nω-nitro-l-arginine methyl ester (L-NAME) attenuated the behavioural symptoms of neuropathic pain in experimentally induced nerve constriction injury in rats (Bonassoli et al. 2013; Yoon et al. 1998).
The mechanistic studies revealed that pre-treatment with substance P, which is known to increase the expression of COX-2, and l-arginine, a NO precursor(Yoon et al. 1998), was found to reverse the analgesic effect of osthole significantly in both neurogenic and inflammatory phases of pain. However, pre-treatment with L-NAME, an inhibitor of NO synthase, did not alter the analgesic effect of osthole. The stimulation of nociceptive neurons through cyclooxygenase (COX) pathway is a major mediator of pain and inflammation. Understandably, the COX enzyme is a target of most of the analgesic drugs (Cashman 1996). Our study revealed that osthole inhibits COX enzyme as confirmed by the reversal of analgesic effect of osthole by substance P which is a well-known stimulator of COX (Hajhashemi et al. 2011) and in immunohistological studies. The interaction of osthole with cyclooxygenase enzyme has been documented in other systems, as well. Studies have revealed that osthole treatment decreases the expression of epidermal growth factor receptor tyrosine kinase (EGFR-TPK), aminopeptidase N, and matrix metalloproteinase 2 which further decreases the expression of COX-2 in bladder cancer (Liu et al. 2016). Osthole is also documented to inhibit COX-2 expression in LPS activated macrophages and to produce reno-protection in a mouse model of accelerated focal segmental glomerulosclerosis (Yang et al. 2014a).
To further strengthen the experimental findings, expression of nitric oxide synthase (NOS) was investigated and the results revealed that formalin treatment increased the expression of NOS, whereas in osthole-treated group, the expression of NOS was significantly less. Literature findings suggest that formalin upregulates the expression of NOS and also increases the levels of NO in the serum (Anbar and Gratt 1997; Lam et al. 1996). NO is one of the major mediators of pain at central as well as peripheral level through modulation of complex pain mediators (Anbar and Gratt 1997; Cury et al. 2011). Formalin administration in a rat paw is reported to upregulate the expression of NOS and increase the NO level in serum (Lam et al. 1996; Yamato et al. 2013). Literature suggests that only minimal amounts of NO are generated by nNOS and eNOS, both of which are constitutive in nature. Activation of iNOS generates a large amount of NO, especially in the diseased states (Ritter et al. 2011). Co-administration of low doses of l-arginine, a precursor of NO, with formalin is known to exaggerate the hyperalgesic effect of the later (Kawabata et al. 1994).
It has also been postulated that inducible NOS is involved in the increased production of chemokines such as tumour necrosis factor α (TNFα) as well as interleukins including IL-6 (Yamato et al. 2013). To verify this pathway, further experiments were carried out by injecting LPS in mice peritoneal cavity. The findings of our experiments suggested that osthole treatment markedly decreased the levels of TNFα and IL-6.
In conclusion, the results of the present studies indicate that the anti-nociceptive and analgesic effect of osthole involves an interplay of several mediators including modulation of NO and COX. Furthermore, osthole reduced the release of inflammatory cytokines TNFα and IL-6 during LPS-induced inflammation. Remarkably, osthole is an interesting molecule that acts at pleiotropic target sites and may provide an interesting lead in developing novel analgesic and anti-inflammatory agents.
References
Anbar M, Gratt BM (1997) Role of nitric oxide in the physiopathology of pain. J Pain Symptom Manag 14:225–254
Andrade SF, Cardoso LGV, Carvalho JCT, Bastos JK (2007) Anti-inflammatory and antinociceptive activities of extract, fractions and populnoic acid from bark wood of austroplenckiapopulnea. J Ethnopharmacol 109:464–471
Basbaum AL, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284
Bhat AS, Tandan SK, Kumar D, Krishna V, Prakash VR (2008) The interaction between inhibitors of nitric oxide synthase and cyclooxygenase in formalin-induced pain in mice: an isobolographic study. Anesth Analg 106:978–984
Bonassoli VT, Contardi EB, Milani H, Oliveira RMWD (2013) Effects of nitric oxide synthase inhibition in the dorsolateral periaqueductal gray matter on ethanol withdrawal-induced anxiety-like behavior in rats. Psycopharmacology 228:487–498
Bushnell MC, Case LK, Ceko M, Cotton VA, Gracely JL, Low LA, Pitcher MH, Villemure C (2015) Effect of environment on the long-term consequence of chronic pain. J Pain 15:S42–S49
Carballo-Villalobos AI, Gonza’lez-Trujano ME, Alvarado-Va’zquez N, Lopez-Munoz FJ (2017) Pro-inflammatory cytokines involvement in the hesperidin antihyperalgesic effects at peripheral and central levels in a neuropathic pain model. Inflammopharmacology 25:265–269
Cashman JN (1996) The mechanisms of action of NSAIDs in analgesia. Drugs 52:13–23
Cha JH, Kim WK, Ha AW, Kim MH, Chang MJ (2017) Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr Res Pract 11:90–96
Crofford LJ (2010) Adverse effects of chronic opioid therapy for chronic musculoskeletal pain. Nat Rev Rheumatol 6:191–197
Cury Y, Picolo G, Gutierrez VP, Ferreira SH (2011) The dual effect of nitric oxide in the nociceptive system. Nitric Oxide 25:243–254
Duarte IDG, Nakamura M, Ferreira SH (1988) Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz J Med Biol Res 21:341–343
Farrar JT (2010) Advances in clinical research methodology for pain clinical trials. Nat Med 16:1248–1293
Goudet C, Chapuy E, Alloui A, Acher F, Pin JP, Eschalier A (2008) Group III metabotropic glutamate receptors inhibit hyperalgesia in animal models of inflammation and neuropathic pain. Pain 137:112–124
Hajhashemi V, Sajjadi SE, Zomorodkia M (2011) Antinociceptive and anti-inflammatory activities of Bunium persicum essential oil, hydroalcoholic and polyphenolic extracts in animal models. Pharm Biol 49:146–151
Hunskaar S, Hole K (1987) The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30:103–114
Ibana H, Yoshigai E, Okuyama T, Murakoshi M, Sugiyama K, Nishino H, Nishizawa M (2015) Antipyretic analgesic drugs have different mechanisms for regulation of the expression of inducible nitric oxide synthase in hepatocytes and macrophages. Nitric Oxide 44:61–70
Janes K, Neumann WL, Salyemini D (2012) Anti-superoxide and anti-peroxynitrite strategies in pain suppression. Biochim Biophys Acta 1822:815–821
Ji HJ, Hu JF, Wang YH, Chen XY, Zhou R, Chen NH (2010) Osthole improves chronic cerebral hypoperfusion induced cognitive deficits and neuronal damage in hippocampus. Eur J Pharmacol 636:96–101
Liu J, Hang W, Zhou L, Wang X, Lian Q (2005) Anti-inflammatory effect and mechanism of osthole in rats. J Chin Med Mater 28:1002–1006
Kawabata A, Manabe S, Manabe Y, Takagi H (1994) Effect of topical administration of l-arginine on formalin-induced nociception in the mouse: a dual role of peripherally formed NO in pain modulation. Br J Pharmacol 112:547–550
Koon HW, Zhao D, Zhan Y, Rhee SH, Moyer MP, Pothoulakis C (2006) Substance P stimulates cyclooxygenase-2 and prostaglandin E2 expression through JAK-STAT activation in human colonic epithelial cells. J Immunol 176:5050–5059
Kulkarni SK (2005) Handbook of experimental Pharmacology, 3rd edn. Vallabh Prakashan, New Delhi, p 127
Lam HH, Hanley DF, Trapp BD, Saito S, Raja S, Dawson TM, Yamaguchi H (1996) Induction of spinal cord neuronal nitric oxide synthase (NOS) after formalin injection in the rat hind paw. Neurosci Lett 210:201–204
Li XX, Hara I, Matsumiya T (2002) Effects of osthole on postmenopausal osteoporosis using ovariectomized rats; comparison to the effects of estradiol. Biol Pharm Bull 25:738–742
Liao PC, Chien SC, Ho CL, Wang EIC, Lee SI, Kuo YH, Jeyashoke N, Chen J, Dong WC, Chao LK, Hua KF (2010) Osthole regulates inflammatory mediator expression through modulating NF-kB, mitogen-activated protein kinases, protein kinase C, and reactive oxygen species. J Agric Food Chem 58:10445–10451
Liu J, Xu R, Zhao X (2016) Mechanisms for effect of osthole on inhibiting the growth and invasion of bladder cancer cells. Eur PMC 41:345–352
Machado GC, Maher CG, Ferreira PH, Day RO, Pinheiro MB, Ferreira ML (2016) Non-steroidal anti-inflammatory drugs for spinal pain: a systematic review and meta-analysis. Ann Rheum Dis 76:210597
Martin SL, Power A, Boyle Y, Anderson IM, Silverdale MA, Jones AK (2017) 5-HT modulation of pain perception in humans. Psychopharmacology 234(19):2929–2939
McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM (2007) TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci 104:13525–13530
OECD Guideline for testing of chemicals (2001) Guideline 423: acute oral toxicity- acute toxic class method. Organization for Economic Cooperation and Development, Paris
Ritter J, Flower RJ, Henderson G, Rang H (2011) Nitric oxide: Chemical mediators. Rang and Dale’s Pharmacology, 7th edn. Elsevier Health Sciences, London, United Kingdom, pp 237–243
Smith WL, Murphy RC (2002) The eicosanoids: cyclooxygenase, lipoxygenase and epoxygenase pathways. New Compr Biochem 36:341–371
Tadiwos Y, Nedi T, Engidawork E (2017) Analgesic and anti-inflammatory activities of 80% methanol root extract of Jasminum abyssinicum hochst. ex. Dc. (Oleaceae) in mice. J Ethnopharmacol 202:281–289
Wandji BA, Bomba FDT, Nkeng-Efouet PA, Piegang BN, Kamanyi A, Nguelefack TB (2018) Antihyperalgesic activity of the aqueous and methanol extracts of the leaves of Pittosporum mannii Hook on CFA-induced persistent inflammatory pain. Inflammopharmacology 26:197–205
Wikel S (2013) Ticks and tick-borne pathogens at the cutaneous interface: host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front Microbiol 19:337
Winter CA, Risely EA, Nuss GW (1962) Carrageenan induced edema in hind paw of the rat as assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 11:544–547
Yamato K, Kataoka T, Nishiyama Y, Taguchi T, Yamaoka K (2013) Antinociceptive effects of radon inhalation on formalin-induced inflammatory pain in mice. Inflammation 36:355–363
Yang SM, Chan YL, Hua KF, Chang JM, Chen HL, Tsai YJ, Chao LK, Feng-Ling Y, Tsai YL, Wu SH, Wang YF, Tsai CL, Chen A, Ka SM (2014) Osthole improves an accelerated focal segmental glomerulosclerosis model in the early stage by activating the Nrf2 antioxidant pathway and subsequently inhibiting NF-κB-mediated COX-2 expression and apoptosis. Free Radic Biol Med 73:260–269
Yang D, Gu T, Wang T, Tang Q, Ma C (2010) Effects of osthole on migration and invasion in breast cancer cells. Biosci Biotechnol Biochem 74:1430–1434
Yoon YW, Sung B, Chung JM (1998) Nitric oxide mediates behavioral signs of neuropathic pain in an experimental rat model. NeuroReport 9:367–372
Zhang ZR, Leung WN, Cheung HY, Chan CW (2015) Osthole: a review on its bioactivities, pharmacological properties, and potential as alternative medicine. Evid Based Complement Altern Med 2015:919616
Acknowledgements
The authors are grateful to the Department of Science and Technology, Government of India, for funding received under EMR (EMR/2016/005878). University Grants Commission, New Delhi, is acknowledged for the financial assistance to Gurjit Singh (UGC-NFSC) and grant under university with potential for Excellence to Guru Nanak Dev University. Statistical analysis performed by Dr. M.S. Bhatti, Associate Professor, Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Singh, G., Bhatti, R., Mannan, R. et al. Osthole ameliorates neurogenic and inflammatory hyperalgesia by modulation of iNOS, COX-2, and inflammatory cytokines in mice. Inflammopharmacol 27, 949–960 (2019). https://doi.org/10.1007/s10787-018-0486-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-018-0486-9