Abstract
Background
Chaerophyllum macropodum Boiss. (popularly known as “Jafari farangi kohestani”) is a predominant medicinal plant traditionally utilized in the treatments of peritoneal inflammation and headache in Persian folk medicine. Here, we have revealed the anti-neuropathic and anti-nociceptive activities of C. macropodum leaves essential oil (CMEO) in addition to uncovering the possible mechanisms of action.
Methods
Formalin-induced paw licking model was used to assess the anti-nociceptive activity of CMEO and its major constituent, terpinolene (TP). The anti-nociceptive activity of these compounds was determined by investigating the roles of various non-opioid and NO-cGMP-K+ channels. Additionally, the anti-neuropathic potential of CMEO and TP was determined using cervical spinal cord contusion/CCS technique.
Results
The CMEO exerted significant anti-nociceptive activity with a remarkable activity seen in the second phase of formalin-induced paw licking model and this activity were remarkably reversed by pre-treatment of naloxone (an opioid antagonist). Pretreatment with several types of NO-cGMP-potassium channel pathway meaningfully reversed the anti-nociceptive potential of CMEO in phase II of formalin model. Moreover, pre-treatment with several antagonists of non-opioid receptors revealed that only the antagonist of TRPV-1, serotonin type 3, 5-HT2, α2 adrenergic, and CB1 receptors (capsaicin, ondansetron, ketanserin, yohimbine, and SR141716A, respectively) reversed CMEO anti-nociception. CMEO and TP also remarkably reversed hyperalgesia and mechanical allodynia in the CCS technique.
Conclusion
The CMEO exerts anti-nociceptive and anti-neuropathic activities via the modulation of NO-cGMP potassium channel pathway, opioid as well as several non-opioid receptor activity. TP might partly contribute to the observed activities of CMEO.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain serves as a protective response by the body and involves alterations in anatomy, physiology, neurochemicals, and psychology. It is characterized as an unpleasant sensory and emotional encounter that poses a hazard of physical injury, making it the primary reason for medical consultations across the globe (Golshani and Mohammadi 2015). The adverse consequences of opioids and other pain medicines, such as NSAIDs, have undergone thorough examination and are controllable with efficiency (Mahmoodi et al. 2016).
In developing nations, it is believed that approximately 80% of the citizens rely on herbs as a form of medical treatment according to the World Health Organization (WHO) (Reis et al. 2022). The pharmaceutical industry's growing reliance on plants emphasizes the urgent need to investigate essential oils as a means of developing more advanced bioactive delivery mechanisms and pharmaceuticals with higher efficacy and fewer adverse effects (Asgari Neamatian et al. 2017).
Apiaceae family provides a large number of plants which are used for different purposes including nutrition, medicine, beverages, spices, repellents, staining, cosmetics, fragrances and industrial uses. This family is rich in phytochemicals and secondary metabolites which are potential source of drugs. Various products and by products derived from Apiaceae family can be considered as easily accessible, cheap, and environmentally friendly raw materials for several industrial and commercial preparations ranging from their uses in the cosmetic and pharmaceutical industries, to their uses as food additives and other industrial applications (Sayed-Ahmad et al. 2017). Chaerophyllum macropodum Boiss (C. macropodum). is an indigenous aromatic biennial shrub belongs to the Apiaceae family. It is locally known in Persian language as jafari frangi kohi, chervil, and chevil. The plant has been traditionally used by the Persian to treat various types of ailments, including a variety of pain (e.g., toothache, back pain, and migraine) (Amiri and Joharchi 2016). Recent studies have proven the antioxidant, antimicrobial, anti-amoebic, and antibacterial (Durmaz et al. 2006; Ebrahimabadi et al. 2010; Nayeri et al. 2022) effects of C. macropodum. The previous studies have indicated that some plants of the same genus as C. macropodum possess in vivo anti-inflammatory effects (Kurkcuoglu et al. 2018). Application of C. macropodum essential oil (CMEO) can be competitive in comparison to the referenced analgesic chemical drugs due to its availability to people in high quantities due to different distribution in Iran, being safer and less side effects, popularity in Iran for using in the pain managements, low economically price, complementary treatment usage (Amiri and Joharchi 2016). This plant also has been used in different countries such as Iraq, Turkey, Pakistan, and Azerbaijan for treatment of headache, fever, eliminating dampness, joint pain, cough, asthma, rheumatic arthralgia, carbuncle, pyogenes infections, regulating vital energy, promoting digestion, relaxing abdominal pain, expelling parasite, feverish conditions, stomach ache and cold (De Silva et al. 1997; Toksoy et al. 2010). In China, the plant leaves has been mainly used for treatment of pallor, fatigability, palpitations, exopathogenic wind-cold (Li 2016). In India also the CMEO was utilized in the industrial applications including lubricants, soaps (for achieving smooth skin), mouthwash, and lotions (Sharma et al. 2008).
In recent decades, numerous studies have reported that terpenes and terpenoids are essential in supporting human health. The content of this compound can be found in several nutritional and health products of humans because it is a source of vitamins A, E, K, and coenzyme Q10. Terpinolene (TP) is one of the most important chemical constituent identified in CMEO, a highly lipophilic monocyclic terpene (Haghi et al. 2010; Dini et al. 2022). Terpinolene has been reported to exert various pharmacological activities such as anti-nociceptive, anti-inflammatory, antioxidant, antifungal, insecticidal, larvicidal as well as anti-tumoral effect that are shown to involve in the inhibitory effect toward the expression of protein kinase 1 based on the previous reports. There are evidences that TP is a powerful weapon against neuropathic pain and inflammatory-associated diseases (Menezes et al. 2023).
However, no attempt has been made to scientifically evaluate the pain-relieving potential of CMEO. Therefore, the objective of the current investigation was to assess the potential anti-nociceptive and anti-neuropathic properties of CMEO and its predominant component, TP. The role of opioids and non-opioids receptors as well as the NO-cGMP-K+ channel pathway in the modulation of CMEO-mediated anti-nociceptive activity was also investigated.
Materials and methods
Preparation of plant
Leaves of C. macropodum were collected in July 2022 from Alvand Mountain in Hamadan, Iran and verified by Dr. Mohammad Zarei. The herbarium section from Islamic Azad University of Tehran (commonly known as Ibn Sina), Iran has confirmed the plant sample (No. 1505).
Extraction of C. macropodum EO
Dried C. macropodum leaves (≈ 2890 g) were subjected to hydro-distillation procedure for two hours using a Clevenger apparatus to extract the essential oil. Afterward, the essential oil was dehydrated with the aid of Na2SO4 and placed in storage at the temperature of 4 °C until used (Ahmadimoghaddam et al. 2020).
Animals
Male Wistar rats (225–240 g; 8 weeks old) were obtained from the Royan-Institute, Tehran, Iran. The animals were kept in plastic cages (n = 4) with tender bedding in the animal house and maintained on a 12:12-h light/dark cycle. Throughout all the inquiries, the examiner was unaware of the specific medication interventions.
Chemicals and drugs
Dose range selected for all chemicals and drugs used in current investigation was based on the preceding investigations (Mahmoodi et al. 2013; Fallahzadeh et al. 2016; Mohammadi and Golshani 2017; Ahmadimoghaddam et al. 2020, 2021; Zarei et al. 2021; Abed et al. 2022a, b; Menezes et al. 2023). Drugs like morphine sulfate (MOR), naloxone or NLX, sodium diclofenac (DIC), diazepam (DZP), and formalin were acquired from Tocris-Bioscience (UK, Bristol) while modulators of NO-cGMP-potassium channels pathway (i.e., arginine-hydrochloride or L-ARG, L-NAME hydrochloride or L-NAME, methylene blue (MB), nitroprusside sodium (SNP), glyburide (GLI, commonly known as glibenclamide), and antagonists of several non-opioid receptors (i.e., SR141716A (SRA; cannabinoid subtype 1 (CB1), SR144528 (SR8; cannabinoid subtype 2 (CB2), ketanserin (KET; serotonin type 2 (5-HT2), ondansetron hydrochloride (OND; serotonin type 3 (5-HT3), GW6471 (GW6; Peroxisome proliferator-activated receptors-α (PPARα), GW9662 (GW9; peroxisome proliferator-activated receptor-γ (PPARγ), prazosin (PRA: α1 adrenergic), yohimbine (YOH; α2 adrenergic), pregabalin (PGB), capsazepine (CAP; transient receptor potential vanilloid subtype 1 (TRPV-1), tranilast (TRA; transient receptor potential vanilloid subtype 2 (TRPV2), SCH23390 (SCH; dopaminergic subtype 1 (D1), and sulpiride (SUL; dopaminergic subtype 2 (D2) were acquired from Sigma Aldrich (St, Louis, USA). Preparative to the experiment, each of CMEO or TP were diluted within 5% dimethyl sulfoxide (DMSO) to the required dose range (Zarei et al. 2021). The solvents solution was formulated by combinations of DMSO/saline/Tween 20 in the ratio of 90:5:5 (v/v).
Formalin-induced paw licking model
The model suggested technique was carried out for evaluation of formalin-induced paw licking model (Gong et al. 2014). Nine groups of rats (n = 7) were used and received vehicle (control/VEH), CMEO (30, 60, and 120 mg/kg, p.o.), TP (5, 10, and 20 mg/kg, p.o.), MOR (1 mg/kg, ip.) or DIC (10 mg/kg, ip.) prior to the administration of formalin solution. Then, 20 min following drug injections and/or 1 h after oral treatment, 50 μl 2.5% formalin was injected to the plantar surface of the left hind paw through a 30-gauge needle microsyringe.
Involvement of NO–cGMP–potassium channel
The rats were pre-treated (ip) with the respective dose of L-ARG (25, 50, and 100 mg/kg), L-NAME (25, 50, and 100 mg/kg), SNP (125, 250, and 500 mg/kg), MB (100, 200, and 400 mg/kg) or GLI (25, 50, and 100 mg/kg) for 10 min followed by the treatment (p.o.) with 120 mg/kg CMEO. Sixty (60) min later, the animals underwent the formalin experiment.
Involvement of other non-opioid receptors
The rats were pre-treated (ip) with CAP (3, 10, and 30 mg/kg), TRA (3, 6, and 12 mg/kg), GW6 (10, 20, and 30 mg/kg), GW9 (3, 10, and 20 mg/kg), KET (3, 10, and 30 mg/kg), OND (2, 4, and 10 mg/kg), SCH (0.25, 0.5, and 1 mg/kg), SUL (0.5, 2, and 4 mg/kg), PRA (0.5, 1, and 2 mg/kg), YOH (0.5, 10, and 20 mg/kg), SRA (1, 3, and 6 mg/kg) or SR8 (5, 10, and 20 mg/kg) for 10 min followed by the treatment (p.o.) with 120 mg/kg CMEO. Sixty (60) min later, the animals underwent the formalin experiment.
The neuropathic pain model
Cervical spinal cord hemicontusion (CCS) was carried out as previously described (Dunham et al. 2010). Rodents were anesthetized with a combination of 6 mg/kg xylazine/60 mg/kg ketamine/6 mg/kg acepromazine. Afterward, partial laminectomy of C5 was performed and the spinal column was fixed in the IH-0400 Impactor apparatus (Precision Systems and Instrumentation, USA). The spinal cord was immediately contused (using standard mouse tip size 1.3 mm) with a force of 200 kdyn (no dwell time), resulting in tissue displacement to a depth of 1600–1800 lm. Finally, the incision was closed in layers and 5 mL of lactated Ringer solution was subcutaneously administered to avoid dehydration.
Allodynia assay
Mechanical allodynia was assessed as previously reported (Zhu et al. 2021) by measuring paw withdrawal thresholds (PWT) for mechanical stimuli using von Frey filaments (Stoelting, USA). Seven calibrated von Frey filaments (i.e., 0.07, 0.16, 0.4, 0.6, 1.0, 1.4, 2.0 g bending force) were used with approximately equal logarithmic incremental bending forces. Rats were acclimatized for at least 20 min on an elevated wire mesh floor protected by a transparent Plexiglas chamber. Then fibers of sequentially increasing rigidity with an initial bending force of 0.07 g were applied to the hind paw plantar surface adjacent to the incision for 5 s with sufficient strength to bend the fiber slightly.
Hyperalgesia assay
As mentioned in the previous investigation (Ahmadimoghaddam et al. 2021), the paw withdrawal delay to thermal radiation has been used to analyze thermal hyperalgesia. Animals were set on a glass surface in a PL-200 Plantar anti-nociceptive checker (China, Chengdu Technology & Market CO. Sichuan) and given at least 10 min to conform to the equipment prior observations. The thermal radiation light origin was placed under the hind paw’s plantar surface and modified strategically to project a 5 mm diameter light spot onto the glass panel. To avoid tissue injury, the cut-off time was set to 12 s.
Enzyme-linked immunosorbent assay (ELISA)
In addition, the spinal cord of CCS-surgery rats treated with CMEO or TP was subjected to the enzyme linked immunosorbent assay (ELISA) to determine the level of IL1β, TNFα and IL2.
Rota-rod assay
The motor performance of rats treated (p.o.) with CMEO (30, 60, and 120 mg/kg) or TP (5, 10, and 20 mg/kg) was evaluated using the rota-rod assay.
Serum biochemical analysis
Serum level of alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, and urea in rats treated once daily for 14 days with VEH, CMEO (30, 60, and 120 mg/kg) or TP (5, 10, and 20 mg/kg) were analyzed.
GCMS analysis of phytoconstituents of CMEO
Phytoconstituents analysis was carried out on CMEO using a GC–MS (Hewlett-Packard 5973) with an HP-5MS column (30 m × 0.25 mm; film thickness, 0.25 µM).
Statistical test
The data were presented as mean ± SEM and analyzed by GraphPad Prism® 16. The statistical significance of difference between groups was assessed by one-way analysis of variance (ANOVA) or two-way followed by Dunnett’s/Bonferroni’s multiple comparison test. The feedback variables were assessed for normality assumptions using Kolmogorov/Smirnov. The trapezoidal rule was utilized to compute the region beneath the curve. If the p values were below 0.05, they were deemed to have notable differences.
Results
Nociceptive pain (formalin-induced model)
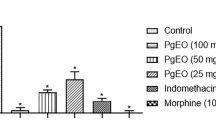
Figure 1(A; neurogenic phase) shows that administration of CMEO (120 mg/kg) or TP (20 mg/kg) exerted significant anti-nociceptive action against the VEH (p < 0.05, p < 0.01, respectively). Standard drugs, DIC and MOR, also exhibited significantly higher anti-nociceptive activity (p < 0.01, p < 0.001, respectively) in comparison to CMEO (120 mg/kg).
In both stages (A, B) of a formaldehyde-induced paw licking test, the use of Chaerophyllum macropodum essential oil (CMEO) was found to alleviate pain scores. CMEO (120 mg/kg) + naloxone (NLX), morphine (Mor), Diclofenac (Dic), Terpinolene/TP, and morphine (Mor) + naloxone/NLX. After conducting One-way ANOVA, the Bonferroni’s tests were performed. n = 7 rats/group. The data are presented as Mean values plus Standard Error of the Mean. aap < 0.01, and aaap < 0.001 vs. Veh (control/vehicle). bbbp < 0.001 vs. CMEO (120 mg/kg)
As can be seen in Fig. 1 (inflammatory-mediated phase), CMEO at all concentrations tested (30, 60, 120 mg/kg) were found to exert significant anti-nociceptive activity (p < 0.01, p < 0.001, p < 0.001, respectively) against VEH treated). MOR (a standard drug) exerted anti-nociceptive activity (p < 0.001) that is greater in intensity than CMEO (120 mg/kg). Interestingly, there was insignificant anti-nociceptive activity between CMEO (120 mg/kg) and DIC. In addition, treatment of rats by TP (10 and 20 mg/kg) significantly reduced the inflammatory pain (p < 0.01, p < 0.001, respectively).
NO–cGMP–potassium channels
In the inflammatory-mediated phase of formalin test (Fig. 2), treatment by specific antagonist such as LAH (i.pl; 50, and 100 μg/paw; p < 0.05, p < 0.001, respectively), GLI (i.pl; 25, 50, and 100 μg/paw; p < 0.01, p < 0.01, p < 0.001, respectively), L-NAME 100 μg/paw (p < 0.001) and/or MB (100, 200 and 400 μg/paw; p < 0.05, p < 0.01, p < 0.001, respectively) significantly altered the anti-nociceptive intensity of CMEO (120 mg/kg, p.o.).
The anti-nociceptive effects of Chaerophyllum macropodum (CMEO) in phase-II of formalin-induced test may be linked to the NO–cGMP–K+ channel pathway. Upon receiving CMEO at a dosage of 120 mg/kg, the rats were administered a local prescription of each antagonist. Nω-Nitro-l-arginine methy lester hydrochloride (L-NAME), Sodium nitroprusside (SNP), Glibenclamide (GLI), l-arginine (LAH), Methylene blue (MB). “The Linear-Log Trapezoidal Method was a technique used to determine the Area Under the Curve or AUC”. ap < 0.05, aap < 0.01, and aaap < 0.001 vs. control group (Veh/vehicle). n = 7 rats/group. After conducting One-way ANOVA, the Bonferroni's tests were performed. The data are presented as Mean values plus Standard Error of the Mean
The involvements of TRPV, serotonin, adrenergic, and cannabinoid systems
Pretreatment with the respective antagonist of several non-opioid receptors revealed that only the antagonist of TRPV-1 (CAP 3, 10, and 30 μg/paw; p < 0.05, p < 0.01, p < 0.001, respectively), serotonin 5-HT3 (OND 2,4, 10 μg/paw; p < 0.01, p < 0.01, p < 0.001, respectively), α2 adrenergic antagonist (YOH 0.5, 10, 20 μg/paw; p < 0.001, at all doses), or CB1 (SRA 3 μg/paw; p < 0.001) receptors significantly reversed analgesics-like activity of 120 mg/kg CMEO as illustrated on Figs. 3, 4, 5.
The Chaerophyllum macropodum essential oil, also known as CMEO, has a potential anti-nociceptive effect at a dosage of 120 mg/kg, which may be attributed to its activation of TRPV and PPAR mechanisms. The antagonist of PPARγ (GW9662 or GW9: 3, 10, 20 μg/paw) and the antagonist of PPARα (GW6471 or GW6: 10, 20, 30 μg/paw), the antagonist of TRPV-1 (capsazepine/Cap: 3, 10, 30 μg/paw,) and the antagonist of TRPV-2 (tranilast/Tra: 3, 6, 12 μg/paw). The data are presented as Mean values plus Standard Error of the Mean. “The Linear-Log Trapezoidal Method was a technique used to determine the Area Under the Curve or AUC”. ap < 0.05, aaap < 0.001 a significant difference to control group (vehicle or Veh). n = 7 rats/group. After conducting One-way ANOVA, the Bonferroni's tests was performed
The anti-nociceptive property of Chaerophyllum macropodum (CMEO), at a dosage of 120 mg/kg, operates through the mechanisms of serotonin and dopamine. Various doses of ondansetron hydrochloride/Ond, a competitive antagonist of serotonin type 3 receptors, and ketanserin/Ket, an antagonist of serotonin type 2 receptors, were administered to the paws in the amounts of 2, 4, 10 μg and 3, 10, 30 μg, respectively. Moreover, to inhibit the activity of D1 and D2 receptors, various amounts of SCH23390//SCH and sulpiride/Sul were administered per paw in dosages of 0.25, 0.5, 1 μg and 0.5, 2, 4 μg, respectively. The data are presented as Mean values plus Standard Error of the Mean. “The Linear-Log Trapezoidal Method was a technique used to determine the Area Under the Curve or AUC”. ap < 0.05, aaap < 0.001 vs. Veh (control/vehicle). n = 7 rats/group. After conducting One-way ANOVA, the Bonferroni's tests were performed
The anti-nociceptive effect of Chaerophyllum macropodum essential oil/CMEO (at a dosage of 120 mg/kg) is attributed to its action on adrenergic and cannabinoid mechanisms. The prazosin (Pra) and yohimbine (Yoh) were administered at varying doses (0.5, 1, and 2 μg/paw for Pra and 0.5, 10, 20 μg/paw for Yoh) to block the α1 and α2 adrenergic receptors, respectively. CB1 and CB2 receptor blockers (SR141716A/SRA and SR144528, respectively) were administered at varying doses per paw (1, 3, and 6 μg/paw for CB1 and 5, 10, and 20 μg/paw for CB2). The data are presented as Mean values plus Standard Error of the Mean. “The Linear-Log Trapezoidal Method was a technique used to determine the Area Under the Curve or AUC”. ap < 0.05, aap < 0.01, and aaap < 0.001 vs. control group (Veh/vehicle). n = 7 rats/group. After conducting One-way ANOVA, the Bonferroni's tests were performed.
The effects of CMEO and TP on the allodynia
Based on the statistics presented in graphs 6A and B, rats that underwent CCS surgery experienced a significant reduction (p < 0.001) in their PWT levels over the 14-day experimental period against rats who did not undergo CCS surgery. Interestingly, 120 mg/kg CMEO significantly reduced allodynia at all day post-surgery (from day 2 with p < 0.05 to day 14 with p < 0.001), whereas 60 mg/kg CMEO exerted meaningful anti-allodynia only on days 4 and 10 post-surgery (p < 0.05). Similarly, TP in 12 and 25 mg/kg dosages also exerted meaningful anti-allodynia activity on days 8 and 10 (p < 0.01; p < 0.001, respectively).
The effects of CMEO and TP on hyperalgesia
CCS-surgery rats pre-treated with VEH and then exposed to thermal radiation exerted significantly higher (p < 0.001) hyperalgesia effect against naïve rodents, which is suggested by a reduction in the level of PWT throughout the 14 days of the experimental duration (Fig. 6C, D). Before undergoing CCS surgery, rodents were given a pre-treatment of CMEO at a dosage of 120 mg/kg. This pre-treatment resulted in a significant reversal of the thermally induced pain response in CCS rats on various days post-surgery, including days 6, 8, 10, 12, and 14 (p values ranging from p < 0.01, p < 0.001, p < 0.05, p < 0.05, and p < 0.01, respectively). Figure 6D illustrated that the PWT level in rodents that underwent surgery (CCS) showed significant improvement with a dose of 20 mg/kg TP, indicating statistical significance (p < 0.05) at day 4, (p < 0.01) at day 6 and day 10, and (p < 0.05) at day 12 following exposure to thermal radiation.
The effects of Chaerophyllum macropodum essential oil (CMEO) and Terpinolene (TP) at varying doses (30, 60, and 120 mg/kg for CMEO, and 5, 10, and 20 mg/kg for TP) were observed in the mechanical allodynia (A, B) and heat hyperalgesia (C, D) through continuous therapy. Vehicle or Veh, Pregabalin (PGB, 30 mg/kg), and Cervical spinal cord contusion (CCS). n = 7 rats per group. After conducting Two-way ANOVA (repeated measures), the Bonferroni's tests were performed. The data are presented as Mean values plus standard error of the mean. ap < 0.05, aap < 0.01, aaap < 0.001 vs. CCS model
Involvements of inflammatory cytokines
Figure 7A–C shows the levels of TNF-α, IL-1β, and IL-2 following treatment of CCS-surgery rats with CMEO. Comparison to VEH-treated rats (normal rats) revealed that the level of all inflammatory cytokines increased significantly (p < 0.001) in CCS-surgery rats. CMEO, at 120 mg/kg, significantly reduced the level of TNF-α (p < 0.001), IL-1β (p < 0.001), and IL-2 (p < 0.05).
The essential oil derived from Chaerophyllum macropodum, also known as CMEO, displayed the ability to reduce the levels of Interleukin-1β, tumor necrosis factor, and Interleukin-2 in rats with spinal cord injuries. Commoditized fragments of the spinal cord in the cervical region have been subjected to lysis-buffer. The cervical spinal cord contusion (CCS) led to a significant increase in the levels of IL-2, IL-1, and TNF in the spinal cord. Nevertheless, this response was suppressed by CMEO. bp < 0.05, bbp < 0.01, and bbbp < 0.001 versus CCS group; aaap < 0.001 versus vehicle/Veh. n = 7 rats per group. After conducting One-way ANOVA, the Bonferroni's tests were performed. The data are presented as Mean values plus Standard Error of the Mean
Monitoring of locomotor motion
Giving rats either CMEO (30, 60, and 120 mg/kg) or TP (5, 10, and 20 mg/kg) by mouth (p.o.) did not change their locomotor activity compared to VEH (Table1).
Blood parameters
Oral pre-treatment with CMEO (30, 60, and 120 mg/kg) or TP (5, 10, and 20 mg/kg) continuously for 7 days caused no significant changes in the blood parameters against VEH pre-treated group (Table 2).
Phytoconstituents analysis of CMEO using GC–MS
Twenty volatile phytochemicals identified from CMEO which represent 97.6% of the total content of CMEO (Table 3). Four main compounds identified in CMEO following the GC–MS analysis was terpinolene (34.1%), myristicin (14.2%), trans-β-ocimene (10.3%), and γ-terpinene (9.7%).
Discussion
The present study was carried out to evaluate the effects of Chaerophyllum macropodum leaves essential oil and its main constituents, TP, in inflammatory and neuropathic pain. Furthermore, it is clearly proved that CMEO also could reduce acute inflammatory pain, partly via the modulation of serotonin, vanilloid, cannabinoid, and adrenergic systems and the involvement of NO–cGMP–K+ channel pathway.
The important of medicinal plants industry, particularly, in Iran has been previously discussed (Sardoei 2022). According to the report, Iran was evaluated as the number one leading country in terms of medicinal plants export in 1998 and the fifth in 2003, and it came to 32 in rankings due to some problems. In 2018, the share of Iran from medicinal plants trade was 440 million dollars, while the total global transaction of medicinal plant import was about 124 billion dollars. This outstanding amount of trade creates unique opportunities for the traders of these products. This information suggests the importance of medicinal plants industry to Iran economy, which triggered the current study. CMEO have been traditionally used by the Iranian to treat various ailments, which include inflammatory-mediated diseases. Unfortunately, no scientific study on its anti-inflammatory and anti-neuropathic activities have been performed; hence no clinical study has been performed too on this plant. The findings reported in this article supported the potential medicinal usages of CMEO to treat inflammatory-mediated diseases; which could be the basis for future clinical study. From this study also, CMEO exerted an activity against the second phase of formalin test indicating its peripheral mechanisms of action as shown with Dic. Hence, it could be developed into a peripheral analgesic drug for clinical use to replace Dic, which have been associated with side effects like gastric ulcer. The identification of terpinolene also could be an added advantage for future drug development since this compound was found to be safe for consumption. Other than that, its anti-neuropathic potential would be an added value in comparison to other similar products (such as pregabalin) available in the market.
Inflammation is a vital defensive mechanism of the body that occurs in response to tissue harm induced by various stimuli. It serves an important role in bringing back homeostasis and facilitating tissue recovery, along with preserving tissue injury. The formalin-induced paw licking model has been divided into two stages: acute and inflammatory phase (Zakaria et al. 2016). The subsequent episode of the formalin model could potentially be instigated by the inflammatory system (Lariviere et al. 2006). Remarkably, treatment of naloxone did not turn around CMEO-induced anti-nociceptive activity in the stage-II of the formalin-induced paw licking model. Therefore, this evidence reinforced the possible involvement of non-opioid pathways in modulation of CMEO anti-nociceptive activity.
Prior investigation elucidated that TP possesses inhibitory properties against acute responses provoked by various inflammatory agents in mouse models (Menezes et al. 2023). TP was found to facilitate a decrease in the duration of licking in both episodes of the formalin-induced model, with a notably substantial impact on the initial phase. Our results are in line with Menezes et al. (2023) findings, although administration of TP in the current study exhibited more pronounced effects in the latter episode of formalin-induced model. Up to this point, the previous study has affirmed anti-nociceptive and anti-inflammatory synergistic activity of TP and diclofenac in a chronic inflammatory model (Macedo et al. 2016). It is widely accepted that diclofenac affected pain by inhibiting the action of cyclooxygenase 1 and 2 that converts arachidonic acid into prostaglandins, thromboxanes, and prostacyclins (Zacher et al. 2008). In the present study, CMEO shows same anti-nociceptive effects when compared to diclofenac (in phase II). It is possible that in the same mechanism, TP and/or CMEO could attenuate inflammatory pain through inhibiting cyclooxygenase mechanism. Moreover, according to the current research, CMEO had a more potent anti-nociceptive impact on the inflammatory episode compared to the initial phase.
Interestingly, the previous study by Macedo et al. (2016), revealed that TP exerted the anti-nociceptive activity through serotoninergic mechanism. In line with the previous study, the current research exhibited that CMEO as an important source of TP could reduce acute inflammatory pain through 5-HT3 receptor of serotonin-dependent mechanism. Furthermore, Sasaki et al (2001) have demonstrated that the intrathecal administration of 5-HT3 receptor agonist declines the occurrence of flinches in the second stage of the formalin-induced model (Sasaki et al. 2001). The 5-HT3 receptors present in the dorsal horn have been observed to exert an inhibitory effect on nociceptive transmission, which occurs in reaction to chemical stimuli related to inflammation (Liu et al. 2002). The activation of 5-HT3 receptors on interneurons by 5-HT agonists resulted in the facilitation of neurotransmitter release, specifically GABA or glycine, within trigeminal neurons. One conceivable hypothesis for delineating the underlying operations of anti-nociceptive outcomes pertaining to 5-HT3 receptor agonists is that its administration leads to elicitation of inhibitory neurotransmitters (Bardoni 2019). Then, pre-treatment of rats by OND as an antagonist of 5-HT3 receptor, possibly could reverse anti-nociceptive effects of CMEO through inhibition of the above mechanism.
The L-arginine–NO–cGMP–KATP channel pathway serves a critical function in modulating the physiological processes underlying inflammatory pain during the phase-II of the formalin-induced model. The anti-nociceptive impacts are initiated by the opening of ATP-sensitive potassium channels, which causes rising intracellular cyclic guanosine monophosphate following the action of nitric oxide (NO) (Abed et al. 2022a). Subsequently, our attention was directed toward investigating the process of NO biosynthesis as a means of elucidating the potential interconnection between CMEO and regulation of L-arginine–NO signaling. In the formalin-induced model (phase II), a significant decrease in pain inhibition was observed subsequent to the administration of L-NAME. The anti-nociceptive function of nitric oxide donors peripherally has been demonstrated to involve the inhibition of specific K+ATP channels (Romero et al. 2012). Previous investigation (Tonussi and Ferreira 1994) used two animal models of pain to show that in addition to inhibiting COX, diclofenac apparently downregulates the function of sensitized peripheral pain receptors by stimulating the L-arginine–NO–cGMP pathway. CMEO apparently has the same effect as the diclofenac-induced activation of ATP-sensitive potassium channels can be inhibited by a NO synthase inhibitor, a guanylate cyclase inhibitor, and an ATP-sensitive potassium channel opener.
To further investigate on the possible mechanisms of action that took place within the second stage of formalin-induced model, rodents were pre-administrated with CAP (the antagonist of TRPV-1), YOH (the antagonist of α2 adrenergic), and SRA (the antagonist of type1 cannabinoid), and was found to return the anti-nociceptive activity of CMEO. In other respects, the use of rats lacking the vanilloid receptor gene (knockout) indicates that the vanilloid mechanism plays a role in both hypersensitivity and pain sensation in response to painful stimuli after damages a part tissue (Ro et al. 2009). Moreover, Bezerra and co-authors conducted the study, in 2021, to investigate the anti-nociceptive properties of triterpene acetyl aleuritolic acid (AAA), which was derived from Croton zehntneri. The findings of this investigation demonstrated that the TRPV-1 and TRPV-2 channel plays a crucial role in mediating the anti-nociceptive action of AAA (Bezerra et al. 2021). The current findings provide empirical support for the preceding research with regard to the involving of TRPV-1 receptors (CAP) in the modulation of nociception. In contrast to the findings of Bezerra et al. (2021), it can be observed that the anti-nociceptive action mediated by CMEO did not rely on the involvement of TRPV-2 receptors.
The considerable anti-nociceptive impact of substances similar to cannabis and the existence of CB1 cannabinoid receptors in regions of the spinal cord and brain involved in pain processing suggests that endogenous cannabinoids, including anandamide, may play a role in regulating pain communications within the CNS. The antagonist SR141716A, which targets the CB1 receptor, has been observed to augment and extend pain like activity triggered by tissue injury. These findings suggest that peripheral CB1 receptors play a critical role in the initiation of intrinsic pain control and indicate that modulating cannabinoid receptor activity may offer therapeutic benefits in managing neuropathic and inflammatory pain (Starowicz et al. 2013). Beaulieu et al. (2000) demonstrated that antagonists of CB1 and CB2 receptor, respectively, elicited a reduction in nociceptive like-behavior in both stages of the formalin-induced model following intraperitoneal administration (Beaulieu et al. 2000). One theory that could explain this phenomenon on a molecular level was presented in a study that showed the cannabinoid CB1 receptors can activate adenylyl cyclase stimulation or inhibition through either Gs or Gi, respectively (Turu and Hunyady 2010). We have recently proven in our lab that Bupleurum falcatum L. essential oil, which belongs to the Apiaceae family, has the ability to alleviate pain and hypersensitivity when taken orally by activating CB1 receptors (Ahmadimoghaddam et al. 2021). The current data align with previous evidence of the involvement of CB1 receptors in the anti-nociceptive properties of this herbal remedy.
Neuropathic pain may result from illnesses or injuries that affect the nervous system. Typical indications of neuropathic pain, encompass hyperalgesia, allodynia and spontaneous pain (Jensen and Finnerup 2014). During the injury that occurred at the C5 level of the spinal cord, researchers were able to observe the presence of hyperalgesia and allodynia (Dietz et al. 2022). There is a general consensus that Cuminum cyminum L. is widely recognized as a valuable source of TP (Koohsari et al. 2020). This medicinal plant has potential to alleviate neuropathic pain by reducing inflammation in the spinal nerves. It highlights the crucial partnership between terpenoids and cannabinoids in achieving this effect. In the same way, the current information showed that utilizing CMEO or TP under CCS circumstances on a temporary or permanent basis can prevent from advancements of neuropathic pain (Putatunda et al. 2014). It is clearly demonstrated that pregabalin as a reference drug could help in reducing neuropathic pain. According to the declared mechanism suggested by previous reports (Colloca et al. 2017), pregabalin blocks the voltage gated calcium channel and hence decrease glutamate and sensory neuropeptides (substance P and gene-related peptide) release at synapses by decreasing Ca2+ influx. Excitatory amino acid transporter activity is increased by pregabalin which caused more decrease in synaptic availability of glutamate. Decreased glutamate levels further inhibited the activation of NMDA and decreased the neuronal firing. Additionally pregabalin also activates the KATP channels, which also contributes to inhibition of neuronal excitation. Pregabalin through all these pathways ultimately provides significant pain relief in various spinal neuropathic pain states. In the present study, administration of CMEO has shown the same anti-neuropathic activity compared to pregabalin (especially in 14th day post spinal surgery). Therefore, CMEO could possibly attenuate neuropathic pain in similar mentioned mechanism.
Research into cytokines plays a crucial role in the progression of knowledge concerning neuropathic pain (Ramesh et al. 2013). Our attention was directed toward the impact of CMEO on cytokines related to inflammation in the spinal cord. The potential of TP to hinder sudden inflammatory reactions and diminish the formation of granulomas in the body is likely due to its ability to disrupt the bodily alterations directed by inflammatory agents like histamine and PGE2 (Menezes et al. 2023). In addition, terpinolene has been shown by researchers to effectively reduce the levels of pro-inflammatory cytokines IL2 and TNFα in a way that is dependent on the dosage used (de Christo Scherer et al. 2019). The use of cytokine antagonists beforehand has diminished the pain related neuropathological symptoms. In a study conducted by Shao and colleagues in 2015, it was demonstrated that administering IL2 and IL1β via intrathecal injection resulted in the development of mechanical allodynia as well as hypersensitivity (Shao et al. 2015). During our study, we noticed that in the CCS model, the levels of cytokines like IL1β, TNFα, and IL2 were reduced in the cervical segments of the spinal cord upon the administration of either CMEO or TP. It is possible that the anti-neuropathic role of CMEO is connected to regulating these key cytokines.
As part of our study, we assessed how CMEO's pain-relieving properties were linked to mobility, using the rota-rod device. Our results indicated that administration of CMEO or TP did not adversely affect the rats' motor function. Given the potential harmful side effects of commonly used pain medications (such as kidney problems and stomach ulcers), we examined the impact of CMEO or TP on biochemical markers. The results are noteworthy as they reveal that there was no noticeable alteration in the biochemical indicators, indicating that the use of CMEO or TP did not result in any adverse effects. Previously conducted studies have aligned with these discoveries, concluding that the TP compound demonstrated no signs of harmful effects over the course of 28 days of treatment. (Menezes et al. 2021).
Conclusion
This study illustrates the ability of CMEO to alleviate neuropathic and nociceptive symptoms after being exposed to chronic and acute models. Moreover, CMEO-mediated anti-nociceptive effect in the inflammatory phase of formalin test, partly, involved the modulation of the serotonin, TRPV, adrenergic, and cannabinoid receptors as well as the NO–Cgmp–K+ channel pathways. The findings suggest that CMEO could be a viable option for creating novel drugs and supplements that can effectively aid in alleviating persistent pain. Therefore, we recommend CMEO as an innovative and captivating option for alleviating neuropathic and inflammatory pain, rooted in the authentic concept of Persian ethno-pharmacological remedies.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Abbreviations
- Cap:
-
Capsazepine
- CCS:
-
Cervical spinal cord contusion
- CMEO:
-
Chaerophyllum macropodum leaves essential oil
- Dic:
-
Diclofenac sodium
- DMSO:
-
Dimethyl sulfoxide
- GC:
-
Guanylate cyclase
- GC–MS:
-
Gas chromatography–mass spectrometry
- GLI:
-
Glibenclamide
- GW6:
-
GW6471
- GW9:
-
GW9662
- KATP:
-
ATP-sensitive potassium channels
- Ket:
-
Ketanserin
- L-NAME:
-
Nω-nitro-l-arginine methyl ester hydrochloride
- MB:
-
Methylene blue
- NMDA:
-
N-Methyl-d-aspartate receptors
- NOS:
-
Nitric oxide synthase
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- Ond:
-
Ondansetron hydrochloride
- PPA:
-
Peroxisome proliferator-activated
- Pra:
-
Prazosin
- SNP:
-
Sodium nitroprusside
- SR1:
-
SR141716A
- TRPV-1:
-
Transient receptor potential vanilloid subtype 1 channel
- Yoh:
-
Yohimbine
References
Abed DZ, Jabbari S, Zakaria ZA, Mohammadi S (2022a) Insight into the possible mechanism (s) involved in the antinociceptive and antineuropathic activity of Descurainia sophia L. Webb ex Prantl essential oil. J Ethnopharmacol 298:115638
Abed DZ, Sadeghian R, Mohammadi S, Akram M (2022b) Thymus persicus (Ronniger ex Rech. F.) Jalas alleviates nociceptive and neuropathic pain behavior in mice: Multiple mechanisms of action. J Ethnopharmacol 283:114695
Ahmadimoghaddam D, Sadeghian R, Ranjbar A, Izadidastenaei Z, Mohammadi S (2020) Antinociceptive activity of Cnicus benedictus L. leaf extract: a mechanistic evaluation. Res Pharm Sci 15(5):463
Ahmadimoghaddam D, Zarei M, Mohammadi S, Izadidastenaei Z, Salehi I (2021) Bupleurum falcatum L. alleviates nociceptive and neuropathic pain: potential mechanisms of action. J Ethnopharmacol 273:113990
Asgari Neamatian M, Yaghmaei P, Mohammadi S (2017) Assessment of the antinociceptive, antiinflammatory and acute toxicity effects of Ducrosia anethifolia essential oil in mice. Sci J Kurd Univ Med Sci 22(3):74–84
Amiri MS, Joharchi MR (2016) Ethnobotanical knowledge of Apiaceae family in Iran: a review. Avicenna J Phytomed 6(6):621
Bardoni R (2019) Serotonergic modulation of nociceptive circuits in spinal cord dorsal horn. Curr Neuropharmacol 17(12):1133–1145
Beaulieu P, Bisogno T, Punwar S, Farquhar-Smith WP, Ambrosino G, Di Marzo V, Rice AS (2000) Role of the endogenous cannabinoid system in the formalin test of persistent pain in the rat. Eur J Pharmacol 396(2):85–92
Bezerra AJN, Silva FCO, da Silva AW, Ferreira MKA, Teixeira AMR, Marinho ES (2021) Antinociceptive effect of triterpene acetyl aleuritolic acid isolated from Croton zehntneri in adult zebrafish (Danio rerio). Biochem Biophys Res Commun 534:478–484
Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D (2017) Neuropathic pain. Nat Rev Dis Primers 16(1):1–9
De Silva T (1997) Industrial utilization of medicinal plants in developing countries. Medicinal plants for forest conservation and health care. FAO, Rome, pp 34–44
De Christo Scherer MM, Marques FM, Figueira MM, Peisino MCO, Schmitt EFP, Kondratyuk TP, Endringer DC, Scherer R, Fronza M (2019) Wound healing activity of terpinolene and α-phellandrene by attenuating inflammation and oxidative stress in vitro. J Tissue Viabil 28(2):94–99
Dietz V, Knox K, Moore S, Roberts N, Corona KK, Dulin JN (2022) Dorsal horn neuronal sparing predicts the development of at-level mechanical allodynia following cervical spinal cord injury in mice. Exp Neurol 352:114048
Dini S, Chen Q, Fatemi F, Asri Y (2022) Phytochemical and biological activities of some Iranian medicinal plants. Pharm Biol 60(1):664–689
Dunham KA, Siriphorn A, Chompoopong S, Floyd CL (2010) Characterization of a graded cervical hemicontusion spinal cord injury model in adult male rats. J Neurotrauma 27(11):2091–2106
Durmaz H, Sagun E, Tarakci Z, Ozgokce F (2006) Antibacterial activities of Allium vineale, Chaerophyllum macropodum and Prangos ferulacea. Afr J Biotechnol 5(19)
Ebrahimabadi AH, Djafari-Bidgoli Z, Mazoochi A, Kashi FJ, Batooli H (2010) Essential oils composition, antioxidant and antimicrobial activity of the leaves and flowers of Chaerophyllum macropodum Boiss. Food Control 21(8):1173–1178
Fallahzadeh AR, Zarei M, Mohammadi S (2016) Preliminary phytochemical screening, analgesic and anti-inflammatory effect of Eryngium pyramidale Boiss. & Husson essential oil in male rat. Entomol Appl Sci Lett 3(5):140–147
Gong N, Huang Q, Chen Y, Xu M, Ma S (2014) Pain assessment using the rat and mouse formalin tests. Bio Protoc 4(21):128–139
Golshani Y, Mohammadi S (2015) Evaluation of antinociceptive effect of methanolic extract of Lallemantia iberica in adult male rats. Armaghane Danesh 19(12):1058–1068
Haghi G, Hatami A, Ghasian F, Hoseini H (2010) Antioxidant activity evaluation and essential oil analysis of Chaerophyllum macropodum Boiss. from central Iran. J Essent Oil Bear 13(4):489–495
Jensen TS, Finnerup NB (2014) Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol 13(9):924–935
Koohsari S, Sheikholeslami MA, Parvardeh S, Ghafghazi S, Samadi S (2020) Antinociceptive and antineuropathic effects of cuminaldehyde, the major constituent of Cuminum cyminum seeds: possible mechanisms of action. J Ethnopharmacol 255:112786
Kurkcuoglu M, Sen A, Bitis L, Birteksoz Tan S, Dogan A, Baser KHC (2018) Chemical composition, anti-inflammatory, antioxidant and antimicrobial activity of essential oil from aerial parts of Chaerophyllum aromaticum L. from Turkey. J Essent Oil Bear 21(2):563–569
Lariviere WR, Sattar MA, Melzack R (2006) Inflammation-susceptible Lewis rats show less sensitivity than resistant Fischer rats in the formalin inflammatory pain test and with repeated thermal testing. J Neurophysiol 95(5):2889–2897
Li TS (2016) Chinese & related North American herbs: phytopharmacology & therapeutic values. CRC Press, Boca Raton, pp 67–69
Liu Z-Y, Zhuang D-B, Lunderberg T, Yu L-C (2002) Involvement of 5-hydroxytryptamine1A receptors in the descending anti-nociceptive pathway from periaqueductal gray to the spinal dorsal horn in intact rats, rats with nerve injury and rats with inflammation. Neuroscience 112(2):399–407
Macedo E, Santos W, Sousa Neto B, Lopes E, Piauilino C, Cunha F, Sousa D, Oliveira F, Almeida F (2016) Association of terpinolene and diclofenac presents antinociceptive and anti-inflammatory synergistic effects in a model of chronic inflammation. Braz J Med Biol Res 49
Mahmoodi M, Mohammadi S, Zarei M (2013) Antinociceptive effect of hydroalcoholic leaf extract of Tribulus terrestris L. in male rat. J Babol Univ Med Sci 15(6):36–43
Mahmoodi M, Mohammadi S, Enayati F (2016) Evaluation of the antinociceptive effect of hydroalcoholic extract of Potentilla reptans L. in the adult male rat. SSU J 24(3):201–210
Menezes IO, Scherf JR, Martins AOBPB, Ramos AGB, Quintans JdSS (2021) Biological properties of terpinolene evidenced by in silico, in vitro and in vivo studies: a systematic review. Phytomedicine 93:153768
Menezes IO, da Silva LYS, Pessoa RT, Ramos AGB, da Cunha FAB (2023) Terpinolene inhibits acute responses triggered by different inflammatory agents in vivo models of mouse. Food Biosci 53:102621
Mohammadi S, Golshani Y (2017) Neuroprotective effects of rhamnazin as a flavonoid on chronic stress-induced cognitive impairment. J Adv Neurosci Res 4(2):30–37
Nayeri T, Bineshian F, Khoshzaban F, Dalimi Asl A, Dayer MS, Ghaffarifar F (2022) The amoebicidal activity of Chaerophyllum macropodum extract on Acanthamoeba genotype T4 in vitro. Sci J Kurd Uni Med Sci 26(7):23–33
Putatunda R, Hala TJ, Chin J, Lepore AC (2014) Chronic at-level thermal hyperalgesia following rat cervical contusion spinal cord injury is accompanied by neuronal and astrocyte activation and loss of the astrocyte glutamate transporter, GLT1, in superficial dorsal horn. Brain Res 1581:64–79
Ramesh G, MacLean AG, Philipp MT (2013) Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediat Inflamm 2013:1
Reis DR, Ambrosi A, Di Luccio M (2022) Encapsulated essential oils: a perspective in food preservation. Future Foods 5:100–102
Ro JY, Lee JS, Zhang Y (2009) Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain 144(3):270–277
Romero TR, Guzzo LS, Perez AC, Klein A, Duarte ID (2012) Noradrenaline activates the NO/cGMP/ATP-sensitive K+ channels pathway to induce peripheral antinociception in rats. Nitric Oxide 26(3):157–161
Sardoei AS (2022) Iranian medicinal plants: from economically to ethnomedicine studies. Int J Adv Biol Biomed Res 10(2):98–116
Sasaki M, Ishizaki K, Obata H, Goto F (2001) Effects of 5-HT2 and 5-HT3 receptors on the modulation of nociceptive transmission in rat spinal cord according to the formalin test. Eur J Pharmacol 424(1):45–52
Sayed-Ahmad B, Talou T, Saad Z, Hijazi A, Merah O (2017) The Apiaceae: ethnomedicinal family as source for industrial uses. Ind Crops Prod 109:661–671
Sharma A, Shanker C, Tyagi LK, Singh M, Rao CV (2008) Herbal medicine for market potential in India: an overview. Acad J Plant Sci 1(2):26–36
Shao Q, Li Y, Wang Q, Zhao J (2015) IL-10 and IL-1β mediate neuropathic-pain like behavior in the ventrolateral orbital cortex. Neuroch Res 40:733–739
Starowicz K, Malek N, Przewlocka B (2013) Cannabinoid receptors and pain. Wiley Interdiscip Rev Memb Transp Signal 2(3):121–132
Toksoy D, Bayramoglu M, Hacisalihoglu S (2010) Usage and the economic potential of the medicinal plants in Eastern Black Sea Region of Turkey. J Environ Biol 31(5):623–628
Tonussi CR, Ferreira SH (1994) Mechanism of diclofenac analgesia: direct blockade of inflammatory sensitization. Eur J Pharmacol 14(2):173–179
Turu G, Hunyady L (2010) Signal transduction of the CB1 cannabinoid receptor. J Mol Endocrinol 44(2):75–85
Zakaria ZA, Jaios ES, Omar MH, Abd Rahman S, Hamid SS, Ching SM, Teh LK, Salleh MZ, Deny S, Taher M (2016) Antinociception of petroleum ether fraction derived from crude methanol extract of Melastoma malabathricum leaves and its possible mechanisms of action in animal models. BMC Complement Alt Med 16(1):488
Zacher J, Altman R, Bellamy N, Brühlmann P (2008) Topical diclofenac and its role in pain and inflammation: an evidence-based review. Curr Med Res Opin 24(4):925–950
Zarei M, Ahmadimoghaddam D, Mohammadi S (2021) Artemisia biennis Willd.: Anti-Nociceptive effects and possible mechanisms of action. J Ethnopharmacol 268:113604
Zhu X, Tang HD, Dong WY, Kang F, Liu A, Mao Y (2021) Distinct thalamocortical circuits underlie allodynia induced by tissue injury and by depression-like states. Nat Neurosci 24(4):542–553
Acknowledgements
The project received funding from the Islamic Azad University, Tehran, Iran, under number: 1400456328.
Author information
Authors and Affiliations
Contributions
Design, study’s conception, perception, and interpretation: SM. Collecting the data: SJ. Technical assistance and critical revision: DZA. Drafting the manuscript: ZAZ.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing for financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Approval for animal ethics was acquired from the Science and Research Branch of Islamic Azad University, located in Tehran, Iran (Ethics approval: REC.1401.382) as well as followed the guidelines of the National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jabbari, S., Abed, D.Z., Zakaria, Z.A. et al. Effects of Chaerophyllum macropodum Boiss. leaves essential oil in inflammatory and neuropathic pain: uncovering the possible mechanism of action. Inflammopharmacol 31, 3203–3216 (2023). https://doi.org/10.1007/s10787-023-01342-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01342-6